Abstract
Background
Many individuals suffering from arthritis and other rheumatic diseases (AORD) supplement pharmacological treatments with psychosocial interventions. One promising approach, guided imagery, , has shown positive results in randomized controlled trials (RCTs) and is a highly scalable treatment for those with AORD.
Objectives
The main purpose of this study was to conduct a systematic review of RCTs that have examined the effects of guided imagery on pain, function, and other outcomes such as anxiety, depression, and quality of life in adults with AORD.
Data Sources
Ten electronic bibliographic databases were searched for reports of RCTs published between 1964 and 2013. Selection criteria included adult participants with AORD who participated in RCTs that used guided imagery as a partial or sole intervention strategy. Risk of bias was assessed using the Cochrane Risk of Bias Assessment Instrument. Results were synthesized qualitatively.
Results
Seven studies representing 306 enrolled and 287 participants who completed the interventions met inclusion criteria. The average age of the participants was 62.9 years (SD=12.2). All interventions utilized guided imagery scripts that were delivered via audio technology. The interventions ranged from a 1-time exposure to 16 weeks in duration. Risk of bias was low or unclear in all but one study. All studies, except one, reported statistically significant improvements in the observed outcomes.
Conclusions
guided imagery appears to be beneficial for adults with AORD. Future theory-based studies with cost benefit analyses are warranted.
Introduction
Arthritis and other rheumatic diseases (AORD) are a leading source of disability for millions of adults. It is estimated that 55.2 million adults in the United States self-report physician diagnosed arthritis, with estimated prevalence expected to reach more than 67 million by the year 2030 (Barbour et al., 2013). Arthritis and other rheumatic diseases include over 100 different conditions that are typically caused by inflammation, swelling, and pain in patients’ joints, ligaments, bones, muscles, and sometimes internal organs throughout the body (NIAMS, 2014). Adults with AORD often experience declines in lifestyle and recreational physical activity and are more prone to depression and anxiety (Covic et al., 2012; Kaplan, Huguet, Newsom, & McFarland, 2003; Murphy, Sacks, Brady, Hootman, & Chapman, 2012; Shih, Hootman, Kruger, & Helmick, 2006). While AORD can impact people of all ages, rheumatoid arthritis (RA), osteoarthritis (OA), and fibromyalgia are the most common AORD conditions experienced by adults, with prevalence estimates in the United States of 1.3 million (Helmick et al., 2008), 27 million (Lawrence et al., 2008), and 5 million (Lawrence et al., 2008) respectively. With an increasing tendency towards an older population, health care costs associated with AORD will likely continue to rise (Hootman & Helmick, 2006), supporting the need for strategies intended to help individuals cope with chronic pain and augment other treatments (Hochberg et al., 2012).
Treatment strategies for AORD generally include a combination of exercise, diet, and medications (NIAMS, 2014). Body weight management is particularly important for patients with AORD in order to reduce stress on painful joints. Pharmacological treatment for AORD depends on the disease being treated and the patient’s individual circumstances. For instance, disease-modifying non-biological and biological medications may be prescribed to patients who have been diagnosed with rheumatoid arthritis (RA) early in the disease (3 to 6 months) without a poor prognosis (Saag et al., 2008). Those with RA or osteoarthritis (OA) may also be prescribed non-steroidal anti-inflammatory drugs (NSAIDs) (MacDonald, 2000) while only duloxetine, milnacipran, and pregabalin are approved by the Food and Drug Administration for the treatment of fibromyalgia (NIAMS, 2014).
Due to the side effects, risks, financial burdens, and patient dissatisfaction with common pharmacological treatments (Nestoriuc, Orav, Liang, Horne, & Barsky, 2010; Page & Henry, 2000; Taylor, Everett, Taylor, Watson, & Taylor-Stokes, 2013; Woolf et al., 2004), many individuals suffering from AORD resort to psychosocial strategies. These may include, but are not limited to, relaxation, mindfulness meditation, or hypnosis (Jensen, 2011). Guided imagery has shown positive results, with respect to AORD-related outcomes, in randomized controlled trials (RCTs) (Baird, Murawski, & Wu, 2010; Baird & Sands, 2004; Baird & Sands, 2006; Fors & Gotestamm, 2000; Fors, Sexton, & Götestam, 2002; Lewandowski, Good, & Draucker, 2005; Menzies, Taylor, & Bourguignon, 2006). Guided imagery can be defined as a quasi-perceptual, multi-sensory, and a conscious experience that resembles the actual perception of some object, scene, or event but occurs in the absence of external stimuli (Thomas, 2014). Also known as “mental simulation” or “visualization,” this cognitive technique has deep historical roots, scientific interest, and popular applications. Psychologists have long used guided imagery to help individuals cope with pain, anxiety, and trauma (Thomas, 2014). Guided imagery interventions with AORD patients often begin with breathing or progressive muscle relaxation exercises and then proceed to images of movement and physical activity free of pain and stiffness (Baird et al., 2010). Importantly, guided imagery is inexpensive, relatively easy to teach, and can be readily applied in both clinical and community-based settings (Baird et al., 2010; Giacobbi, Dreisbach, Thurlow, Anand, & Garcia, 2014).
