Abstract
SIRT1, a NAD+ dependent histone deacetylase, has been shown to act as a key regulator of angiogenesis. The purpose of this study was to determine the effects of resveratrol (RSV, a SIRT1 activator) on the vascular endothelial growth factor receptor 2 (VEGFR2) signaling pathway and to establish its relevance to choroidal neovascularization (CNV), a blinding complication of age-related macular degeneration. Western blot and ELISA assay showed that RSV inhibited hypoxia-inducible factor (HIF)-1α accumulation and VEGF secretion induced by cobalt chloride (CoCl2) through SIRT1 in human retinal pigment epithelial (hRPE) cells. Furthermore, RSV down-regulated VEGFR2 phosphorylation and activation induced by VEGF in endothelial cells via SIRT1. Thus, the inhibitory effect of RSV on the HIF-1α\VEGF\ VEGFR2 signaling axis is mediated, at least in part, through SIRT1. The results suggest that targeting SIRT1 could have therapeutic potential for the treatment of CNV.
Keywords: RPE, SIRT1, Resveratrol, VEGFR2, HIF-1α, CNV
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world. Choroidal neovascularization (CNV) is a serious complication of late AMD, characterized by growth of immature choroidal blood vessels through Bruch membrane, where they can leak fluid or hemorrhage under the retina [1]. The retinal pigment epithelium (RPE) is a monolayer of cells that resides between Bruch membrane and the neural retina and it plays a central role in the pathogenesis of CNV. The RPE synthesizes and secretes several cytokines and growth factors that are important regulators of angiogenesis. Among them, vascular endothelial growth factor (VEGF) is considered to be a key factor for the induction of angiogenesis [2]. As a result, treatment with VEGF inhibitors is the current standard of care for CNV [3]. VEGF signaling is predominately mediated through activation of VEGF receptor 2 (VEGFR2) on endothelial cells, stimulating their cell proliferation and migration, thus promoting new vessel formation [4].
Resveratrol (RSV) is a natural polyphenol compound enriched in several plants and red wine. Previous studies have revealed its protective effects on the eye. It has been shown that RSV has anti-oxidant, anti-apoptotic, anti-inflammatory, and anti-angiogenic properties [5]. Many of the activities of RSV are mediated through sirtuin1 (SIRT1), which in turn acts by deacetylation of transcription factors and other cellular proteins [6]. Recent reports have shown that RSV can suppress hypoxia-induced VEGF expression and secretion in an immortalized endothelial cell line derived from monkey retina/choroid [7], human adult RPE cells [8] and macrophages [9]. Furthermore, systemic application of RSV inhibits angiogenesis in murine CNV models [9, 10]. In this study, we show for the first time, that the inhibition of VEGF/VEGFR2 signaling in low passage human RPE and endothelial cells is mediated, at least in part, through SIRT1, providing evidence for using RSV or targeting SIRT1 as a potential therapeutic approach for CNV.
2. Methods
2.1 Cells culture and Reagents
The Institutional Review Board of the University of Southern California approved our use of human RPE cells and conformed to the Declaration of Helsinki. HRPE cells were isolated from fetal human eyes of 18-20 weeks gestation (Advanced Bioscience Resources, Inc, Alameda, CA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Fisher Scientific, Pittsburgh, PA) with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO), and 10% heat-inactivated fetal bovine serum (FBS; Irvine Scientific, Santa Ana, CA). Second to fourth passage cells were used. Human umbilical vein endothelial cells (HUVECs) were purchased from ATCC (Manassas, VA) and were cultured in endothelial growth medium (EGM™ Bullet Kit, #CC-3124, Lonza, Switzerland) and used from passages 2 to 8.
Cobalt chloride (CoCl2; Sigma-Aldrich Co., MO, USA) solution was freshly prepared. RSV (Sigma) was dissolved in dimethylsulfoxide (Sigma) at a concentration of 100mM.
2.2 siRNA transfection
HRPE cells or HUVECs were seeded in 6-well plates transfecting with SIRT1 siRNA (10nM, sc-40986, Santa Cruz Biotechnology, Inc., Texas, USA) or control siRNA (10nM, sc-37007, Santa Cruz Biotechnology) using Lipofectamine RNAiMAX (Life Technologies, NY, USA).
