Abstract
BACKGROUND
Sickle cell disease is characterized by hemoglobin (Hb) polymerization upon deoxygenation. Polymerization causes the sickle cells to become rigid and misshapen (sickling). Red blood cell (RBC) dehydration greatly increases polymerization. Cycles of sickling and unsickling cause an influx of calcium that leads to loss of potassium via the calcium-activated Gardos channel which dehydrates the cells leading to increased polymerization. In this study effects of NO and its congeners on RBC deformability were examined, focusing on sickle red blood cells.
STUDY DESIGN AND METHODS
Red blood cells from patients with sickle cell disease and from non-patients were exposed to various compounds that release NO or its congeners. Intracellular calcium was increased using a calcium ionophore or cycling of oxygen tension for sickle red blood cells. Deformability was measured by laser-assisted osmotic gradient ektacytometry.
RESULTS
Consistent with a previous report, sodium nitroprusside (SNP) was found to protect against calcium-induced loss of deformability in normal red blood cells, but (contrary to some previous reports) no effect of any NO donors was observed when calcium influx was not induced. Importantly, in studies of deoxygenation-induced dehydration of sickle RBCs, SNP resulted in substantial improvements in deformability (p=0.036) and hydration (p=0.024). Sodium nitrite showed similar trends. SNP was shown to have no effect on calcium influx, but reduced potassium efflux.
CONCLUSION
These data suggest SNP and perhaps certain nitrogen oxides (like nitrite) inhibit the Gardos channel and may be able to protect sickle cells from dehydration and thereby improve outcome in the disease.
Introduction
Sickle cell disease is a hemoglobinopathy characterized by hemolysis and vaso-occlusive crises caused by a mutation in the sixth amino acid of the β-globin subunit of hemoglobin wherein hydrophilic glutamate is replaced by hydrophobic valine. This mutation promotes polymerization of hemoglobin chains upon deoxygenation and formation of the T- quaternary state of hemoglobin. Polymerization distorts the normal discoid shape of an RBC sometimes forming the distinctive “sickle” shape that leads to vasoocclusion and episodes of painful crises.1
This deoxygenation-induced polymerization of sickle hemoglobin (HbS) creates a more permeable membrane2 susceptible to diffusion of cations such as Na+, K+, Mg2+, and, most notably, Ca2+. It has been shown that repeated cycles of sickling and unsickling lead to activation of the Gardos channel (KCa3.1), a calcium-activated potassium efflux channel and thus a key component of RBC dehydration and reduced deformability.3 Upon activation, K+ and water leave the cell at a rate limited by Cl− permeability, leading to RBC acidification and dehydration.4 The K:Cl cotransporter (KCC) –mediated KCl loss combined with K+ efflux via the Gardos channel result in rapid dehydration of the sickle red blood cell (sRBC).3 Dehydration increases the intracellular concentration of hemoglobin which enhances the rate of polymerization.56 Consequently, considerable research has been focused on the Gardos channel as a target for SCD therapies.7
Vasoocclusive and proinflammatory episodes are accompanied by increases in cytokine expression that have been shown to exhibit a positive correlation to the increase in dehydration in SCD.8,9 These cytokines lead to stimulation of a membrane oxidoreductase, protein disulfide isomerase (PDI), which has been shown to exist in higher concentrations in sRBC membranes compared with those on healthy RBCs. A recent study found a correlation between PDI redox status and Gardos Channel activity.10 Oxidized PDI leads to disulfide formation on the substrate protein. Reduced PDI leads to breaking of disulfides on the substrate protein (and subsequent potential rearrangement of substrate protein disulfides). The work of Romero et al suggests that formation of disulfide bonds in PDI (oxidized) leads to a less active Gardos channel.10 Thus, disulfide formation would decrease Gardos activity. Similarly, nitrosation could lead to reduced Gardos channel activity. In addition to PDI being a probable target for thiol oxidation and/or nitrosation reactions, the Gardos Channel is also susceptible since residing in its transmembrane domain are nine cysteine residues, four of which are located adjacent to the pore.11 The effects of nitric oxide (NO) on RBC deformability have been studied extensively with mixed results.12,13,14 One previous study examined effects on normal RBC deformability with SNP treatment prior to Gardos channel activation via addition of extracellular calcium and ionophore A23187, and it was determined that this treatment prevents loss of RBC deformability representative of cell dehydration.14
Sodium nitroprusside [Fe(CN)5NO]2−2Na+ (SNP) is neither a nitro compound, nor a prusside, however the name has been commonly accepted.15 In vivo, SNP is able to donate either NO or NO+, however in vitro studies have been unable to show any significant yield of either product. It has been proposed that NO is produced from the reaction of SNP and hemoglobin.16 Under aerobic conditions SNP reacts with a thiolate anion to form a disulfide17 (see appendix). Formation of disulfides has been suggested as a factor in reducing activity of the Gardos Channel activity.10,14
Nitrite reacts with deoxygenated Hb to form methemoglobin and NO18, and reacts with oxygenated Hb to form methemoglobin.18 However, several other nitrogen oxides and NO congeners are also thought to be produced in these nitrite/Hb reactions.18 These reactions can lead to thiol modification (see appendix for more details).18
We hypothesized that in addition to preventing the calcium-induced dehydration in healthy cells facilitated by ionophore A23187, SNP would protect sRBC from cyclic deoxygenation-induced dehydration and deformability loss and that nitrite may have similar effects.
Materials and Methods
All procedures involving human subjects were approved by the Wake Forest School of Medicine Internal Review Board.
