Abstract
Antimicrobial peptides (AMPs) play an important role as a host defense against microbial pathogens and are key components of the human innate immune response. Neisseria meningitidis frequently colonizes the human nasopharynx as a commensal but also is a worldwide cause of epidemic meningitis and rapidly fatal sepsis. In the human respiratory tract, the only known reservoir of N. meningitidis, meningococci are exposed to human endogenous AMPs. Thus, it is not surprising that meningococci have evolved effective mechanisms to confer intrinsic and high levels of resistance to the action of AMPs. This article reviews the current knowledge about AMP resistance mechanisms employed by N. meningitidis. Two major resistance mechanisms employed by meningococci are the constitutive modification of the lipid A head groups of lipooligosaccharides by phosphoethanolamine and the active efflux pump mediated excretion of AMPs. Other factors influencing AMP resistance, such as the major porin PorB, the pilin biogenesis apparatus, and capsular polysaccharides, have also been identified. Even with an inherently high intrinsic resistance, several AMP resistance determinants can be further induced upon exposure to AMPs. Many well-characterized AMP resistance mechanisms in other Gram-negative bacteria are not found in meningococci. Thus, N. meningitidis utilizes a limited but highly effective set of molecular mechanisms to mediate antimicrobial peptide resistance.
Graphical abstract
1. Introduction
Neisseria meningitidis, the meningococcus, is a Gram negative aerobic encapsulated diplococcal β-proteobacterium. Meningococci are carried asymptomatically by 5 to 10% of the overall population in non-epidemic periods and are transmitted from a carrier by aerosol droplets or respiratory secretions. N. meningitidis is unique among the major bacterial agents of meningitis in that it causes epidemic as well as endemic (sporadic) disease. Approximately 500,000 cases of invasive meningococcal disease have occurred annually worldwide, with at least 50,000 deaths and as many survivors suffering neurological sequelae [1]. The meningococcus causes a range of disease: rapid onset meningitis and severe sepsis (meningococcemia), septic arthritis, pneumonia, purulent pericarditis, conjunctivitis, otitis, sinusitis, and urethritis. Meningococci are classified by serologic typing based on the biochemical composition of the capsular polysaccharides (serogroup), major outer membrane porin proteins (serotype), other outer membrane proteins (serosubtype), and lipooligosaccharide (immunotype). Of the 12 serogroups identified, almost all of invasive cases are caused by meningococci that express one of six capsular polysaccharides (serogroups A, B, C, X, Y, and W) and most epidemic and endemic cases of meningococcal disease are caused by a limited number of clonal groups defined genetically using multilocus sequence typing (MLST). The US licensed vaccines against N. meningitidis are based on capsular polysaccharides (CPS) with the more recent development of CPS-protein conjugate vaccines for different combinations of serogroups A, C, Y and W [2-5]. New serogroup B vaccines using sub-capsular surface antigens are now approved in the US, Europe, Australia and Canada.
2. Antimicrobial peptides
Antimicrobial peptides play an important role in host defense against microbial infection. In addition to being major components of the innate immune response, AMPs also have many potential roles in inflammatory responses by inducing the secretion of chemokines and cytokines [6]. Antimicrobial peptides are peptides of 12–50 amino acids with excess of basic amino acids (arginine, lysine and histidine), thus resulting in a net positive charge (cationic). AMPs also generally have significant portion of hydrophobic amino acids residues and are amphipathic to facilitate interaction with bacterial membranes. Based on their structural characteristics, AMPs are classified into different categories [7]. The most common classes are β-sheet peptides stabilized by disulfide bonds such as β-defensins [8, 9], and amphipathic α-helices formed upon contact with membranes such as α –defensins, cathelicidin and LL-37 [10-12]. Less common are extended peptides with a predominance of one or two amino acids (e.g. proline, tryptophan or histidine) and peptides with loop structures formed by either a single disulfide bond such as bactenecins. A cyclic lipopeptide, polymyxin B (PMB), has long been used as a model compound to define the mechanisms by which AMPs kill bacteria and how bacteria develop resistance to antimicrobial actions of AMPs.
The initial electrostatic interaction of the positively charged AMPs with the negatively charged lipopolysaccharides of the outer leaflet of the outer membrane is believed to initiate the self-promoted uptake of AMPs in Gram-negative bacteria [13]. Subsequently, both electrostatic and hydrophobic interactions between AMPs and the inner membrane phospholipids are critical for AMP’s antimicrobial activity that disrupts membrane integrity. As the membrane-peptide complexes are insoluble and non-crystalline, solid-state NMR studies of AMPs [14] have been used to obtain structure, dynamics, orientation, and oligomeric states of AMPs in a membrane environment [15, 16] as well in lipopolysaccharide micelles [17-20]. These biochemical studies provide important information about the mechanism of action of AMPs at molecular level. Further, recent studies suggested that AMPs are also able to act on intracellular targets following their translocation across the inner membrane either as a main mode of action or as additive effects combining with membrane disruption [13, 21]. Expression of AMPs is widespread in many cell types. AMPs are constitutively produced by phagocytic cells such as macrophages and neutrophils [6]. For example, defensins have been shown to be the most abundant protein species in neutrophils [22]. Mucosal epithelial cells also constitutively expressed AMPs and AMP production can be further induced following exposure to bacterial determinants [23]. AMPs can also be formed by proteolytic digestion of larger cationic proteins such as lactoferricin, a proteolytic cleaved product from the N-terminus of lactoferrin [24].