Given the overlap between the various psychosocial strategies used to treat AORD (Jensen, 2011), systematic reviews of one or more of these techniques helped inform the present review. One team of researchers systematically reviewed 12 randomized controlled trials (RCTs) with participants who were diagnosed with fibromyalgia (n = 5), osteoarthritis (n = 2), rheumatoid arthritis (n = 1), neck pain (n = 2), pain in the upper limbs (n = 1) and diffuse long term pain (n = 1). The studies included interventions that used relaxation techniques, massage, biofeedback, the provision of information, and cognitive behavioral techniques for the treatment of musculoskeletal pain (Persson, Veenhuizen, Zachrison, & Gard, 2008). While the authors concluded that relaxation training could be effective at pain reduction, their results should be interpreted with caution due to the heterogeneity of techniques used in the studies reviewed.
Another systematic review with meta-analysis that focused on fibromyalgia patients included six RCTs that tested the efficacy of hypnosis and guided imagery on pain, sleep, fatigue, depressed mood, and health-related quality of life (Bernardy, Fuber, Klose, & Hauser, 2011). The authors of this review included studies that combined imagery and hypnosis and pointed out that both approaches attempt to promote changes in emotion, sensation, perception, thought, and behavior by offering suggestion. While meta-analytic results showed significant reductions in pain, these observations were tempered by low methodological quality of the studies reviewed (adequacy of randomization, blinding of outcome assessor, and lack of intent to treat analyses). Effect sizes on the other outcomes considered in this meta-analysis were not calculated due to limited data. Collectively, the mixed results from these previous systematic reviews and meta-analyses demonstrate the need for more careful characterization of the psychosocial interventions included by previous authors.
The purpose of this study was to conduct a systematic review of RCTs that have examined the effects of guided imagery in adults with AORD in order to determine whether this intervention approach is effective at reducing pain, increasing function, or improving other outcomes such as anxiety, depression, and quality of life. A secondary purpose was to characterize the theoretical underpinnings and nature of imagery exposure by participants in RCTs used to treat AORD outcomes. The present study extends previous systematic reviews on the use of psycho-social strategies for the treatment of AORD by focusing on studies that used guided imagery and coding key methodological information not addressed in previous systematic reviews (e.g., theoretical frameworks, length of intervention, and cost-benefit analyses). The coded studies were not meta-analyzed due to methodological heterogeneity between studies.
METHODS
Study Eligibility Criteria
Inclusion criteria included the following: 1) RCTs with a comparison group; 2) adult participants ages 18 years and older; 3) use of guided imagery as the sole or partial intervention strategy; 4) focus on AORD; 5) publications in English from January 1, 1960 to June 1, 2013; and 6) results reported for pain, physical function, anxiety, depression, or quality of life . Studies were limited to RCTs because this research approach is the only way to control, by study design, for known confounders and the observation that nonrandomized approaches tend to overestimate treatment effects (Sacks, Chalmers, & Smith, 1982; Schulz, Chalmers, Hayes, & Altman, 1995).
Data Sources
Citations were retrieved from searching 10 electronic bibliographic databases (Academic Search Complete, Medline from Ebscohost, PsycInfo, Scopus, SPORTDiscus, Cochrane Central Register of Controlled Clinical Trials, Cumulative Index to Nursing and Allied Health Literature, Physiotherapy Evidence Database, Web of Science, and Eric), (2) cross referencing from retrieved studies, including systematic reviews and meta-analyses, and (3) hand searching specific journals. Variations of specific keywords were tested for relevancy to our topic and whether truncation would work best in different databases. Searched keywords included random, mental imagery, guided imagery, visualization, and relaxation: also used were randomly, randomized, and randomized to increase possible retrieval. The fifth author, a Health Sciences librarian, conducted all searches in consultation with the research team. An example of the search strategy used for one of the electronic databases (Medline from Ebscohost) is available upon request to the corresponding author. No attempt was made to search for unpublished data since previous research has suggested that these efforts may not be worth the effort (van Driel, de Sutter, Maeseneer, & Christiaens, 2009).
Study Selection
Studies were selected by the first three authors who independently reviewed all studies in close consultation with study personnel. During and between periodic meetings, the studies were reviewed for accuracy and consistency. If consensus could not be achieved, the last author was consulted and asked for a recommendation. A list of included and excluded studies, incorporating reasons for exclusion, was stored in a Microsoft Excel 2013 file.
Coding Sheet and Data Extraction
The codebooks were developed by the first author working closely with the senior investigator (GAK) on the team. The major categories of variables coded included the following: 1) study characteristics (year of publication, journal); 2) participant demographics; 3) length of intervention; 4) mode of intervention delivery; and 5) primary and secondary outcomes measured. Three doctoral students (MS, JS, AMJ) independently coded studies that met the inclusion criteria. Risk of bias was evaluated using the Cochrane risk of bias tool and included six known sources of bias in RCTs (Higgins & Green, 2009): 1) blinding of study personnel or participants to group assignment; 2) sequence generation of group assignment; 3) allocation of participants to treatment groups; 4) incomplete outcome data; 5) incomplete outcome reporting; 6) and other sources of bias (Higgins & Green, 2009). An important part of the coding process was to observe the length, nature, timing, and mode of intervention delivery for the reviewed studies. This decision was based on the flexibility for intervention delivery with guided imagery as this treatment approach can be delivered in person, by paper, or using electronic methods. Likewise, studies were examined as to whether cost-benefit analyses were conducted. Finally, studies were coded on apriori theoretical frameworks and if previous theorizing informed the content of imagery exposure.
Data Synthesis
Because of the expected heterogeneity with respect to such things as study design, participant characteristics, intervention and outcome variables being measured, an a priori decision was made to assess all results qualitatively.