2.3 Western blot assay
Cells (hRPE and HUVECs) were lysed in RIPA buffer (Cell Signaling Technology, Inc., MA, USA) and proteins were separated using 4-15% Tris-HCl polyacrylamide gradient gels (Bio-Rad) at 120 V. The proteins were transferred to polyvinylidene fluoride transfer membrane (Millipore, Billerica, MA). The membranes were blocked (5% milk) and incubated with primary antibodies overnight at 4°C. The membranes were washed and incubated with the relevant horseradish peroxidase (HRP)-conjugated secondary antibodies (Vector Laboratories, Inc. Burlingame, CA) for 1 hour at room temperature. Protein bands were developed by enhanced chemiluminescent (ECL) substrates (Thermo Scientific, Rockford, IL). All the results were calculated by mean pixel density x band area in arbitrary units using Image J software. GAPDH (MAB374, Millipore) was used a loading control. Antibodies detecting HIF-1α (#3716), VEGFR2 (#2479) and Phospho-VEGFR2 (#2478) were from Cell Signaling and SIRT1 (sc-15404) from Santa Cruz.
2.4 ELISA
VEGF concentrations were measured by ELISA (Quantikine; R&D Systems).
2.5 Statistical analysis
All experiments were repeated at least three times. Data are presented as mean±SEM. Differences were analyzed by ANOVA or Student's t test and P<0.05 was accepted as significant.
3. Results and discussion
3.1 Resveratrol inhibits HIF-1α expression and VEGF secretion in hRPE cells through activation of SIRT1
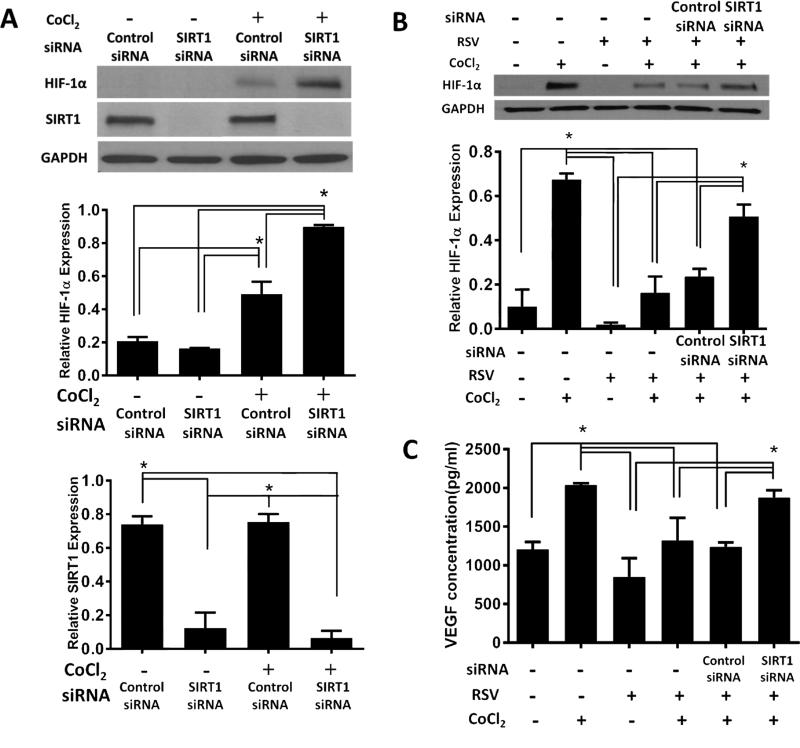
Based on preliminary experiments on cell viability and toxicity, we selected 100μM CoCl2 and 50μM RSV as optimal doses. Expression of SIRT1 protein in hRPE cells was significantly suppressed by SIRT1 siRNA transfection (Fig. 1A). CoCl2 was used to mimic hypoxia, a condition that plays an important role in the pathogenesis of CNV [11]. Treatment with CoCl2 resulted in increased protein expression of HIF-1α protein and suppression of SIRT1 further enhanced this effect (Fig. 1A). Importantly, pretreatment of hRPE cells with RSV significantly inhibited HIF-1α protein expression induced by CoCl2 (Fig. 1B) and knocking down SIRT1 attenuated the inhibitory effects of RSV on HIF-1α expression (Fig. 1B).