3. AMP resistance mechanisms in N. meningitidis
Following acquisition through close contact with a carrier, meningococci overcome clearance and other local specific and nonspecific mucosal host defenses in order to colonize the upper respiratory mucosal surfaces (e.g., the nasopharynx). Colonization of N. meningitidis may also result in invasion of epithelial surfaces, access to the bloodstream and the production of systemic and focal infections. As the only natural reservoir of N. meningitidis is the human nasopharynx, meningococci constantly encounter endogenous antimicrobial defense including antimicrobial peptides during colonization and infection. Thus, it is not surprising that meningococci have developed mechanisms for conferring intrinsic and/or inducible resistance to the action of AMPs. AMP resistance mechanisms have been well-characterized in various Gram negative bacteria to include (i) efflux pumps that export AMP from the periplasmic and intracellular compartments [25]; (ii) structural modifications of lipopolysaccharide (LPS) and lipooligosaccharide (LOS) to reduce interaction with AMPs; (iii) modulation of outer membrane permeability to limit entry and/or enhance excretion of AMPs; and (iv) proteases that degrade AMPs [26, 27]. Here we summarize the current knowledge of AMP resistance mechanisms as well as other characteristics that influence AMP resistance in N. meningitidis (Figure 1) in the order of importance. The inducible effects of AMPs on some of these resistance determinants will also be discussed.
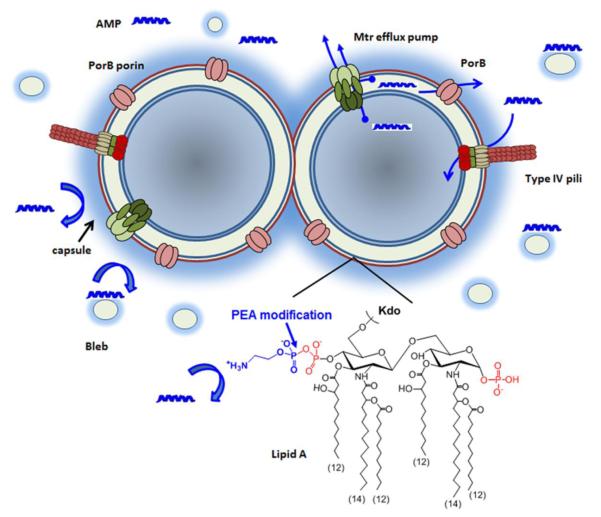
Figure 1.
A schematic summary of cellular factors influencing antimicrobial peptide resistance in Neisseria meningitidis. AMPs can initiate the self-promoted uptake through electrostatic interactions with negatively charged lipopolysaccharides of the outer membrane or through the secretin apparatus of type IV pili. The constitutive PEA modification of lipid A head groups reduces electrostatic interactions of AMPs with the cell envelop, while both capsule and bleb act to sequester AMPs from reaching the cell surface. The Mtr efflux pump can expel AMPs from either periplasm or cytoplasm by active efflux, and the major outer membrane porin, PorB, also likely functions as an excretion channel.
3.1 Lipooligosaccharide (LOS)
Lipopolysaccharide (LPS) is the major component of the outer leaflet of the outer membrane of Gram-negative bacteria. Meningococcal LPS is a lipooligosaccharide (LOS) that is structurally similar to LPS, but does not have repeating O-antigens. Both LOS and LPS have a conserved inner core region composed of heptose and 3-deoxy-D-manno-2-octulosonic acid (KDO) attached to a lipid A moiety. The meningococcal lipid A structure has a symmetrical distribution of acyl chain (C12 and C14) attachments to the di-galactosamine backbone (Figure 1) and thus differs from that described for E. coli with an asymmetrical distribution of C14 and C16 acyl chains [28]. One of the best-characterized mechanisms of AMP resistance is remodeling of LPS [29]. It is believed that AMPs interacts with phosphorylated head groups of lipid A, and modification of the lipid A head groups correlates with increased PMB resistance. Such structural modifications that have been shown to affect CAMP resistance include i) removal of the phosphate head groups of the lipid A disaccharides [30, 31]; (ii) modifications of lipid A head groups by the addition of positively charged moieties, such as aminoarabinose [32-35], glucosamine [36], galactosamine [37] or phosphoethanolamine (PEA) [38, 39]; and (iii) alteration in the degree of lipid A acylation such as the formation of hepta-acylated lipid A [40, 41].