RESULTS
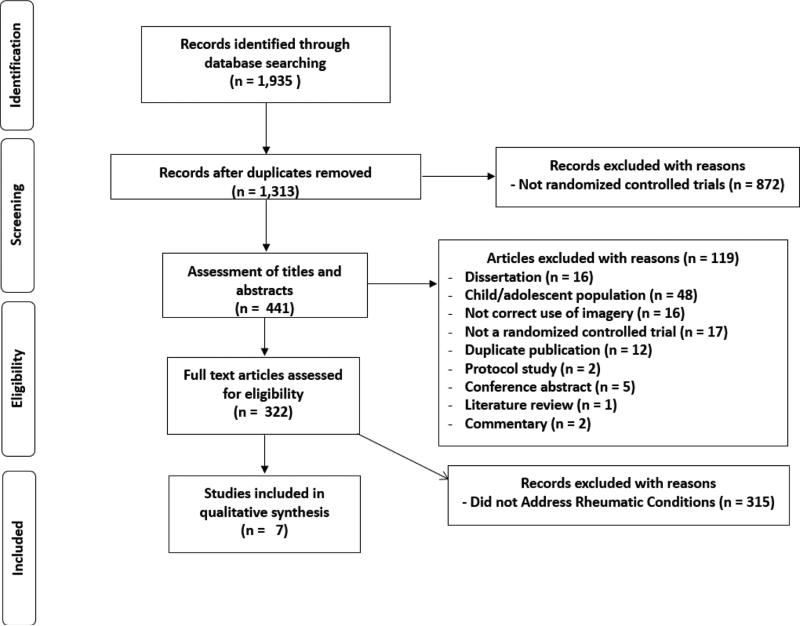
The characteristics of the studies reviewed are shown in Table 1. Of the 1,313 studies reviewed, 7 met the inclusion criteria. The studies included 16 groups (9 intervention and 7 control) representing 306 individuals, with 8 men and 282 women randomized to the various study arms (Baird and colleagues, 2004, 2006, 2010; Fors et al., 2000, 2002; Lewandowski, et al., 2004, Menzies et al., 2006). The selection process and reasons for exclusion are shown in Figure 1, while a full list of excluded studies are available upon request to the corresponding author.
Table 1.
Study characteristics and theoretical bases
| Study | Country | Participants | Imagery Intervention | Theoretical Underpinnings |
|---|---|---|---|---|
| Baird et al., 2004 | United States | 28 women over 65 years of age diagnosed with OA were assigned to GI plus PMR (Mage = 72.1) or usual care (Mage = 74.8). | 12 weeks listening to audio files twice daily. | Psychoneuromuscular theory. |
| Baird et al., 2006 | United States | Same sample as above. | 12 weeks listening to audio files twice daily. | Biopsychosocial and Psychoneuromuscular theories. |
| Baird et al., 2010 | United States | 30 older adults, gender breakdown unclear, who self-reported OA, moderate to severe pain, and mobility difficulties were randomized to GI plus relaxation (Mage = 71.9) or a sham intervention (Mage 62.6) (planned rest). | 16 weeks listening to audio files (12 minutes) twice daily. | Biopsychosocial and Psychoneuromuscular theories. |
| Fors et al., 2000 | Norway | 58 women (Mage 45.7) diagnosed with fibromyalgia were randomized to patient education (n = 22), guided imagery (n = 17) or a pain related talk group (n = 19). | 30 minutes “audio instructions.” | Not discussed. |
| Fors et al., 2002 | Norway | Same as above with 3 dropouts in the latter group. | 4 weeks; audio files | Hyper-vigilance theory; Adaptation theory. |
| Lewandowski et al., 2004 | United States | 37 women and 7 men between 34 to 90 years of age (median age = 61) who self-reported pain diagnoses from arthritis, fibromyalgia, and other conditions were randomized to guided imagery (n = 21) or control groups (n = 21). | 7 minute audio tape, 3 times a day for 4 days. | Science of “unitary beings.” |
| Menzies et al., 2006 | United States | 47 females and 1 men (Mage = 49.6) were randomized GI or usual care. | 6 weeks; audio files | Not discussed. |
Note 1: GI = guided imagery; Note 2 = PMR = progressive muscle relaxation; Note 2: OA = osteoarthritis; Note 3: AIMS – 2 = Arthritis Impact Measurement Scale – 2; Note 4: VAS = visual analog scale. Note 4: STAI-T = State-Trait Anxiety Inventory – Trait. Note 5: BDI = Beck Depression Inventory. Note 6: ATQ-30 = Automatic Negative Thoughts Inventory
Figure 1.
Flow diagram for selection of studies.
Imagery Exposure
Imagery exposure was compared to control conditions and was described by the authors as usual care plus journaling (Baird et al., 2004; Baird et al., 2006), a sham intervention involving planned rest, daily logs of medication use, weekly pain ratings (Baird et al., 2010), pain related talk with a therapist (Fors et al., 2000), or usual care (Fors et al., 2002; Lewandowski et al., 2004; Menzies et al., 2006).
Participant Characteristics
The sample sizes of the 7 studies ranged from 28 to 58 participants with an average age of 62.9 years. The breakdown by gender was disproportionate as only 2 of the 7 studies included men (Lewandowski et al., 2004; Menzies et al., 2006). One study included contradictory reports of gender breakdown in the published manuscript (Baird et al., 2010).