Direct effects of NO donors
To test the effects of NO on RBC deformability, whole blood from 4 healthy volunteers was incubated with NO donors and agents that could affect nitric oxide synthase, and their deformability was measured via osmotic gradient ektacytometry. Whole blood was drawn from healthy volunteers into vacutubes with sodium heparin (BD, 367871). One milliliter samples were incubated for one hour at room temperature with 10 μM SNP (MP Biomedicals, 152061), 10 μM DEA-NONOate (Cayman, 82100), 1 mM L-NAME (Cayman, 80210) or 3 mM L-arginine (Sigma, A5006) as these concentrations of these compounds were previously shown to have significant effects on RBC deformability19. After one hour the samples were placed on ice and scanned in the ektacytometer.
Protective effect of SNP in normal RBCs
To confirm the protective effects of SNP on preventing calcium-induced RBC dehydration shown previously14 we replicated the experiments with washed RBC, 20% Hct. As in the experiments conducted by Rifkind and colleagues, calcium chloride (Sigma, C7902) combined with ionophore A23187 (Sigma, C7522) was used to initiate red cell dehydration. The experiments were then repeated with whole blood and higher concentrations of SNP, calcium and A23187. Blood was drawn from 3 healthy volunteers into sodium heparin and used immediately. One milliliter samples of whole blood were treated with 80 μM SNP and incubated at 37°C for 60 minutes before 10 μM ionophore A23187 and 20 μM Calcium Chloride were added, and the samples were incubated an additional 30 minutes. Samples were placed on ice and deformability was measured.
Sickle RBCs
Whole blood was drawn intravenously from 13 patients with sickle cell disease into sodium heparin tubes. Upon arrival of the samples (6–30 hours after draw), whole blood was aliquoted into four 1 ml samples. Whole blood (rather than washed red blood cells) was used in these experiments to more closely replicate physiological conditions. SNP (80 μM final concentration) was added to sample 3 and 10 μM nitrite was added to sample 4. All four samples were incubated at 37°C for 30 minutes, then 2 mM calcium chloride was added to samples 2–4. In some experiments these samples were put under positive nitrogen pressure to deoxygenate samples and prevent air from leaking in for 3 hours and then placed in an incubator at 37°C overnight. The samples then underwent cycles of oxygenation and deoxygenation at room temperature for another 3 hours with samples being placed in the incubator for five minutes in between each cycle to induce sickling. In other experiments the samples were prepared in the same manner but were simultaneously degassed and incubated at 37°C for 4 hours. The samples remained in the incubator while undergoing the same oxygenation and deoxygenation cycles described above. In both cases, the last cycle was reoxygenation with air. Sample 1 was kept as a control sample that was not degassed (did not undergo cycles of oxygenation and deoxygenation) but was incubated at 37°C alongside the other samples (Table 1). Though it is possible that some irreversibly sickled cells (ISCs) had already formed in the samples before experiments began, the aim of this study was to see if SNP could lessen deformability loss (whether due to formation of ISC or otherwise) due to hypoxia and Gardos activity. The extent to which ISCs were present before starting in vitro experiments would tend to decrease effects of SNP (assuming SNP cannot reverse ISC formation, but only prevent it). The variation in time between blood draws and initial in vitro manipulation of samples had no effect on the results.
Table 1.
Preparation of sickle RBC samples. SNP (80 μM) was added to sample 3 or sodium nitrite (10 μM) added to sample 4 and were incubated for 30 minutes at 37°C. Calcium chloride (2 mM) was then added to samples 2–4. The gray cells represent the samples that underwent hypoxia-reoxygenation cycling and when in the sample preparation the cycling was performed. Sample 1 remained a control and was not cycled.
| +80 μM SNP | +10 μM NO2− | +2 mM Ca2+ | ||
|---|---|---|---|---|
| Sample | 1 (n = 13) | |||
| 2 (n = 13) | X | |||
| 3 (n = 8) | X | X | ||
| 4 (n = 5) | X | X | ||
RBC deformability
Deformability of RBCs is extremely important for proper circulation through the vasculature as the diameter of the RBC is around 8 μm and the capillaries can be as narrow as 2 μm. In addition to alterations in cytoskeletal integrity, the increase in mean corpuscular hemoglobin concentration associated with SCD increases the cytoplasmic viscosity, rendering the cells less flexible.20 Osmotic gradient ektacytometry is a laser diffraction technique that quantifies deformability by the relation
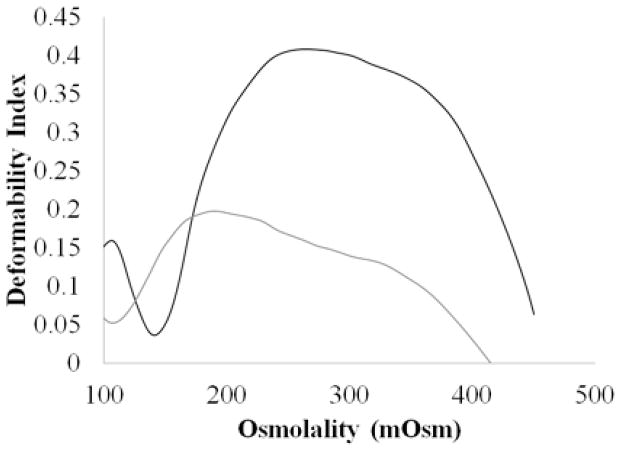
where L and W are RBC length and width, respectively, and DI represents the deformability index.21 Cells are suspended in a viscous medium and exposed to a shear stress in a viscometer that causes them to elongate. Osmotic gradient ektacytometry subjects cells to increasing osmolality that causes the cells to swell and shrink according to the extracellular osmotic pressure. Healthy RBCs have a maximum deformability (DI Max) at around 290 mOsm, while dehydration of the cells causes a shift to the left (lower osmolality) in DI Max. Since sickle RBC are so dehydrated they have a much lower DI Max with a corresponding osmolality shifted of the maximum peak to the left (lower osmolality).