Among the different structural modifications of the lipid A head group identified in Gram negative bacteria, only the PEA modification has been demonstrated in N. meningitidis. This is consistent with the fact that meningococci only encodes the PEA transferase LptA [42] and the gene cassette encoding the aminoarabinose modification machinery is absent in the meningococcal genomes. In contrast to the lipid A of E. coli and Salmonella enterica, which may be modified by PEA after induction by certain environmental conditions [38], meningococcal lipid A is constitutively substituted with PEA [43, 44], which is a key factor that defines the intrinsic high level resistance of meningococci to PMB [39]. An lptA mutation caused ~ 250 fold reduction in PMB resistance to reach levels similar to those of E. coli. In comparison, an mtr mutation that inactivates major efflux pumps in meningococci resulted in a 16-fold reduction [39], demonstrating the LptA-mediated PEA decorations of lipid A is vital to meningococcal resistance. Recently, a poly-T8 tract in the lptA coding sequence was shown to phase vary at a frequency of ~10−5 in N. gonorrhoeae, and the frame shift resulted in truncated LptA and PMB sensitivity [45]. Such a poly-T8 tract is also present in meningococcal lptA, but it is as yet unknown whether it varies at a significant frequency. LptA is anchored to the periplasmic face of the cytoplasmic membrane by a transmembrane domain and utilizes the phosphatidylethanolamine lipid as its substrate. The crystal structure of LptA soluble domain was solved and shown to contain 5 disulfide bonds [46], suggesting that LptA is stabilized by disulfide bonds. Indeed. the presence of DsbA3, one of three DsbA proteins encoded in meningococci, supported LptA stability because lacking DsbA3 had a measurable decrease in the amount of PEA decoration on lipid A head groups [47]. Further, combinations of multiple dsbA mutations displayed an additive increase in sensitivity to PMB, indicating that all three oxidoreductases were needed for either LptA-dependent and/or independent pathways that lead to PMB resistance [47]. The constitutive modifications aiming to reduce negative charges in the LPS molecules appear to be a major determinant of antimicrobial peptide resistance in several high AMP resistant bacteria. In addition to N. meningitidis that utilizes constitutive PEA modification, Burkholderia sp. also constitutively produce LPS with aminoarabinose substitution [48] and is resistant to AMPs at a level similar to meningococci.
Additional structural features of lipid A contributing to PMB resistance have been characterized. While O-antigens [49] and inner core of LPS in Burkholderia cenocepacia [50] contribute to PMB resistance, further truncation of the outer or inner core oligosaccharides in meningococcal LOS, however, did not affect PMB susceptibility. An outer membrane localized acyltransferase PagP, which transfers palmitate from phospholipid to lipid A to generate heptaacylated lipid A, is important for inducible AMP resistance in Salmonella and E. coli [40, 51], indicating that increasing lipid A acylation is an AMP resistance mechanism [39]. Varying lipid A acylation patterns in LPS could result in different outer membrane permeability and is a probable underlying AMP resistance mechanism. Although this hepta-acylation mechanism is absent in N. meningitidis, decreased acylation of lipid A indeed reduced AMP resistance, correlating with the effect of varying degrees of lipid A acylation on AMP resistance. Mutations in the late acyl transferases, lpxL1 or lpxL2, responsible for adding the acyloxyacyl laurate chains to the 2 and 2′ positions of lipid A [52], resulted in penta-acylated lipid A and reduced PMB resistance [39]. Naturally occurring lpxL1 mutations via different insertion/deletion events have been identified in many invasive meningococcal clinical isolates [53]. The resulting underacylated lipid A was shown to have low endotoxin activity with reduced proinflammatory cytokine induction [53]. Such variants, although become less resistant to AMPs, likely cause reduced AMP production, thus aiding meningococci to evade the innate immune system.
Finally, mutation in the ABC transporter system (lptH) responsible for LOS export [54] was also identified in a transposon mutagenesis study to further reduce AMP resistance [39]. A lptH mutant contained significantly lower cellular levels of LOS and released high levels of proteins into the medium [54]. The apparent leakiness of the lptH mutant, as indicated by their enhanced sensitivity toward vancomycin [54], likely allows AMP to reach its target membrane more readily, leading to higher PMB sensitivity.
3.2 Efflux pumps
Several efflux pumps including FarA/B [55]. MacA/B [56], NorM [57] and MtrC/D/E [58], have been largely characterized in N. gonorrhoeae to mediate resistance toward various antimicrobial agents and meningococci encode orthologs of these gonococcal systems [59]. The Mtr pump is formed by the outer membrane MtrE, the membrane fusion protein MtrC and the inner membrane protein MtrD and belongs to the resistance-nodulation-division (RND) efflux pump family. Only the Mtr pump was shown to modulate gonococcal susceptibility to several structurally unrelated AMPs, such as the β-sheet peptide PG-1 and the α-helical peptide LL-37 [60]. However, human defensin HNP-2 is not a substrate of the gonococcal Mtr pump as an mtr mutant is equally resistance to this AMP [60]. The contribution of Mtr efflux pumps to AMP resistance in N. meningitidis has been clearly demonstrated as we have shown that the meningococcal Mtr pump decreases susceptibility to PMB, LL-37, and PG-1 [39]. In a transposon random mutagenesis screen, more than half of the PMB sensitive mutants are due to various insertions within the mtrCDE operon, supporting the importance of Mtr pump in AMP resistance [39]. These mutants displayed ~16-fold reduction in PMB resistance.
Meningococci, in general, exhibit ~ 5-fold higher PMB resistance compared to gonococci, partially due to genetic polymorphisms of the mtr locus between the two species. In N. gonorrhoeae the Mtr efflux pump is regulated by a divergently transcribed repressor, MtrR, and is inducible by its substrates through a transcriptional activator, MtrA [61] that is not universally present in all gonococcal strains. However, in N. meningitidis, the Mtr pump is not inducible and is highly expressed due to various mutations in the mtrR coding sequence [62]. In addition, there are polymorphisms within the meningococcal mtr promoter region, including an insertion of a ~150-bp Correia element or an IS1301 element together with the Correia element. These genetic variations within the promoter region showed different promoter activities [62] that lead to varied Mtr pump expression levels.