Outcomes
All 7 studies relied on self-report surveys to measure the primary and secondary outcomes shown in Table 2. These outcomes included pain in 4 of the studies (Baird et al., 2010, Fors et al., 2000, Lewandowski et al., 2004, Menzie et al., 2006), psychological well-being in 3 studies (Baird et al., 2004, Baird et al., 2006, Baird et al., 2010), anxiety and depression in 2 (Fors et al., 2000, 2002) and 1 study, respectively (Fors et al., 2002). Other outcomes included the meaning of pain (Lewandowski et al., 2004), fibromyalgia impact questionnaire, arthritis self-efficacy (Menzies et al., 2006), and journaling to measure medication usage (Baird et al., 2010).
Table 2.
Outcome measures and authors' conclusions
| Study | Outcome Measures | Authors' Conclusions |
|---|---|---|
| Baird et al., 2004 | AIMS - 2; mobility survey not reported. | Significant reductions in pain and mobility difficulties at week 12 compared to control group. |
| Baird et al., 2006 | Health related quality of life (AIMS – 2). | Significant increases in health related quality of life at week 12 compared to control group. |
| Baird et al., 2010 | Numeric rating scales of pain; mobility difficulty (AIMS - 2 (short forms); the Western McMasters Osteoarthritis Scale; journals to measure medication use. | Significant reductions in pain compared to control group after 4 months; Significant improvements in mobility from baseline to month 2; Significant reductions in prescribed analgesics from baseline to month 4 and total medication use from baseline to month 2 and month 2 to 4. |
| Fors et al., 2000 | Pain and anxiety using a VASs. | Patient education and guided imagery groups experienced significant reductions in pain and anxiety. |
| Fors et al., 2002 | Anxiety (STAI-T); depression (BDI); and automatic negative thoughts (ATQ-30). | Significant reductions in pain for those in the pleasant imagery condition compared to control group; no difference between attention imagery and control group. |
| Lewandowski et al., 2004 | Pain using a VAS and the McGill Pain Questionnaire; Power as Knowing Participation in Change Tool; Imagery Ability Questionnaire; Marlow-Crowne Social Desirability Scale. | Perceived pain improved in the treatment group. |
| Menzies et al., 2006 | McGill Pain Questionnaire (short form); impact scores; Fibromyalgia Impact Questionnaire; Arthritis Self-Efficacy Scale. | Significant reductions in FIQ scores in group exposed to imagery compared to controls; self-efficacy for managing pain and other symptoms of FM increased significantly with intervention group compared to controls. |
Statistically significant results supporting the use of guided imagery were observed in all 7 studies. In Baird et al., (2010), statistically significant reductions in pain and medication usage were accompanied by increased function and mobility with their 16-week intervention. Results by Fors and colleagues (2000, 2002) revealed acute reductions for pain and anxiety in a laboratory setting immediately following exposures to imagery while their efforts in 2002 reflected daily exposure to imagery and reductions in pain but not anxiety. Lewandowski's (2004) four day study revealed that participants exposed to guided imagery reported significantly reduced pain, as compared to the control group, during the last two days of the study. The only study to use a disease specific measurement scale produced statistically significant reductions in fibromyalgia symptoms and coping self-efficacy resulting from listening to a 20 minute audio file once daily for 21 days (Menzies, 2006). Finally, none of the 7 studies performed a cost-benefit analysis.
Intervention Characteristics and Theoretical Underpinnings
Baird and colleagues (2004, 2006) combined guided imagery with progressive muscle relaxation while other investigators combined different variations of relaxation exercises within their imagery interventions (Baird et al., 2010; Fors et al., 2002; Lewandowski et al., 2004; Menzies et al., 2006). Only one study did not report the use of relaxation exercises embedded within the imagery intervention (Fors et al., 2000).
All 7 interventions involved scripts delivered with audio files. Researchers in one of the 7 studies used a combination of researcher instructions and audio delivery of the imagery scripts (Fors et al., 2000). Fors and colleagues (2000) required participants to visit a clinical setting for administration of their intervention with sessions lasting 8 weeks. The remaining 6 studies involved home-based imagery practice (Baird and colleagues, 2004, 2006, 2010; Fors et al., 2002; Lewandowski et al., 2004; Menzies et al., 2006).
Between pre- and post-testing, the imagery interventions lasted 30 minutes (Fors et al., 2000) to 16 weeks (Baird et al., 2010). In a pair of 12-week investigations, participants were instructed to listen to their imagery audio files twice daily but no information was provided about the length of the audio files (Baird et al., 2004; Baird et al., 2006). It is important to note that Baird and colleagues (2004, 2006) relied on the same sample but reported outcomes related to self-reported pain and physical function in the earlier manuscript and psychological well-being in the latter. Similarly, Fors and colleagues (2000, 2002) used the same sample with one focusing on the acute effects of imagery exposure to a 30 minute audio file administered in a lab setting as previously described (Fors et al., 2000): this sample of participants and group assignments were then part of a prospective 4 week home-based intervention using the same audio files that consisted of once daily practice (Fors et al., 2002). A 16 week investigation also used twice daily practice with an imagery audio file that lasted 12 minutes (Baird et al., 2010). Lewandowski, 2004 and Menzies 2005 lasted 7 weeks using a 7 minute audio file practiced 3 times daily (Lewandowski et., 2004), and 6 weeks using an audio file lasting 20 minutes practiced once daily, respectively (Menzies et al., 2006).