A refurbished Technicon osmotic gradient ektacytometer (Technicon Instrument Corp.) was used in all experiments to measure the deformability of the RBC. A 20 centipoise (cP) carrier solution pH 7.4 was made from 31 g/L polyvinylpyrrolidone (PVP) (Sigma, PVP360), 0.24 g/L potassium phosphate monobasic (Sigma, S0751) and 0.90 g/L potassium phosphate dibasic (Fisher, BP332). Sodium chloride (Sigma, S7653) was used to make solutions of 40 mOsm (“low”), 290 mOsm (“sample”) and 750 mOsm (“high”). RBCs (150 μl) were diluted into a 4 ml sample solution and pumped into the ektacytometer where they were exposed to an increasing osmotic gradient produced by mixtures of low and high osmolarity solution. The cells are exposed to shear of 160 dynes/cm2 in a couette viscometer and the diffraction pattern recorded. Data was fit using a custom MATLAB program (R2012a, Mathworks) with a Savitsky-Golay noise reduction filter of polynomial order n=3.
Calcium influx
Given the limited availability of samples of sRBCs, RBCs from three healthy volunteers were washed 3 times (1500×g, 10 min 18°C) with PBS Ca2+ Mg+ buffer (Sigma) containing 5 mM glucose. 40 μL of washed RBC pellets were suspended with 1.2 mL of the buffer, and labeled with the calcium fluorophore, Fluo-3,AM (Life Technologies). Fluo-3,AM-labeled RBCs were diluted with the same buffer to a final hematocrit of 0.1%. Each RBC suspension was then divided into 1 mL aliquots, and treated with selected drugs.
SNP was initially added at selected concentrations of 20, 40, 60 or 180 μmol/L. Triplicates (195 μL) of each sample were then transferred into a dark 96-well plate. Baseline Fluo-3,AM fluorescence (intracellular calcium) was monitored for 30 min with 1 min intervals using a fluorescent plate reader (Bio-Tek). After baseline evaluation, 5 μL of A23187 (0.2 mM; final concentration in RBCs was 5 μM) were injected to all samples, and RBC Fluo-3,AM fluorescence was monitored for additional 90 min.
Potassium Efflux
To determine if the Gardos Channel plays a role in this mechanism, potassium efflux was quantified using atomic emission spectroscopy. For these experiments, whole blood was again drawn from three healthy volunteers directly into sodium heparin and used immediately. Blood was centrifuged at 1000 g for 10 minutes and the supernatant and buffy coat discarded. The cells were resuspended to 40% Hct with 0.01 M phosphate buffer pH 7.4, 290 mOsm. This process was repeated twice more or until supernatant was clear. RBC suspensions were aliquoted into three 1 ml samples. Sample 3 was treated with 80 μM SNP and incubated along with samples 1 and 2 at 37°C for 60 minutes. A solution containing 2 mM calcium chloride and 1 mM A23187 was diluted 100x into samples 2 and 3 and incubated alongside the control for an additional 30 minutes. Sample 1 was mock-treated as a control. Five hundred microliters were drawn, centrifuged and supernatants diluted 500x into Millipore water from each sample immediately after addition of the calcium mixture and again at t=0 minutes, t=10 minutes and t=30 minutes to measure potassium efflux as a function of time.
Statistics
Data from experiments examining effects of calcium and cycles of sickling and unsickling are presented as percent recovery, defined by the equation
| (5) |
where X represents either DI Max or Osm Max, and %Rec is the percent recovered using SNP compared to calcium treatment. The percent recovery using nitrite was calculated similarly. All data was evaluated by a student paired t-test with p<0.05 defining significance.
Results
Healthy RBCs have a maximum deformability (DI Max) at around 290 mOsm, while dehydration of the cells causes a shift to the left in DI Max. Since sickle RBC are susceptible to dehydration they have a much lower DI Max with a corresponding osmolality shifted of the maximum peak to the left (lower osmolality) (Figure 1). Our goal in this study was to see if agents could be employed to improve sickle RBC deformability and hydration that results from cycles of sicking and unsickling.
Figure 1.
Osmotic deformability curves for healthy (black) and sickle (gray) RBCs. Healthy RBCs are much more deformable with DI Max around 290 mOsm, while dehydrated sickle RBCs are much more rigid and Osm Max much lower.