3.3 Outer membrane proteins
As restricting access of AMPs to its cytoplasmic membrane targets confers resistance, one logical strategy in Gram negative bacteria is to reduce outer membrane permeability. In addition to LPS/LOS mediated outer membrane permeability changes described above, outer membrane porins may function as entry/excretion channels for AMPs and thus influence the levels of AMP crossing the outer membrane barrier. Indeed, PorB, one of the two major porins of N. meningitidis, affects AMP resistance because a porB mutant is 16-fold more sensitive to PMB [39]. This phenotype differs from the observation that a porB mutation increased resistances to several antibiotics such as ciprofloxacin and cephalosporins [63]. Since the porB mutant is not more susceptible to other Mtr efflux pump substrates, the increased PMB sensitivity was not caused by a decrease in the efflux function or levels of the Mtr pump. A general outer membrane permeability increase in the absence of PorB is also not likely as this would correlate with an increase in antibiotic sensitivity. Thus, the increased AMP sensitivity of the porB mutant suggests that excretion of AMPs by PorB is possibly an active AMP resistance mechanism.
On the other hand, the pilin secretion apparatus may act as an entry point for AMPs. A transposon screening for enhanced PMB resistance in N. meningitidis identified five mutants that all mapped in the pilMNOPQ locus [39], predicted to encode proteins involved in type IV pilus biogenesis. A mutant form of the pilus secretin protein PilQ in N. gonorrhoeae that allows increased entry of antimicrobial compounds has also been identified [64].
Outer membrane proteins may also play a role in sequestering AMP from reaching its targets. Two such examples have been characterized in N. meningitidis. First, fHBP-deficient strains are more sensitive to killing by LL-37 [65]. Factor H-binding protein (fHBP), an outer membrane lipoprotein, enables innate immune system evasion of N. meningitidis by binding to the inhibitor of the complement alternative pathway, factor H, and is one of the recombinant vaccine antigens present in serogroup B meningococcal vaccine [66].The fHBP protein does not have proteolytic activity against LL-37, does not affect efflux of LL-37 and no decreased outer membrane stability is detected in the mutant [65]. As the mutant is more resistant to killing by LL-37 in the presence of 2% NaCl and low pH, it was suggested that fHBP likely mediate electrostatic interaction with LL-37 as a sequestration mechanism to prevent contact with the cell membrane. Second, lactoferrin binding protein B (LbpB), a surface-exposed membrane bound lipoprotein, was shown to provide protection against lactoferricin [67] and the cathelicidin related antimicrobial peptide (mCRAMP), but not against LL37 [68]. LbpB works together with LbpA to constitute host-specific lactoferrin receptors and can be selectively released from the bacterial surface [69]. The negatively charged amino acid clusters of LbpB are responsible for the protective effect against lactoferricin [67].
3.4 Capsular polysaccharides
N. meningitidis is encapsulated and expresses one of the 12 capsular polysaccharide (CPS) structures (e.g. serogroup). Capsule is the most critical meningococcal virulence determinant during bacteremia, meningitis and other invasive meningococcal disease, as it imparts antiphagocytic and antibactericidal properties to the meningococcus [43, 70-72]. An additional mechanism by which the capsule protects meningococci is to provide increased resistance to AMPs as unencapsulated serogroup B (α-2, 8-polysialic acid capsule) strains were more susceptible than encapsulated meningococci to defensins (β defensing 1&2, HNP-1&2) cathelicidins (LL-37, CRAMP, CRAMP-18), protegrin PG-1, and polymyxin B [73]. Similarly, a contribution from capsule to LL-37 resistance was shown in an encapsulated serogroup C strain that expresses α-2, 9-polysialic acid capsule [74]. Further, this study showed that after exposure to nonlethal concentrations of LL-37, higher proportion of unencapsulated meningococci accumulated LL-37 on the surface compared to the encapsulated wild-type strain [74], suggesting that CPS reduces the binding of LL-37 to the bacterial surface. All disease-causing meningococcal serogroups express negatively charged capsular polysaccharides and the nonproductive electrostatic interaction between CPS and AMP could serve as a sequestering mechanism to limit AMP reaching its targets. Thus, CPS acts as a shield to reduce the binding of AMPs to bacterial surface. As a correlation, CPS-mediated AMP resistance in Klebsiella pneumoniae was shown to be unrelated to the CPS chemical composition but was dependent on the amount of CPS expressed [75].
3.5 Bleb and biofilm
Meningococci are characterized by frequent vesiculation (blebing) of the outer membrane [76] that appears to contribute to rapid initiation of the inflammatory cascades of meningococcal diseases. Blebs, which contain DNA, outer membrane proteins and LOS, have been shown to be major constituents of the biofilm matrix and the ability to produce blebs shown to be crucial to biofilm formation in N. gonorrhoeae [77]. Contributions of biofilm to AMP resistance have been characterized in other bacteria [78] and likely also play a role in meningococcal AMP resistance. N. meningitidis has been shown to form biofilm on abiotic surfaces as well as on epithelial cells [79-81]. Blebing may potentially function as another sequestering mechanism of AMP evasion through the unproductive electrostatic interactions between LOS/DNA within blebs and AMPs, either in the presence or absence of biofilm.