The nature and content of the imagery scripts and authors’ reporting of information varied between studies. One study developed personalized scripts that were based on interviews of participants and included descriptions of specific joints and movements that caused pain and being in a relaxing place (Baird et al., 2004). Two other studies lead by Baird and colleagues (2006, 2010) also co-developed imagery scripts with participants that were characterized by attempts to focus participants’ attention to imagine physical movements without stiffness, pain, or hesitancy. The remaining four studies developed universal imagery scripts whereby all research participants were exposed to the same imagery scripts in the intervention (Fors and colleagues 2000, 2002; Lewandowski et al., 2004; Menzies et al., 2006). Only one study mentioned embedding music within the audio file (Fors et al., 2002). No information was provided about the voice characteristics within audio files in any of the studies reviewed.
Theoretical underpinnings of the imagery interventions were diverse and ranged from no discussion of theoretical bases (Fors et al., 2000; Menzies et al., 2006), an ambiguous presentation in one study (Lewandowski et al., 2004), to somewhat more sophisticated theorizing in others (Baird et al., 2004, 2006, 2010). Psychoneuromuscular (Jacobsen, 1932) and biopsychosocial (Engel, 1977) theoretical models were used in one series of studies with the same lead author (Baird et al., 2004, 2006, 2010). Briefly, psychoneuromuscular theory predicts that guided imagery may stimulate neurological pathways between the motor cortex of the brain to implicated musculoskeletal systems in a similar manner, albeit a lower amplitude, to when the movements are actually performed. The links between the guided imagery scripts used by Baird and colleagues (2004, 2006, 2010) with psychoneuromuscular theory focused on the specific movements that caused pain rather than the pain itself. Biopsychosocial theory (Engel, 1977) was the framework for addressing sensations of pain by Baird and colleagues (2004, 2006, 2010). This theory predicts that multiple factors affect pain sensation and imagery may initiate adaptive cognitive processes such as active coping, refocusing attention, distraction (Schoenfeld-Smith et al., 1996), reduced autonomic responses, reduced muscle contractions, and other responses similar to those occurring when individuals experience stress. An examination of ancillary material provided by Baird et al. (2004) showed clear connections between the imagery scripts and their theoretical frameworks.
Hyper-vigilance and adaptation theories informed theorizing in one study representing approach versus avoidance imagery (Fors et al., 2002). The former theory suggests that pain would increase with time when an individual focuses on the experience of pain and that chronic pain sufferers are overly focused on pain (Chapman, 1986). In contrast, adaptation theory suggests that pain would diminish when an individual repeatedly focuses on pain perceptions (Naliboff, Cohen, Schandler, & Heinrich, 1981). Hyper-vigilance and adaptation are more accurately viewed as hypotheses or contrasting perspectives on the pain experience and not theories. These contrasting pain perspectives were experimentally manipulated by Fors et al. (2002).
Finally, science of unitary beings (Rogers, 1992) was used as a conceptual model in one study (Lewandowski et al., 2004). This viewpoint holds that the experience of chronic pain is a lifestyle that evolves as a dynamic interplay between the person and environment and maintaining power, relationships, harmony, and control are ways of adapting and evolving as a person with pain. The imagery scripts used in this investigation targeted relaxation, sensory images related to pain, and sensory images intended to create personal change.
Risk of Bias
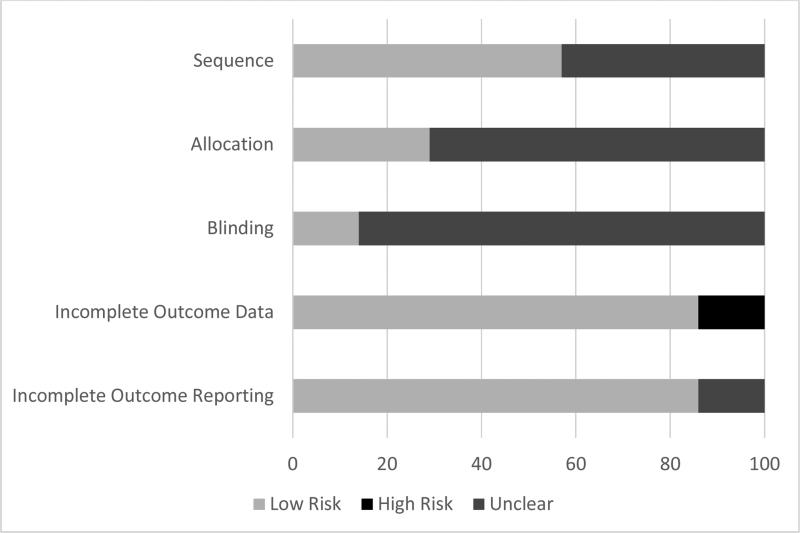
Risk of bias results are shown in Table 3 and Figure 2. As can be seen, a large percentage of items across all categories were classified as being at an unclear or low risk of bias. For sequence generation, four studies were at a low risk of bias (Baird et al., 2006, Fors et al., 2002, Lewandowski et al., 2004, Menzies et al., 2006) while the remaining three were at an unclear risk of bias (Baird et al., 2004, Baird et al., 2010, Fors et al., 2000). For allocation concealment, two studies were considered to be at low risk of bias (Fors et al., 2002, Menzies et al., 2006) while 5 were considered to be at an unclear risk (Baird et al., 2004, Baird et al., 2006, Baird et al., 2010, Fors et al., 2000, Lewandowski et al., 2004). Six studies were classified as being at an unclear risk of bias for blinding (Baird et al., 2004, 2006, 2010, Fors et al., 2000, Lewnandowski et al., 2005, Menzies et al., 2006) while one was considered low risk (Fors et al., 2000). For incomplete outcome data, six studies were classified as being at a low risk of bias (Baird et al., 2004, 2006, 2010, Fors et al., 2002, Lewnandowski et al., 2005, Menzies et al., 2006) while one was considered to be at a high risk (Fors et al., 2000). Finally, 6 studies were coded as low risk of bias for incomplete outcome reporting (Baird et al., 2006, 2010, Fors et al., 2000, 2002, Lewnandowski et al., 2005, Menzies et al., 2006) with one being unclear (Baird et al., 2004).