Fig. 1.
(A) Effects of SIRT1 on the expression of HIF-1α in hRPE cells. Cells were transfected using specific SIRT1 or control siRNA for 24 hours following by treatment with or without CoCl2 (100μM) for another 3 hours. Representative Western blots and densitometry analysis are shown. SIRT1 protein expression was significantly inhibited by siRNA (*p<0.05). HIF-1α protein expression was increased after adding CoCl2 (*p<0.05). Knocking down SIRT1 further augmented this increase (*p<0.05). (B) RSV inhibits HIF-1α through SIRT1. HRPE cells were transfected using specific SIRT1 or control siRNA following by treatment with or without RSV for 16 hours before co-treatment with or without CoCl2 for another 3 hours. RSV impaired HIF-1α accumulation induced by CoCl2 and knocking down SIRT1 attenuated the inhibitory effect of RSV on HIF-1α expression (*p<0.05). (C) RSV decreases VEGF secretion through SIRT1. Methods as in Fig.1B. Secreted VEGF protein in hRPE cell supernatant was analyzed by ELISA. RSV inhibited VEGF secretion induced by CoCl2 and knocking down SIRT1 attenuated the inhibitory effect of RSV on VEGF expression (*p<0.05).
VEGF (VEGF165) is an important target gene of HIF1 [12]. VEGF levels in the hRPE supernatants were increased under hypoxia but decreased after pretreatment with RSV (Fig.1C) as shown previously [8]. This decrease was prevented by knocking-down SIRT1, demonstrating that RSV inhibition of VEGF expression in hRPE cells was mediated mainly through SIRT1.
3.2 Resveratrol suppresses VEGFR2 phosphorylation in HUVECs via SIRT1
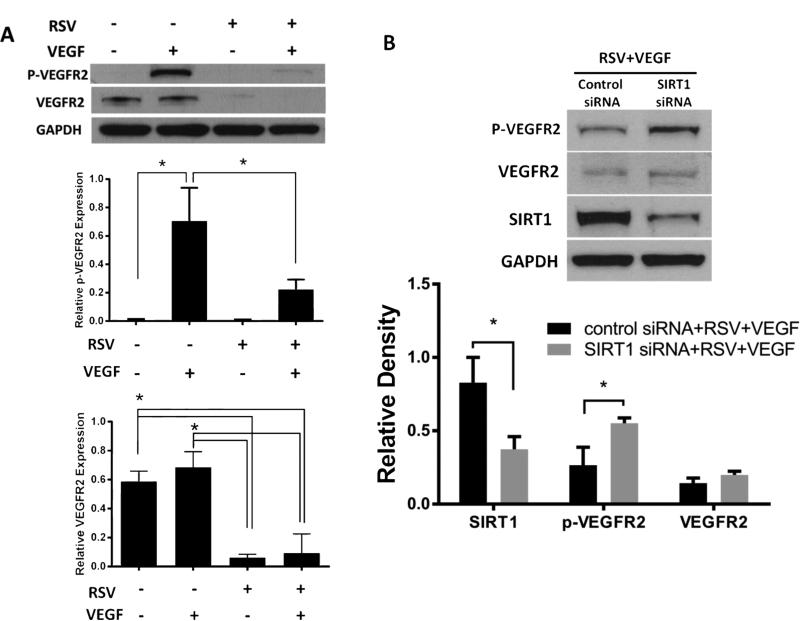
Next, we explored the expression and activation of VEGFR2 after RSV treatment in endothelial cells (HUVECs). Although it would be more physiologically relevant to study choroidal endothelial cells (CEC), we were unable to utilize CEC in these experiments due to their heightened sensitivity to transfection reagents, resulting in excessive cell death (results not shown). As expected, VEGF induced the phosphorylation of VEGFR2 without influencing total VEGFR2 expression (Fig.2A). Pretreating the cells with RSV reduced total VEGFR2 expression (Fig.2A); importantly, phosphorylation of VEGFR2 stimulated by VEGF was also inhibited (Fig.2A). This result is consistent with the report that a RSV derivative, Trans-3,4,5,4'-tetramethoxystilbene (DMU-212), also suppressed VEGF-induced phosphorylation of VEGFR2 in HUVECs [12]. To further establish a role for SIRT1 in this process, we used SIRT1 siRNA to knock-down SIRT1 expression. Here, we (Fig.2B) showed, for the first time, that knocking down SIRT1 significantly reversed the inhibitory effects of RSV on VEGFR2 phosphorylation induced by VEGF (p<0.05) , suggesting RSV inhibits phosphorylation of VEGFR2 in HUVECs, at least in part, via SIRT1.