4. Response to AMP exposure in N. meningitidis
Without constitutive and intrinsically high levels of resistance, bacteria targeted by AMPs have to be able to detect and respond to AMPs and the efficiency of these processes are important for the survival of bacteria in the host [82]. Well-characterized response mechanisms are the PhoP/PhoQ and PmrA/PmrB two-component system-mediated inducible resistance to AMPs that resulted in modifications of lipid A head groups by aminoarabinose and PEA and formation of hepta-acylated lipid A [83, 84]. The fact that both PEA modification of the LOS lipid A head groups and the Mtr efflux pump were constitutively expressed at high levels likely reduces the dependence on inducible AMP resistance in N. meningitidis. However, inducible responses to AMP exposure in N. meningitidis have been shown in several studies. A mutation in the MisR/MisS two-component system caused a 2-fold reduction in lptA expression [44]. Although no quantitative changes in PEA modification levels of lipid A were detected, the misR/misS mutants are more sensitive to PMB, suggesting that other genes controlled by this two-component system are involved in AMP resistance. Exposures to PG-1 and LL-37 at concentrations close to the MIC elicit an increase (~1.5-2 fold) on mtrCDE transcript levels in N. meningitidis [73]. As these two AMPs are not structurally related, the up-regulation is unlikely promoted by sensing specific AMPs, but potentially by sensing perturbation of the inner and/or outer membrane. Another study showed an up-regulation of mtrD upon a 30 minute exposure to sublethal dose of LL-37 [74]. Interestingly, exposure to LL-37 also further induces expression of CPS genes in this study [74]. A global transcriptome study performed by treating a serogroup B meningococcal strain with a sublethal concentration of CRAMP, a mouse LL-37 homolog, for 1 hour found a total of 21 genes being differentially expressed greater than 2-fold [85]. Up-regulated genes encode proteins involved cell envelope processing such as pilin glycosylation; while genes involved in energy metabolism are down-regulated. Among many affected genes with unknown functions [85], two encoding conserved hypothetical proteins were further characterized. A mutation in either NMB0741 or NMB1828 caused ~3-log lower bacteremia in an adult mouse model of infection and the NMB1828 protein binds LL-37 in vitro [85].
5. Summary
Considerable progress has been made in identification of the molecular basis of antimicrobial peptide resistance mechanisms in N. meningitidis and many other bacterial pathogens. Resistance to antimicrobial peptides is multifactorial as pathogens use multiple mechanisms to resist AMPs. The contribution and the relative importance of each resistance mechanism vary in different bacterial species. N. meningitidis utilizes several efficient strategies to defend against AMPs. The critical mechanism is constitutive LOS modification by PEA, which is further enhanced by efficient excretion of AMPs by efflux pumps. Other resistance mechanisms, such as CPS and outer membrane protein expression, appear to play a more limited role but contribute to the total high level of resistance. As AMPs are actively being explored as a new class of antimicrobial therapeutics [86, 87], expanding our understanding of AMP resistance mechanisms in bacterial pathogens is essential.
Highlights.
N. meningitidis is highly resistant to antimicrobial peptides (AMPs)
Meningococcal AMP resistance mechanisms are summarized.
Constitutive modification of lipid A by phosphoethanolamine is most critical.
AMP excretion by efflux pumps contributes to meningococcal AMP resistance.
Acknowledgement
This work was supported in part by NIH Grant 5R01 AI40247 awarded to DSS and by NIH Grant R56AI 061031 awarded to YT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wilder-Smith A. Meningococcal vaccine in travelers. Current opinion in infectious diseases. 2007;20:454–460. doi: 10.1097/QCO.0b013e3282a64700. [DOI] [PubMed] [Google Scholar]
- [2].C. Centers for Disease Prevention, Licensure of a meningococcal conjugate vaccine (Menveo) and guidance for use - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. Morbidity and mortality weekly report. 2010;59:273. [PubMed] [Google Scholar]
- [3].Lorick SA, Fishbein D, Weintraub E, Wortley PM, Lee GM, Zhou F, Davis R. Uptake of meningococcal conjugate vaccine among adolescents in large managed care organizations, United States, 2005: demand, supply and seasonality. BMC infectious diseases. 2009;9:175. doi: 10.1186/1471-2334-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clark TA, Stern E, Pondo T, Arnold K, Harrison LH, Vello M. The effect of quadrivalent (A, C, Y,W-135) meningococcal conjugate vaccine on serogroup-specific carriage of Neisseria meningitidis. 16th International pathogenic Neissera conference; Rotterdam, Netherlands. 2008. [Google Scholar]
- [5].Zimmer SM, Stephens DS. Meningococcal conjugate vaccines. Expert Opin. Pharmacother. 2004;5:855–863. doi: 10.1517/14656566.5.4.855. [DOI] [PubMed] [Google Scholar]