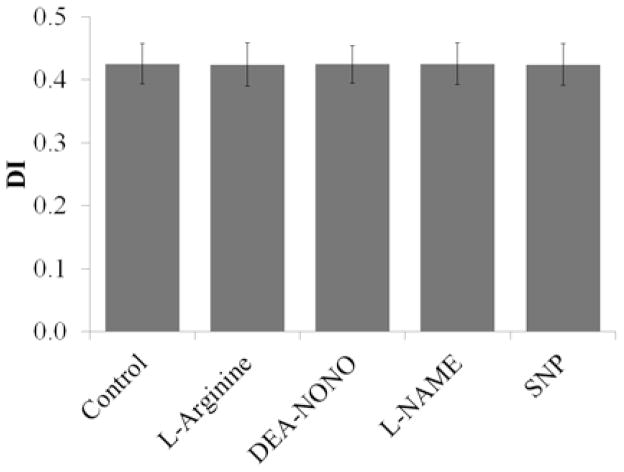
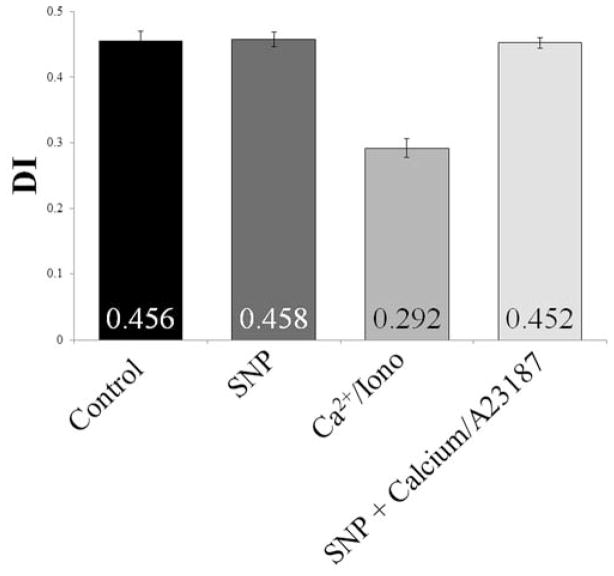
As previously reported by some14, but contrary to reports from others19, NO donors and agents that could affect nitric oxide synthase in the absence of excess calcium influx did not have an effect on DI Max (p≥0.30, Figure 2). SNP did, however, protect against loss in deformability due to calcium-induced dehydration in healthy cells. SNP alone had no effect on deformability (DI Max=0.458) compared with the mock-treated (control) cells (DI Max=0.456). The addition of calcium and A23187 caused a dramatic decrease in deformability (DI Max=0.292), however if the cells were pretreated with SNP before the addition of the calcium/ionophore solution, the deformability of the cells was comparable to that of the control or the cells treated with SNP alone (Figure 3).
Figure 2.
Average DI Max for healthy RBC treated with NO donors and agents that could affect nitric oxide synthase. NO donors DEA-NONOate and SNP (10 μM), NOS substrate L-arginine (3 mM) and NOS inhibitor (1 mM) were added to 1 ml fresh whole blood and incubated for 1 hour at room temperature (n=4, each trial from a different donor).
Figure 3.
Average DI Max for healthy RBC treated with or without SNP and/or the calcium with calcium ionophore A23187. From left to right: control; +80 μM SNP; +20 μM Ca2+ +10 μM A23187; +80 μM SNP +20 μM Ca2+ +10 μM A23187 (n=3, each trials from a different donor)
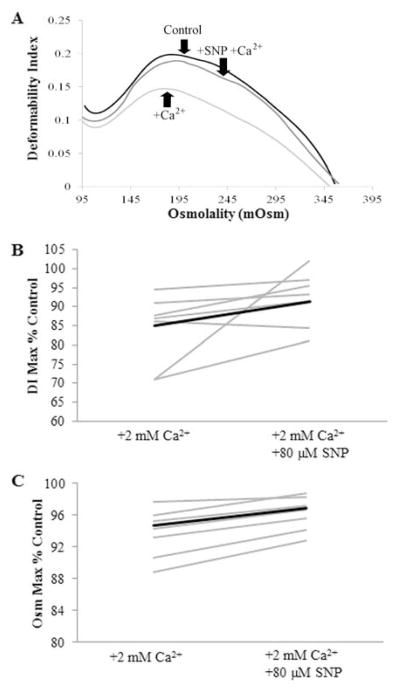
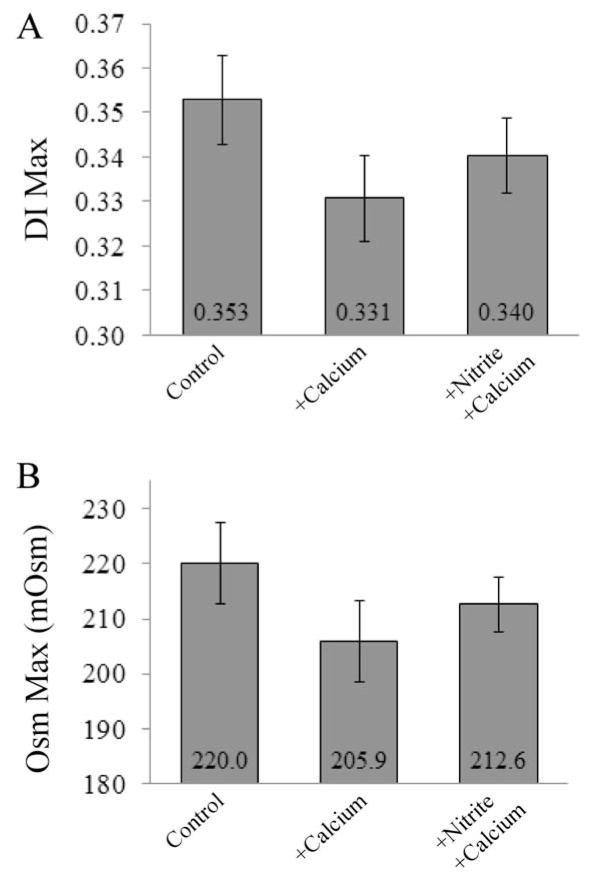
Sickle cells pretreated with SNP before inducing sickling and unsickling by oxy-deoxy cycling showed a significant improvement in DI Max when compared with untreated cycled cells. Representative data are shown in Figure 4A. Only one patient showed a decrease in deformability of just over 14% with the addition of SNP. In addition to preventing deformability loss, preincubation of the cells with SNP showed a significant improvement in Osm Max (Figure 4C). Though we did not directly measure O hyper, the osmolality at which the deformability is at half its maximum22, it followed the same trend as Osm Max – a leftward shift in dehydrated cells. The increased deformability combined with a rightward shift in osmolality is consistent with the hypothesis of a protective effect of SNP from calcium-induced dehydration.