Table 3.
Cochrane Collaboration risk of bias
| Risk of bias | Baird et al., 2004 | Baird et al., 2006 | Baird et al., 2010 | Fors et al., 2000 | Fors et al., 2002 | Lewandowski et al., 2004 | Menzies et al., 2006 |
|---|---|---|---|---|---|---|---|
| Sequence | Unclear | Low | Unclear | Unclear | Low | Low | Low |
| Allocation | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low |
| Blinding | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Incomplete outcome data | Low | Low | Low | High | Low | Low | Low |
| Incomplete outcome reporting | Unclear | Low | Low | Low | Low | Low | Low |
Figure 2.
Pooled Cochrane Collaboration risk of bias
Within each study, one was categorized as low risk across all risk of bias domains (Fors et al., 2002), one was classified as low risk across four domains (Menzies et al., 2006), two were classified as low risk across two domains (Baird et al., 2004, 2010), and two were classified as low risk across three domains (Baird et al., 2006, Lewndowski et al., 2004). One study was considered to be at a high risk of bias in one domain (Fors et al., 2000). With the exception of one study (Fors et al., 2002), all others included at least one domain that was classified as being at an unclear risk of bias (Baird et al., 2004, Baird et al., 2006, Baird et al., 2010, Fors et al., 2000, Lewandowski et al., 2005, Menzies et al., 2006).
DISCUSSION
Overall Findings
The current systematic review provides evidence, with certain qualifiers, that guided imagery is an effective intervention for the treatment of AORD-related health conditions. Specifically, all seven studies reported results that support the use of guided imagery as a therapeutic tool for the treatment of pain (Baird et al., 2004, 2010, Fors et al., 2000, 2002, Lewandowski, 2004), improved psychological well-being (Baird et al., 2006, Menzies et al., 2006), improved mobility (Baird et al., 2004, 2010), reductions in anxiety (Fors et al., 2000), and increased self-efficacy managing pain and fibromyalgia symptoms (Menzies et al., 2006). The reviewed studies were relatively short in duration, presumably inexpensive, and administered in either a clinic or home-based setting. Given, the overall findings, these results suggest that guided imagery is worthy of further investigation given its observed benefits and potential cost-effectiveness in the treatment of adults with AORD.
Implications for Research
Several implications for future research are gleaned from this systematic review. First, there is a need for larger and longer-term RCTs that examine the impact of guided imagery on AORD outcomes. Such trials could be creatively administered using various technology platforms that include the internet, telephone, or the use of pre-recorded compact discs (CD). Indeed, these technologies create possibilities of population-based trials that should include more male participants and individuals from diverse socio-demographic backgrounds. These trials should explore potential dose-response effects and include follow-up measures to evaluate the impact of continued guided imagery practice over longer periods of time. In addition, to effectively implement GI treatment, the feasibility and acceptability of GI practices within populations of interest need to be well established via empirical future studies.
Greater methodological rigor should also be considered particularly with regard to the use of outcome measures and dose/response relationships. For instance, objective measures could be used in future RCTs that test the impact of guided imagery on AORD and may include assessments of strength, gait, balance, endurance, and other functional outcomes that can be readily administered in both clinical and community-based settings. These variables should be measured while blinding study personnel to group assignment of participants. Future investigators should also assess participant compliance with assigned imagery exposures in order to measure dose/response relationships and evaluate potential causal links between guided imagery exposure and outcomes. While a variety of procedures are possible, investigators could administer part or all of the imagery intervention in a laboratory setting where participants are administered audio scripts and time of exposure is measured.
The above recommendations highlight qualifiers and shortcomings of the RCTs in the present review. First, there was an unclear risk of bias for several of the studies with respect to study design (e.g., blinding, sequence generation, allocation concealment) (Baird et al., 2004, 2010; Fors et al., 2000). These concerns should be addressed in future RCTs with clear reporting of study design information consistent with CONSORT guidelines (Moher et al., 2010). A second shortcoming of the reviewed studies was that two sets of studies used the same sample that was reported in separate publications. Baird and colleagues (2004) reported improvements in pain and mobility while improved psychological well-being was reported in the second publication (Baird et al., 2006). Fors and colleagues (2000, 2002) also reported data from the same randomized participants in separate publications, however their second study involved a longitudinal comparative analysis with participants originally allocated to the three treatment arms. As discussed above, a third shortcoming of the reviewed studies was reliance on self-report for all the major outcomes which raises the possibility of response biases.
As discussed in this review, many of the RCTs used guided imagery along with relaxation exercises. From a scientific standpoint, imagery interventions should not include relaxation techniques so that the independent impact of guided imagery can be evaluated. Despite this scientific ideal, researchers typically combine imagery with relaxation because of common cognitive processes between psycho-social techniques for the treatment of pain (Jansen, 2011). Therefore, an important question for this review is how guided imagery is distinct from, but also complementary to, mindfulness meditation (MM), hypnosis, and relaxation techniques for the treatment of pain related to AORD. Guided imagery, like mindfulness meditation, hypnosis, and relaxation techniques, directs cognitive attention and increases awareness of individuals’ thoughts, feelings, and behaviors through acceptance of the pain and self-efficacy building visualizations (Jansen, 2011). Each of these techniques involves deliberate practice and requires conscious efforts to focus one's mind on breathing and or visualization. Autonomy is a central theme in these techniques as one could presumably choose to engage in guided imagery, relaxation, or mindfulness at any time or place: hypnosis requires induction and likely cannot be practiced in as many settings as the other techniques. Each of these techniques can also be self-taught or instructor guided. Guided training typically involves pre-written scripts, prompts, or outlines to facilitate individualized meditation and imagery. All of these methods can be safely and inexpensively delivered to various populations as a supplement to medication to provide comfort, relaxation, and a means of coping with pain and adversity.