- [6].Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- [7].Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends in biotechnology. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- [8].Dhople V, Krukemeyer A, Ramamoorthy A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta. 2006;1758:1499–1512. doi: 10.1016/j.bbamem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- [9].Sudheendra US, Dhople V, Datta A, Kar RK, Shelburne CE, Bhunia A, Ramamoorthy A. Membrane disruptive antimicrobial activities of human beta-defensin-3 analogs. European journal of medicinal chemistry. 2015;91:91–99. doi: 10.1016/j.ejmech.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- [11].Wildman K.A. Henzler, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- [12].Ramamoorthy A, Lee DK, Santos JS, Henzler-Wildman KA. Nitrogen-14 solid-state NMR spectroscopy of aligned phospholipid bilayers to probe peptide-lipid interaction and oligomerization of membrane associated peptides. J Am Chem Soc. 2008;130:11023–11029. doi: 10.1021/ja802210u. [DOI] [PubMed] [Google Scholar]
- [13].Hale JD, Hancock RE. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert review of anti-infective therapy. 2007;5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- [14].Ramamoorthy A. Beyond NMR spectra of antimicrobial peptides: dynamical images at atomic resolution and functional insights. Solid state nuclear magnetic resonance. 2009;35:201–207. doi: 10.1016/j.ssnmr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee DK, Bhunia A, Kotler SA, Ramamoorthy A. Detergent-Type Membrane Fragmentation by MSI-78, MSI-367, MSI-594, and MSI-843 Antimicrobial Peptides and Inhibition by Cholesterol: A Solid-State Nuclear Magnetic Resonance Study. Biochemistry. 2015;54:1897–1907. doi: 10.1021/bi501418m. [DOI] [PubMed] [Google Scholar]
- [16].Lee DK, Brender JR, Sciacca MF, Krishnamoorthy J, Yu C, Ramamoorthy A. Lipid composition-dependent membrane fragmentation and pore-forming mechanisms of membrane disruption by pexiganan (MSI-78) Biochemistry. 2013;52:3254–3263. doi: 10.1021/bi400087n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Domadia PN, Bhunia A, Ramamoorthy A, Bhattacharjya S. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: role of the helical hairpin conformation in outer-membrane permeabilization. J Am Chem Soc. 2010;132:18417–18428. doi: 10.1021/ja1083255. [DOI] [PubMed] [Google Scholar]
- [18].Bhunia A, Domadia PN, Torres J, Hallock KJ, Ramamoorthy A, Bhattacharjya S. NMR structure of pardaxin, a pore-forming antimicrobial peptide, in lipopolysaccharide micelles: mechanism of outer membrane permeabilization. J Biol Chem. 2010;285:3883–3895. doi: 10.1074/jbc.M109.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bhunia A, Ramamoorthy A, Bhattacharjya S. Helical hairpin structure of a potent antimicrobial peptide MSI-594 in lipopolysaccharide micelles by NMR spectroscopy. Chemistry. 2009;15:2036–2040. doi: 10.1002/chem.200802635. [DOI] [PubMed] [Google Scholar]
- [20].Bhattacharjya S, Ramamoorthy A. Multifunctional host defense peptides: functional and mechanistic insights from NMR structures of potent antimicrobial peptides. The FEBS journal. 2009;276:6465–6473. doi: 10.1111/j.1742-4658.2009.07357.x. [DOI] [PubMed] [Google Scholar]
- [21].Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- [22].Rice WG, Ganz T, Kinkade JM, Jr., Selsted ME, Lehrer RI, Parmley RT. Defensin-rich dense granules of human neutrophils. Blood. 1987;70:757–765. [PubMed] [Google Scholar]
- [23].Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci U S A. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gifford JL, Hunter HN, Vogel HJ. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009;69:1555–1623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- [27].Gruenheid S, Le Moual H. Resistance to antimicrobial peptides in Gram-negative bacteria. FEMS Microbiol Lett. 2012;330:81–89. doi: 10.1111/j.1574-6968.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- [28].Kahler CM, Stephens DS. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin) Crit. Rev. Microbiol. 1998;24:281–334. doi: 10.1080/10408419891294216. [DOI] [PubMed] [Google Scholar]
- [29].Lopez J.L. Anaya, Meza J.E. Lopez, Zarzosa A. Ochoa. Bacterial resistance to cationic antimicrobial peptides. Critical reviews in microbiology. 2013;39:180–195. doi: 10.3109/1040841X.2012.699025. [DOI] [PubMed] [Google Scholar]
- [30].Wang X, Ribeiro AA, Guan Z, Abraham SN, Raetz CR. Attenuated virulence of a Francisella mutant lacking the lipid A 4′-phosphatase. Proc Natl Acad Sci U S A. 2007;104:4136–4141. doi: 10.1073/pnas.0611606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tran AX, Whittimore JD, Wyrick PB, McGrath SC, Cotter RJ, Trent MS. The lipid A 1-phosphatase of Helicobacter pylori is required for resistance to the antimicrobial peptide polymyxin. J Bacteriol. 2006;188:4531–4541. doi: 10.1128/JB.00146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nummila K, Kilpelainen I, Zahringer U, Vaara M, Helander IM. Lipopolysaccharides of polymyxin B-resistant mutants of Escherichia coli are extensively substituted by 2-aminoethyl pyrophosphate and contain aminoarabinose in lipid A. Mol. Microbiol. 1995;16:271–278. doi: 10.1111/j.1365-2958.1995.tb02299.x. [DOI] [PubMed] [Google Scholar]