Figure 4.
SNP effects on deformability of sickle red blood cells A. Osmotic deformability curves showing improvement in DI Max and Osm Max when sickle cells were precincubated with SNP. Sickle whole blood (black, DI Max=0.198) underwent several cycles of sickling and unsickling in the presence of 2 mM extracellular calcium (light gray, DI Max=0.147) to promote dehydration along with potential formation of irreversibly sickled cells. Samples that were pretreated with 80 μM SNP were protected from this calcium-induced dehydration (dark gray, DI Max=0.189). B. Percent control is the fraction of DI Max with Ca2+ and/or SNP to that of the control. All but one of the patients (grey) showed an increase in DI Max (p=0.036, DI Maxcalcium vs DI MaxSNP; n=7). The average (black) percent recovery of deformability loss with addition of SNP was 42.7 ± 36.7 % C. Percent control is the fraction of Osm Max with Ca2+ and/or SNP to that of the control. All of the patients’ RBC showed an increase in osmolality at DI Max when pretreated with SNP (p=0.024, Osm Maxcalcium vs Osm MaxSNP; n=8 patients). The average percent recovery in osmolality was 81.5 ± 71.1%.
When 10 μM sodium nitrite was used in place of SNP, there was an insignificant trend towards reduced dehydration regarding both DI Max and Osm Max. There was a percent recovery in DI Max of 25.4% (p=0.11; Figure 5A) and Osm Max of 32.5% (p=0.092; Figure 5B) (n = 5 patients).
Figure 5.
Nitrite effects on deformability and hydration of sickle red blood cells A. Average DI Max for sickled RBC treated with or without sodium nitrite (10 μM) and/or calcium chloride (2 mM). Nitrite had a small though insignificant protective effect against hypoxia-induced deformability loss (p=0.11; n=5 patients). B. Average Osm Max for sickled RBC treated with or without sodium nitrite (10 μM) and/or calcium chloride (2 mM). Nitrite had a small though insignificant protective effect against hypoxia-induced dehydration (p=0.092; n=5 patients).
The addition of SNP did not have an effect on calcium influx in healthy RBCs treated with calcium and ionophore A23187 (Figure 6A), however it reduced potassium efflux in ([K+]e) healthy RBC as shown by atomic emission spectroscopy (Figure 6B). At the end of the 30 minute incubation with 20 μM Ca2+ and 10 μM A23187, potassium efflux was reduced drastically in the samples that had been pretreated with 80 μM SNP. Samples pretreated with SNP showed an average [K+]e of 6.07 mM which was slightly higher than the control ([K+]e = 1.65 mM), but much less than cells treated with calcium and A23187 alone ([K+]e = 20.2 mM) for a total reduction in potassium efflux of 74.2±19.2% (n=3). While measurements of calcium influx and potassium efflux were conducted using RBC from healthy donors, it is likely that the effects would translate to sRBC.
Figure 6.
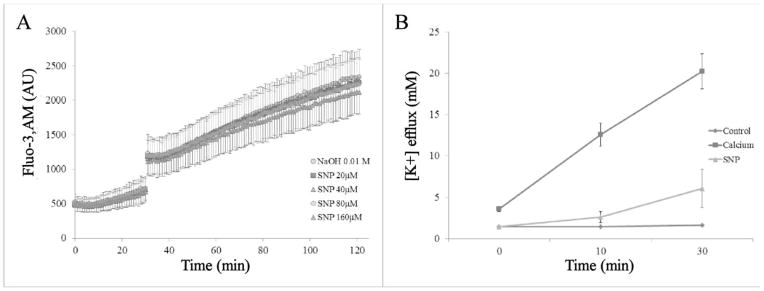
Effect of SNP on ion transport A. RBC Calcium influx in response to A23187 (5 μM) in the presence or absence of SNP: RBCs labeled with the calcium fluorophore, Fluo-3,AM, were treated with SNP at selected concentrations between 20 μM and 160 μM. Control RBCs were not treated with SNP or A23187. NaOH 0.01 M was the vehicle control for the SNP treatments. RBC Fluo-3,AM was monitored for 30 min, then A23187 was injected, and fluorescence was monitored for additional 90 min (n=3). B. Pretreating 40% Hct washed RBC (blue) with SNP (green) before addition of calcium and ionophore A23187 (red) blunted potassium efflux by 74.2±19.2% after 30 minutes (p=0.003, n=3, each trial from a different donor).