Implications for Nursing Practice
The results of this review provide justification for nursing professionals to use of Guided imagery in clinical settings. Nursing personnel could facilitate the use of guided imagery with television, CDs, and the internet. Given the close association noted by other authors (Giacobbi et al., 2014) regarding links between verbalizations of experience and guided imagery, nurses could ask patients to describe various physical activities and movements that are conducted free of pain and stiffness. If patients respond with great detail to these questions it is likely they are engaging in mental imagery. Time permitting, nursing or other health personnel could co-develop guided imagery scripts with patients in order to maximize the impact of imagery on patient outcomes. Self-efficacy building statements should be embedded into the scripts. If possible, clinical personnel could maintain libraries of guided imagery scripts that can be used, shared, and re-used over time. A more efficient process would be to use guided imagery scripts with demonstrated effectiveness in research studies by creating audio scripts with documented effect. Standardized scripts could also be edited and personalized for individual patients.
Potential Limitations of the Current Study
The current study is subject to several potential limitations. For example, only published studies were included in the review process. Thus, the results may have been influenced by publication bias as studies that did not reach publication were not included. It is also possible that other inclusion and exclusion criteria may have led to selection bias. These include language (only those published in English), age limit (only studies with participants 18 years of age and above), and the limitations of the time frame for publication. Many of the studies included in the review used self-report questionnaires to collect data. As a result, the findings of the current study are also subject to errors in self-report questionnaires such as self-report bias. The generalizability of these findings might also be limited based on the participants included in each of the studies. Finally, while the researchers followed the Cochrane Collaboration guidelines for coding risk of bias (Higgins & Green, 2009), there is still a substantial amount of subjectivity in these assessments. As a result, others may have made different decisions in how selected items should be assessed.
Conclusions
The results of this qualitative systematic review suggest that guided imagery may improve selected outcomes in adults with AORD. However, additional, well-designed randomized controlled trials are needed in order to more fully substantiate AORD outcomes from the use of guided imagery. However, practitioners are encouraged to implement guided imagery in clinical settings using various technologies.
Acknowledgments
GA Kelley was partially funded by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under award number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baird CL, Murawski MM, Wu J. Efficacy of guided imagery with relaxation for osteoarthritis symptoms and medication intake. Pain Management Nursing: Official Journal Of The American Society Of Pain Management Nurses. 2010;11(1):56–65. doi: 10.1016/j.pmn.2009.04.002. doi: 10.1016/j.pmn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Baird CL, Sands L. A pilot study of the effectiveness of guided imagery with progressive muscle relaxation to reduce chronic pain and mobility difficulties of osteoarthritis. Pain Management Nursing. 2004;5(3):97–104. doi: 10.1016/j.pmn.2004.01.003. doi: 10.1016/j.pmn.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Baird CL, Sands LP. Effect of Guided Imagery with Relaxation on Health-Related Quality of Life in Older Women with Osteoarthritis. Research In Nursing & Health. 2006 doi: 10.1002/nur.20159. [DOI] [PubMed] [Google Scholar]
- Barbour KE, Stevens JA, Helmick CG, Luo YH, Murphy LB, Hootman JM, Sugerman DE. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation-United States. MMWR. 2013;62(44):869–873. [PMC free article] [PubMed] [Google Scholar]
- Bernardy K, Fuber N, Klose P, Hauser W. Efficacy of hypnosis/guided imagery in fibromyalgia syndrome--a systematic review and meta-analysis of controlled trials. BMC Musculoskelet Disord. 2011;12:133. doi: 10.1186/1471-2474-12-133. doi: 10.1186/1471-2474-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C, editor. Pain, perception, and illusion. Raven Press; New York: 1986. [Google Scholar]
- Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, Conaghan PG, Tennant A. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry. 2012;12:6–6. doi: 10.1186/1471-244X-12-6. doi: 10.1186/1471-244X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel GL. The need for a new medical model: A challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Fors EA, Gotestamm KG. Patient education, guided imagery and pain related talk in fibromyalgia coping. European Journal of Psychiatry. 2000;14(4):233–240. [Google Scholar]
- Fors EA, Sexton H, Götestam KG. The effect of guided imagery and amitriptyline on daily fibromyalgia pain: a prospective, randomized, controlled trial. Journal Of Psychiatric Research. 2002;36(3):179–187. doi: 10.1016/s0022-3956(02)00003-1. [DOI] [PubMed] [Google Scholar]
- Giacobbi PR, Jr., Dreisbach KA, Thurlow NM, Anand P, Garcia F. Mental imagery increases self-determined motivation to exercise with university enrolled women: A randomized controlled trial. Psychology of Sport and Exercise. 2014;15:374–381. doi: dx.doi.org/10.1016/j.psychsport.2014.03.004. [Google Scholar]