- [33].McCoy AJ, Liu H, Falla TJ, Gunn JS. Identification of Proteus mirabilis mutants with increased sensitivity to antimicrobial peptides. Antimicrob Agents Chemother. 2001;45:2030–2037. doi: 10.1128/AAC.45.7.2030-2037.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moskowitz SM, Ernst RK, Miller SI. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol. 2004;186:575–579. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tamayo R, Ryan SS, McCoy AJ, Gunn JS. Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella enterica serovar typhimurium. Infect. Immun. 2002;70:6770–6778. doi: 10.1128/IAI.70.12.6770-6778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shah NR, Hancock RE, Fernandez RC. Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation. Antimicrob Agents Chemother. 2014;58:4931–4934. doi: 10.1128/AAC.02590-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee H, Hsu FF, Turk J, Groisman EA. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J Bacteriol. 2004;186:4124–4133. doi: 10.1128/JB.186.13.4124-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tzeng YL, Ambrose KD, Zughaier S, Zhou X, Miller YK, Shafer WM, Stephens DS. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 2005;187:5387–5396. doi: 10.1128/JB.187.15.5387-5396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, Hackett M, Miller SI. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- [41].Bishop RE. The lipid A palmitoyltransferase PagP: molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol. 2005;57:900–912. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- [42].Cox AD, Wright JC, Li J, Hood DW, Moxon ER, Richards JC. Phosphorylation of the lipid a region of meningococcal lipopolysaccharide: identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 2003;185:3270–3277. doi: 10.1128/JB.185.11.3270-3277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kahler CM, Martin LE, Shih GC, Rahman MM, Carlson RW, Stephens DS. The (a2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 1998;66:5939–5947. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tzeng YL, Datta A, Ambrose KD, Davies JK, Carlson RW, Stephens DS, Kahler CM. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. J. Biol. Chem. 2004;279:35053–35062. doi: 10.1074/jbc.M401433200. [DOI] [PubMed] [Google Scholar]
- [45].Kandler JL, Joseph SJ, Balthazar JT, Dhulipala V, Read TD, Jerse AE, Shafer WM. Phase-variable expression of lptA modulates the resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides. Antimicrob Agents Chemother. 2014;58:4230–4233. doi: 10.1128/AAC.03108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wanty C, Anandan A, Piek S, Walshe J, Ganguly J, Carlson RW, Stubbs KA, Kahler CM, Vrielink A. The structure of the Neisserial Lipooligosaccharide phosphoethanolamine transferase A (LptA) required for resistance to polymyxin. J Mol Biol. 2013 doi: 10.1016/j.jmb.2013.06.029. [DOI] [PubMed] [Google Scholar]
- [47].Piek S, Wang Z, Ganguly J, Lakey AM, Bartley SN, Mowlaboccus S, Anandan A, Stubbs KA, Scanlon MJ, Vrielink A, Azadi P, Carlson RW, Kahler CM. The role of oxidoreductases in determining the function of the neisserial lipid a phosphoethanolamine transferase required for resistance to polymyxin. PLoS One. 2014;9:e106513. doi: 10.1371/journal.pone.0106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Loutet SA, Valvano MA. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Frontiers in cellular and infection microbiology. 2011;1:6. doi: 10.3389/fcimb.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Banemann A, Deppisch H, Gross R. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect. Immun. 1998;66:5607–5612. doi: 10.1128/iai.66.12.5607-5612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Loutet SA, Flannagan RS, Kooi C, Sokol PA, Valvano MA. A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to antimicrobial peptides and bacterial survival in vivo. J Bacteriol. 2006;188:2073–2080. doi: 10.1128/JB.188.6.2073-2080.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CR. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 2000;19:5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 2001;69:5981–5990. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fransen F, Heckenberg SG, Hamstra HJ, Feller M, Boog CJ, van Putten JP, van de Beek D, van der Ende A, van der Ley P. Naturally occurring lipid A mutants in neisseria meningitidis from patients with invasive meningococcal disease are associated with reduced coagulopathy. PLoS Pathog. 2009;5:e1000396. doi: 10.1371/journal.ppat.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bos MP, Tommassen J. The LptD chaperone LptE is not directly involved in lipopolysaccharide transport in Neisseria meningitidis. J Biol Chem. 2011;286:28688–28696. doi: 10.1074/jbc.M111.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee EH, Shafer WM. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol Microbiol. 1999;33:839–845. doi: 10.1046/j.1365-2958.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- [56].Rouquette-Loughlin CE, Balthazar JT, Shafer WM. Characterization of the MacA-MacB efflux system in Neisseria gonorrhoeae. J Antimicrob Chemother. 2005;56:856–860. doi: 10.1093/jac/dki333. [DOI] [PubMed] [Google Scholar]
- [57].Rouquette-Loughlin C, Dunham SA, Kuhn M, Balthazar JT, Shafer WM. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J Bacteriol. 2003;185:1101–1106. doi: 10.1128/JB.185.3.1101-1106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- [59].Shafer WM, Veal WL, Lee EH, Zarantonelli L, Balthazar JT, Rouquette C. Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis. J Mol Microbiol Biotechnol. 2001;3:219–224. [PubMed] [Google Scholar]