Fig. 2.
(A) RSV suppresses VEGFR2 expression and phosphorylation in HUVECs. Cells were pretreated with or without RSV for 16 hours before co-treatment with or without VEGF (25 mg/ml) for another 10 minutes. Western blots with densitometry analysis were performed. Data show that VEGF induced phosphorylation of VEGFR2 (*p<0.05) without influencing total VEGFR2 expression. RSV significantly decreased total VEGFR2 (*p<0.05) and VEGFR2 phosphorylation induced by VEGF (*p<0.05). (B) RSV suppresses VEGFR2 phosphorylation in HUVECs via SIRT1. Methods as in Fig.2B. Knocking down SIRT1 resulted in increased phospho-VEGFR2 expression compared with control siRNA group (*p<0.05).
In summary, we demonstrate that RSV can disrupt HIF-1α-mediated up-regulation of VEGF expression under hypoxia through activation of SIRT1 in RPE cells. We further show that RSV inhibits VEGF induced phosphorylation of VEGFR2 in endothelial cells via SIRT1. Thus RSV inhibits the HIF-1α/VEGF/VEGFR2 signaling axis in choroidal neovascularization-related cells, at least in part, through SIRT1. These results provide further support for evaluation of SIRT1 as a potential therapeutic target for CNV.
Acknowledgments
Grant information: NIH grants EY01545, EY03040; Research to Prevent Blindness, Inc.; China Scholarship Council (CSC program)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grossniklaus HE, Green WR. Choroidal neovascularization. American journal of ophthalmology. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Strauss O. The retinal pigment epithelium in visual function. Physiological reviews. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 3.Veritti D, Sarao V, Lanzetta P. Neovascular age-related macular degeneration. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 2012;227(Suppl 1):11–20. doi: 10.1159/000337154. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Wang H, Jiang Y, Hartnett ME. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. The American journal of pathology. 2014;184:1230–1239. doi: 10.1016/j.ajpath.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bola C, Bartlett H, Eperjesi F. Resveratrol and the eye: activity and molecular mechanisms. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2014 doi: 10.1007/s00417-014-2604-8. DOI 10.1007/s00417-014-2604-8. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni SS, Canto C. The molecular targets of Resveratrol. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbadis.2014.10.005. DOI 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Balaiya S, Murthy RK, Chalam KV. Resveratrol inhibits proliferation of hypoxic choroidal vascular endothelial cells. Molecular vision. 2013;19:2385–2392. [PMC free article] [PubMed] [Google Scholar]
- 8.Nagineni CN, Raju R, Nagineni KK, Kommineni VK, Cherukuri A, Kutty RK, Hooks JJ, Detrick B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-related Macular Degeneration. Aging and disease. 2014;5:88–100. doi: 10.14366/AD.2014.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai N, Kubota S, Tsubota K, Ozawa Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. The Journal of nutritional biochemistry. 2014 doi: 10.1016/j.jnutbio.2014.05.015. DOI 10.1016/j.jnutbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Kanavi MR, Darjatmoko S, Wang S, Azari AA, Farnoodian M, Kenealey JD, van Ginkel PR, Albert DM, Sheibani N, Polans AS. The sustained delivery of resveratrol or a defined grape powder inhibits new blood vessel formation in a mouse model of choroidal neovascularization. Molecules. 2014;19:17578–17603. doi: 10.3390/molecules191117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Progress in retinal and eye research. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen LK, Qiang PF, Xu QP, Zhao YH, Dai F, Zhang L. Trans-3,4,5,4′-tetramethoxystilbene, a resveratrol analog, potently inhibits angiogenesis in vitro and in vivo. Acta pharmacologica Sinica. 2013;34:1174–1182. doi: 10.1038/aps.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]