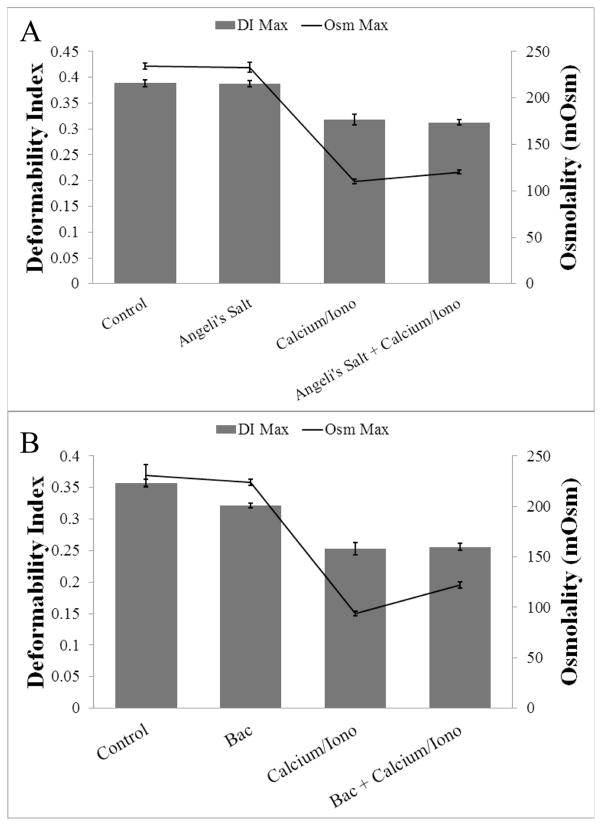
Given previously published results showing effects of oxidizing agents on Gardos channel activity10, we tested effects of several other oxidizing and nitrosating agents besides SNP and nitrite. Angeli’s Salt, a nitroxyl donor, did not demonstrate a protective effect against calcium-induced dehydration in healthy cells (Figure 7A). Bacitracin, a non-specific inhibitor of PDI, also showed no significant effect (Figure 7B). Hydrogen sulfide and a mixture of DEA-NONOate with cyanide were also tested but damaged the RBCs too severely to obtain good curves (data not shown). Thus, SNP and perhaps nitrite seem to have unique reactivity.
Figure 7.
Effect of other nitrosating and oxidizing agents on calcium-induced RBC dehydration in healthy RBCs. A. RBCs were pretreated with Angeli’s Salt (100 μM) before addition of calcium chloride (20 μM) and A23187 (10 μM). Gray bar graphs show average DI Max ± standard deviation (from left to right: 0.389±0.007, 0.388±0.006, 0.318±0.010, 0.313±0.006) and black line graph shows average Osm Max ± standard deviation (from left to right (in mOsm): 234.5±3.4, 232.9±5.6, 109.9±2.6, 120.3±1.9) (n=3 donors). B. RBCs were pretreated with Bacitracin (3 mM) before addition of calcium chloride (20 μM) and A23187 (10 μM). Gray bar graphs show average DI Max ± standard deviation (from left to right: 0.357±0.006, 0.321±0.004, 0.253±0.010, 0.256±0.005) and black line graph shows average Osm Max ± standard deviation (from left to right (in mOsm): 230.9±10.7, 223.5±3.1, 94.2±2.3, 122.1±2.9) (n=3 donors).
Discussion
Consistent with some previous reports14, but contrary to others12, we did not see an effect of NO donors alone, NO precursor L-arginine or NOS inhibitor L-NAME on RBC deformability. One explanation for the contradictory results could be the different shear stresses used, though one would think that changes in cellular dehydration, membrane stiffness, and surface-to-volume ratio would affect all DI at all shear stresses. While our experiments were performed at a constant high shear (160 dyn/cm2), other experiments were performed at a variable increasing shear and, in one study using SNP and Deta NONOate, only results at low shear were shown to have an effect12. In another study, DI max was calculated by fitting DI versus shear curves23. It is not specified which fitting method was applied in these studies, but a common fitting technique, Lineweaver-Burke method, is known to be inaccurate at low shear.24 The result of Bor-Kucukatay et al using Deta NONOate (half-life of 56 hours at room temperature) is also perplexing given how much NO must have been released in those experiments. After incubation for one hour at room temperature, the amount of NO provided by 1 μM Deta NONOate (which reversed decrease in deformability in the presence of NOS inhibitors and trended to increase deformability in absence of inhibitors12) would be about 10 nM. This small amount challenges mechanistic explanations. In any case, the effects of these agents, when observed to have an effect, are very small compared to those when calcium is allowed to enter cells.
We confirmed the results of Rifkind and colleagues14 showing a protective effect of SNP on calcium-induced dehydration of healthy RBC using osmotic gradient ektacytometry. In new, important experiments we found that SNP protected against loss of deformability and dehydration in RBCs from patients with SCD after cycles of hypoxia and reoxygenation. The deformability index and osmolality at peak deformability were both significantly improved when cells were pretreated with SNP before calcium influx via ionophore A23187 or cyclic hypoxia/oxygenation as was the case with sRBCs. Nitrite trended to have similar effects as SNP.
In the case of our experiments with healthy cells, we employed a calcium ionophore that is thought to bind to calcium on side of the membrane and diffuse through the membrane and then release the calcium on the other side. In our experiments with sickle cells we do not add an ionophore and calcium is thought to leak in through native channels due to cycles of sickling and unsickling which is associated with cellular deformation due to polymerization25. Due to these different mechanistic pathways of calcium influx, we believe that SNP does not influence calcium entry into the cell. This hypothesis is supported by our fluorescence experiments showing only a mild effect of SNP at high concentrations on calcium influx into healthy RBC. Rifkind et al measured intracellular calcium directly and showed reduced calcium uptake in cells pretreated with SNP, and these authors therefore attributed the protective effect of SNP to inhibition of CA uptake. However, our experiments measuring real-time calcium influx via fluorescence microscopy do not support this hypothesis.