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, National Arthritis Data W. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis & Rheumatism. 2008;58(1):15–25. doi: 10.1002/art.23177. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane handbooks for reviews of interventions. 2009 [Google Scholar]
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Research. 2012;64(4):465–474. doi: 10.1002/acr.21596. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis & Rheumatism. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- Jacobsen E. Electrophysiology of mental activities. American Journal of Psychology. 1932;44:677–694. [Google Scholar]
- Jensen MP. Psychosocial approaches to pain management: An organizational framework. Pain. 2011;152(4):717–725. doi: 10.1016/j.pain.2010.09.002. doi: http://dx.doi.org/10.1016/j.pain.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Huguet N, Newsom JT, McFarland BH. Characteristics of physically inactive older adults with arthritis: results of a population-based study. Preventive Medicine. 2003;37(1):61–67. doi: 10.1016/s0091-7435(03)00059-8. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski W, Good M, Draucker CB. Changes in the meaning of pain with the use of guided imagery. Pain Management Nursing. 2005;6(2):58–67. doi: 10.1016/j.pmn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- MacDonald TM. Epidemiology and pharmacoeconomic implications of non-steroidal anti-inflammatory drug-associated gastrointestinal toxicity. Rheumatology. 2000;39(suppl.2):13–20. doi: 10.1093/rheumatology/39.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- Menzies V, Taylor AG, Bourguignon C. Effects of guided imagery on outcomes of pain, functional status, and self-efficacy in persons diagnosed with fibromyalgia. The Journal of Alternative and Complementary Medicine. 2006;12(1):23–30. doi: 10.1089/acm.2006.12.23. doi: 10.1089/acm.2006.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LB, Sacks JJ, Brady TJ, Hootman JM, Chapman DP. Anxiety and depression among US adults with arthritis: prevalence and correlates. Arthritis Care & Research. 2012;64(7):968–976. doi: 10.1002/acr.21685. doi: 10.1002/acr.21685. [DOI] [PubMed] [Google Scholar]
- Naliboff B, Cohen M, Schandler S, Heinrich R. Signal detection for chronic back patients and cohort controls to radiant heat stimuli. Journal of Abnormal Psychology. 1981;90:271–274. doi: 10.1037//0021-843x.90.3.271. [DOI] [PubMed] [Google Scholar]
- Nestoriuc Y, Orav EJ, Liang MH, Horne R, Barsky AJ. Beliefs about medicines predict non-specific side effects in rheumatoid arthritis patients. Arthritis Care & Research. 2010;62(6):791–799. doi: 10.1002/acr.20160. doi: 10.1002/acr.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAMS . Arthritis and Rheumatic Diseases. National Institutes of Health; Bethesda, Maryland: 2014. Retrieved from http://www.niams.nih.gov/Health_Info/Arthritis/arthritis_rheumatic_qa.asp#3. [Google Scholar]
- Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients. Archives of Internal Medicine. 2000;160:774–784. doi: 10.1001/archinte.160.6.777. [DOI] [PubMed] [Google Scholar]
- Persson AL, Veenhuizen H, Zachrison L, Gard G. Relaxation as treatment for chronic musculoskeletal pain – a systematic review of randomised controlled studies. Physical Therapy Reviews. 2008;13(5):355–365. doi: doi:10.1179/174328808X356366. [Google Scholar]
- Rogers ME. Nursing science and the space age. Nursing Science Quarterly. 1992;5:27–34. doi: 10.1177/089431849200500108. [DOI] [PubMed] [Google Scholar]
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Furst DE. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Care & Research. 2008;59(6):762–784. doi: 10.1002/art.23721. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- Sacks H, Chalmers TC, Smith H. Randomized versus historical controls for clinical trials. American Journal of Medicine. 1982;72(2):233–240. doi: 10.1016/0002-9343(82)90815-4. [DOI] [PubMed] [Google Scholar]
- Schoenfeld-Smith K, Petroski GF, Hewett JE, Johnson JC, Wright GE, Smarr KL. A biopsychosocial model of disability in rheumatoid arthritis. Arthritis Care & Research. 1996;9:368–375. doi: 10.1002/1529-0131(199610)9:5<368::aid-anr1790090505>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers L, Hayes RJ, Altman DG. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association. 1995;273(5):408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Shih M, Hootman JM, Kruger J, Helmick CG. Physical Activity in Men and Women with Arthritis: National Health Interview Survey, 2002. American Journal of Preventive Medicine. 2006;30(5):385–393. doi: 10.1016/j.amepre.2005.12.005. doi: http://dx.doi.org/10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Taylor SD, Everett SV, Taylor TN, Watson DJ, Taylor-Stokes G. A measure of treatment response: patient and physician satisfaction with traditional NSAIDs for osteoarthritis control. Open Access Rheumatology: Research and Reviews. 2013;5:69–76. doi: 10.2147/OARRR.S41940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NJT. Zalta EN, editor. Mental Imagery. The Stanford Encyclopedia of Philosophy. 2014 (Fall, 2014 ed.) [Google Scholar]
- van Driel ML, de Sutter A, Maeseneer JD, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. Journal of Clinical Epidemiology. 2009;62(8):838–e833. doi: 10.1016/j.jclinepi.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Woolf AD, Zeidler H, Haglund U, Carr AJ, Chaussade S, Cucinotta D, Martin-Mola E. Musculoskeletal pain in Europe: its impact and a comparison of population and medical perceptions of treatment in eight European countries. Annals of Rheumatic Diseases. 2004;63:342–347. doi: 10.1136/ard.2003.010223. doi: 10.1136/ard.2003.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]