- [60].Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U S A. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rouquette-Loughlin C, Stojiljkovic I, Hrobowski T, Balthazar JT, Shafer WM. Inducible, but not constitutive, resistance of gonococci to hydrophobic agents due to the MtrCMtrD-MtrE efflux pump requires TonB-ExbB-ExbD proteins. Antimicrob. Agents Chemother. 2002;46:561–565. doi: 10.1128/AAC.46.2.561-565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rouquette-Loughlin CE, Balthazar JT, Hill SA, Shafer WM. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol. Microbiol. 2004;54:731–741. doi: 10.1111/j.1365-2958.2004.04299.x. [DOI] [PubMed] [Google Scholar]
- [63].Peak IR, Jennings CD, Jen FE, Jennings MP. Role of Neisseria meningitidis PorA and PorB expression in antimicrobial susceptibility. Antimicrob Agents Chemother. 2014;58:614–616. doi: 10.1128/AAC.02506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 2004;186:730–739. doi: 10.1128/JB.186.3.730-739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Seib KL, Serruto D, Oriente F, Delany I, Bobie J. Adu, Veggi D, Arico B, Rappuoli R, Pizza M. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun. 2009;77:292–299. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McNeil LK, Zagursky RJ, Lin SL, Murphy E, Zlotnick GW, Hoiseth SK, Jansen KU, Anderson AS. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev. 2013;77:234–252. doi: 10.1128/MMBR.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Morgenthau A, Beddek A, Schryvers AB. The negatively charged regions of lactoferrin binding protein B, an adaptation against anti-microbial peptides. PLoS One. 2014;9:e86243. doi: 10.1371/journal.pone.0086243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Morgenthau A, Partha SK, Adamiak P, Schryvers AB. The specificity of protection against cationic antimicrobial peptides by lactoferrin binding protein B. Biometals. 2014;27:923–933. doi: 10.1007/s10534-014-9767-y. [DOI] [PubMed] [Google Scholar]
- [69].Roussel-Jazede V, Jongerius I, Bos MP, Tommassen J, van Ulsen P. NalP-mediated proteolytic release of lactoferrin-binding protein B from the meningococcal cell surface. Infect Immun. 2010;78:3083–3089. doi: 10.1128/IAI.01193-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Joiner KA. Complement evasion by bacteria and parasites. Annu. Rev. Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- [71].Moxon ER, Kroll JS. The role of bacterial polysaccharide capsules as virulence factors. Curr. Top. Microbio. Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- [72].Ram S, Mackinnon FG, Gulati S, McQuillen DP, Vogel U, Frosch M, Elkins C, Guttormsen HK, Wetzler LM, Oppermann M, Pangburn MK, Rice PA. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 1999;36:915–928. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- [73].Spinosa MR, Progida C, Tala A, Cogli L, Alifano P, Bucci C. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect. Immun. 2007;75:3594–3603. doi: 10.1128/IAI.01945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jones A, Georg M, Maudsdotter L, Jonsson AB. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J. Bacteriol. 2009;191:3861–3868. doi: 10.1128/JB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun. 2004;72:7107–7114. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Devoe IW, Gilchrist JE. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973;138:1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Falsetta ML, Steichen CT, McEwan AG, Cho C, Ketterer M, Shao J, Hunt J, Jennings MP, Apicella MA. The Composition and Metabolic Phenotype of Neisseria gonorrhoeae Biofilms. Frontiers in microbiology. 2011;2:75. doi: 10.3389/fmicb.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Otto M. Bacterial evasion of antimicrobial peptides by biofilm formation. Current topics in microbiology and immunology. 2006;306:251–258. doi: 10.1007/3-540-29916-5_10. [DOI] [PubMed] [Google Scholar]
- [79].Neil RB, Shao JQ, Apicella MA. Biofilm formation on human airway epithelia by encapsulated Neisseria meningitidis serogroup B. Microbes Infect. 2009;11:281–287. doi: 10.1016/j.micinf.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [80].Lappann M, Vogel U. Biofilm formation by the human pathogen Neisseria meningitidis. Medical microbiology and immunology. 2010;199:173–183. doi: 10.1007/s00430-010-0149-y. [DOI] [PubMed] [Google Scholar]
- [81].Yi K, Rasmussen AW, Gudlavalleti SK, Stephens DS, Stojiljkovic I. Biofilm Formation by Neisseria meningitidis. Infect. Immun. 2004;72:6132–6138. doi: 10.1128/IAI.72.10.6132-6138.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shprung T, Peleg A, Rosenfeld Y, Cuot P. Trieu, Shai Y. Effect of PhoP-PhoQ activation by broad repertoire of antimicrobial peptides on bacterial resistance. J Biol Chem. 2012;287:4544–4551. doi: 10.1074/jbc.M111.278523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gunn JS. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 2008;16:284–290. doi: 10.1016/j.tim.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [84].Ernst RK, Guina T, Miller SI. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 2001;3:1327–1334. doi: 10.1016/s1286-4579(01)01494-0. [DOI] [PubMed] [Google Scholar]
- [85].Frigimelica E, Bartolini E, Galli G, Grandi G, Grifantini R. Identification of 2 hypothetical genes involved in Neisseria meningitidis cathelicidin resistance. J. Infect. Dis. 2008;197:1124–1132. doi: 10.1086/533456. [DOI] [PubMed] [Google Scholar]
- [86].Steckbeck JD, Deslouches B, Montelaro RC. Antimicrobial peptides: new drugs for bad bugs? Expert opinion on biological therapy. 2014;14:11–14. doi: 10.1517/14712598.2013.844227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]