To test the involvement of the Gardos Channel in these experiments involving SNP, we measured potassium efflux by atomic emission spectroscopy. Samples pretreated with SNP before addition of calcium/A23187 showed a reduction in potassium efflux of over 74%. These findings support the hypothesis of SNP interacting with the Gardos Channel directly or with neighboring proteins influencing Gardos Channel activity. SNP does not affect CA influx, yet diminishes potassium efflux and associated RBC dehydration.
There are many strong arguments supporting the hypothesis of an increased oxidative state in SCD. This imbalance between oxidant and antioxidant activity takes a toll on the cell and the surrounding tissue, leading to increases in cytokine expression and other adhesion molecules that further exacerbate the injurious conditions. Recently, it was discovered that activation of the ET-1 type B receptor led to increased activity of a ubiquitous oxidoreductase, PDI, residing in the RBC membrane that has been shown to have an effect on Gardos Channel activity in SAD sickle transgenic mice.10 This same group found that impermeable oxidizing agents have the same effect on PDI activity as specific PDI inhibitors in reducing Gardos Channel activity. Similarly, they found that thiol blocker phenylarsine oxide (PAO) had a smaller effect on blocking potassium efflux through the Gardos Channel. We propose that SNP interacts with either PDI or thiols in the Gardos Channel directly through nitrosation or oxidation, preventing [K+] efflux and corresponding RBC dehydration. However, other oxidizing or nitrosating agents tested did not have similar activity (Figure 7). SNP thus appears to have unique reactivity in the red cells that leads to Gardos channel inactivation. Further studies are needed to fully determine the mechanism by which this unique reactivity of SNP protects against calcium-induced dehydration of the cells.
It appears that NO alone is not capable of protecting against RBC stiffness caused by increased intracellular calcium as shown by us and Rifkind’s group who, in addition to some of the NO donors we used, tested nitroglycerine and acetyl-d-l-penicillamine at concentrations similar to those of SNP.14 No effect was seen. Sodium ferrocyanide (Fe(CN)6−4) and sodium cyanide, both of which resemble SNP structurally, also had no effect. A biotin switch assay and the Cu2+/ascorbate-reductive chemiluminescence assay were performed to detect any S-nitrosylation of thiols along the Gardos Channel, however none were detected. Lastly, Rifkind and coworkers tested glutathione and S-nitrosocysteine, neither of which had an effect. The unique reactivity of SNP is likely to modify PDI or the Gardos Channel directly through nitrosation and/or oxidation of thiols in ways that other nitrosating agents do not. Though our results with nitrite were not significant by student t-test, the trend we saw showing a protective effect similar to that of SNP supports our hypothesis of nitrosation or oxidation of PDI or Gardos Channel thiols.
Our results showing a protective effect of SNP warrant additional research. While the dose of SNP (80 μM) used in these experiments is substantially higher than that which has been used clinically without adverse effects26, it has been demonstrated that an infusion of 2 μg·kg−1·min−1 yielding a concentration of 20 μM resulted in no adverse effects27. Others have found that toxicity is associated with the rate of infusion, but less so with the duration26. Thus, chronic use is better. With this in mind, the total SNP deliverable to a patient may be comparable or higher than that used in this study. Further work is required to see if such infusions would result in clinical benefit.
The Gardos Channel is not a new target for potential therapies in sickle cell disease, as some specific blockers have been discovered and tested in recent years.7 Nevertheless, new therapeutic agents that inhibit Gardos channel activity are worth exploring. In addition, the finding that PDI and its oxidative state affect the activity of the Gardos Channel and RBC dehydration introduces a number of potential therapies.
Acknowledgments
We would like to thank George Donati of the chemistry department at Wake Forest University for his time and skills to help with the atomic absorption experiments and also Suchitra Barge of University of Pittsburgh Medical Center in providing sickle blood samples.
This work was supported by NIH grants HL058091 and HL098032. AB acknowledges support from T32 GM095440.
Appendix
Under aerobic conditions SNP reacts with a thiolate anion to form a disulfide17 as in equation (1).
| (1) |
Nitrite reacts with deoxygenated Hb to form methemoglobin and NO (eq. 218).
| (2) |
The intermediate species formed in eq. 2 represents NO bound to methemoglobin. This species, the ferric (Fe3+) nitrosyl is known to have character of the ferrous nitosonium (NO+). The NO+could nitrosate thiols.
Nitrite also reacts with oxygenated Hb to form methemoglobin18 (eq. 3).
| (3) |
Footnotes
Conflicts of Interest: Dr. Kim-Shapiro and Gladwin are listed as co-authors on a patent application for use of nitrite in cardiovascular diseases.
References
- 1.Bunn HF. Pathogenesis and Treatment of Sickle Cell Disease. New England Journal of Medicine. 1997;337:762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Franck P, Bevers E, Lubin B, Comfurius P, Chiu D, Kamp JOd, Zwaal R, Deenen Lv, Roelofsen B. Uncoupling of the membrane skeleton from the lipid bilayer. The cause of accelerated phospholipid flip-flop leading to an enhanced procoagulant activity of sickled cells. J Clin Invest. 1985;75:183–90. doi: 10.1172/JCI111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apovo M, Beuzard Y, Galacteros F, Bachir D, Giraud F. The involvement of the Ca-dependent K channel and of the KCl co-transport in sickle cell dehydration during cyclic deoxygenation. BBA Mol Basis of Dis. 1994;1225:255–8. doi: 10.1016/0925-4439(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 4.Bookchin RM, Lew VL. Sickle red cell dehydration: mechanisms and interventions. Curr Opin Hematol. 2002;9:107–10. doi: 10.1097/00062752-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Ferrone FA, Hofrichter J, Eaton WA. Kinetics of sickle hemoglobin polymerization: I. Studies using temperature-jump and laser photolysis techniques. Journal of Molecular Biology. 1985;183:591–610. doi: 10.1016/0022-2836(85)90174-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferrone FA, Hofrichter J, Eaton WA. Kinetics of Sickle Hemoglobin Polymerization .2. A Double Nucleation Mechanism. Journal of Molecular Biology. 1985;183:611–31. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- 7.McNaughton-Smith GA, Burns JF, Stocker JW, Rigdon GC, Creech C, Arrington S, Shelton T, de Franceschi L. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J Med Chem. 2008;51:976–82. doi: 10.1021/jm070663s. [DOI] [PubMed] [Google Scholar]
- 8.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92:2551–5. [PubMed] [Google Scholar]
- 9.Rybicki AC, Benjamin LJ. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood. 1998;92:2594–6. [PubMed] [Google Scholar]
- 10.Prado GN, Romero JR, Rivera A. Endothelin-1 receptor antagonists regulate cell surface-associated protein disulfide isomerase in sickle cell disease. FASEB J. 2013;27:4619–29. doi: 10.1096/fj.13-228577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tharp DL, Bowles DK. The intermediate-conductance Ca2+-activated K+ channel (KCa3. 1) in vascular disease. Cardiovasc Hematol Agents Med Chem. 2009;7:1–11. doi: 10.2174/187152509787047649. [DOI] [PubMed] [Google Scholar]
- 12.Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1577–H84. doi: 10.1152/ajpheart.00665.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–51. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 14.Barodka V, Mohanty JG, Mustafa AK, Santhanam L, Nyhan A, Bhunia AK, Sikka G, Nyhan D, Berkowitz DE, Rifkind JM. Nitroprusside inhibits calcium-induced impairment of red blood cell deformability. Transfusion. 2014;54:434–44. doi: 10.1111/trf.12291. [DOI] [PubMed] [Google Scholar]
- 15.Williams DLH. Nitrosation Reactions and the Chemistry of Nitric Oxide. 1. Amsterdam: Elsevier; 2004. [Google Scholar]
- 16.Friederich J, Butterworth J. Sodium Nitroprusside - 20 years and counting. Anesthesia and Analgesia. 1995;81:152–62. doi: 10.1097/00000539-199507000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Mulvey D, Waters WA. Reduction of alkaline aqueous disodium pentacyanonitrosylferrate(2-)(sodium nitroprusside) and kinetic features of its colour reaction with thiols. Journal of the Chemical Society, Dalton Transactions. 1975:951–9. [Google Scholar]
- 18.Patel RP, Hogg N, Kim-Shapiro DB. The potential role of the red blood cell in nitrite-dependent regulation of blood flow. Cardiovascular Research. 2011;89:507–15. doi: 10.1093/cvr/cvq323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhoda MD, Apovo M, Beuzard Y, Giraud F. Ca2+ permeability in deoxygenated sickle cells. Blood. 1990;75:2453–8. [PubMed] [Google Scholar]
- 20.Mohandas N, Chasis J. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Seminars in Hematology. 1993;30:171–92. [PubMed] [Google Scholar]
- 21.Groner W, Mohandas N, Bessis M. New optical technique for measuring erythrocyte deformability with the ektacytometer. Clinical Chemistry. 1980;26:1435–42. [PubMed] [Google Scholar]
- 22.Bareford D, Jennings PE, Stone PC, Baar S, Barnett AH, Stuart J. Effects of hyperglycaemia and sorbitol accumulation on erythrocyte deformability in diabetes mellitus. Journal of Clinical Pathology. 1986;39:722–7. doi: 10.1136/jcp.39.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grau M, Pauly S, Ali J, Walpurgis K, Thevis M, Bloch W, Suhr F. RBC-NOS-Dependent S-Nitrosylation of Cytoskeletal Proteins Improves RBC Deformability. PLoS ONE. 2013;8:e56759. doi: 10.1371/journal.pone.0056759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyeres P, Rabai M, Toth A, Kesmarky G, Marton Z, Alexy T, Toth K. Reviewing data reduction methods for ektacytometry. Clinical Hemorheology & Microcirculation. 2011;47:143–50. doi: 10.3233/CH-2010-1375. [DOI] [PubMed] [Google Scholar]
- 25.Brugnara C. Cation Homeostasis. In: Embury SHRH, Mohandas N, Steinberg MH, editors. Sickle Cell Disease: Basic Principles and Clinical Practice. New York: Raven Press; 1994. [Google Scholar]
- 26.Lockwood A, Patka J, Rabinovich M, Wyatt K, Abraham P. Sodium nitroprusside-associated cyanide toxicity in adult patients - fact or fiction? A critical review of evidence and clinical relevance. OAJCT. 2010;2010(2):133–48. [Google Scholar]
- 27.Schulz V, Gross R, Pasch T, Busse J, Loeschcke G. Cyanide toxicity of sodium nitroprusside in therapeutic use with and without sodium thiosulphate. Klin Wochenschr. 1982;60:1393–400. doi: 10.1007/BF01716244. [DOI] [PubMed] [Google Scholar]