Abstract
Group IIA secretory phospholipase A2 (sPLA2-IIA) of mammalian species is unique among the many structurally and functionally related mammalian sPLA2 in their high net positive charge and potent (nM) antibacterial activity. Toward the Gram-positive bacteria tested thus far, the global cationic properties of sPLA2-IIA are necessary for optimal binding to intact bacteria and penetration of the multi-layered thick cell wall, but not for the degradation of membrane phospholipids that is essential for bacterial killing. Various Gram-positive bacterial species can differ as much as 1000-fold in sPLA2-IIA sensitivity despite similar intrinsic enzymatic activity of sPLA2-IIA toward the membrane phospholipids of the various bacteria. D-alanylation of wall- and lipo-teichoic acids in Stapylococcus aureus and sortase function in Streptococcus pyogenes increase bacterial resistance to sPLA2-IIA by up to 100-fold apparently by affecting translocation of bound sPLA2-IIA to the cell membrane. Action of the sPLA2-IIA and other related sPLA2 against Gram-negative bacteria is more dependent on cationic properties of the enzyme near the amino-terminus of the protein and collaboration with other host defense proteins that produce alterations of the unique Gram-negative bacterial outer membrane that normally represents a barrier to sPLA2-IIA action.
Introduction
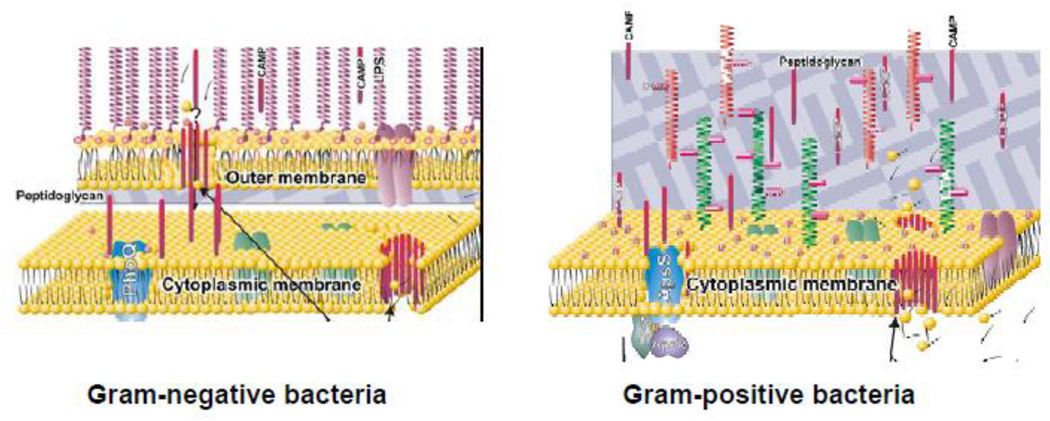
Acute inflammatory responses of mammalian hosts to invading bacteria include recruitment of polymorphonuclear leukocyte neutrophils from the bloodstream and mobilization of extracellular antimicrobial compounds that may originate from a variety of intravascular and extravascular sources (1–3). The latter often includes a secretory (Group IIA phospholipase A2 (sPLA2-IIA)) that can function both independently against many Gram-positive bacteria and in concert with other host defense systems toward both Gram-negative and Gram-positive bacteria to increase digestion and killing of these bacteria (1, 2, 4–14). The sPLA2-IIA is a member of a family of low Mr (14,000–19,000) secretory PLA2 with close overall structural and functional (enzymatic) similarity to related enzymes present in the venoms of snakes and insects and also in plants (15). Hallmarks of these proteins include a highly compact three-dimensional structure that is stabilized by 6–8 disulfide bonds and a calcium-dependent catalytic machinery mediating stereospecific hydrolysis of glycerophospholipids at the sn-2 position yielding free fatty acids and 1-acyl-lyso-phospholipids. Among the ten different secretory sPLA2 expressed by humans, however, there are marked differences in the regulation of expression, sites of extracellular mobilization, and preferred biological targets consistent with distinct physiological roles of the various sPLA2. The sPLA2-IIA is unique in its very high net charge (up to +17) and antibacterial potency. At nM concentrations, the sPLA2-IIA can attack both Gram-positive and Gram-negative bacteria, though the latter typically requires the assistance of other host defense proteins to facilitate access of the sPLA2 to phospholipids in the Gram-negative bacterial envelope (Fig. 1). In contrast, host cells are highly resistant to sPLA2-IIA, at least under normal resting conditions, as are viruses whose envelopes are derived from host cell membranes. Together, the unique antibacterial properties of the sPLA2-IIA and its target cell selectivity suggest specific molecular and structural determinants of the antibacterial action of sPLA2-IIA. This review will mostly focus on those properties that influence the action of this enzyme on Gram-positive bacteria.
Figure 1.
Schematic structure of envelopes of Gram-negative (left) and Gram-positive (right) bacteria. Note the unique asymmetry to lipid arrangement in the outer membrane of Gram-negative bacteria, with lipopolysaccharides (LPS) occupying the outer leaflet and phospholipids mainly restricted to the inner leaflet. Figure is adapted from (47).
Actions of purified sPLA2-IIA against Gram-positive bacteria
Testing of the antimicrobial spectrum of sPLA2-IIA has not been exhaustive but sufficient to reveal the susceptibility of many different Gram-positive bacterial species to doses of extracellular sPLA2-IIA achievable in certain settings in vivo (11, 12, 16–19). Bacillus subtilis is among the most sensitive to sPLA2-IIA, with as little as 0.1–1 nM (1.5–15 ng/ml) of human sPLA2-IIA sufficient to produce 1–3 logs killing of 106 bacteria/ml within 1 hour of incubation. Similar effects on S. aureus and Streptococcus agalactiae (20) (Group B streptococci; GBS) generally (but see below) require 10–100× higher sPLA2-IIA concentrations. Other Gram-positive bacterial species (e.g., Staphylococcus epidermidis, Streptococcus pyogenes, Enterococcus faecalis) can also be killed by sPLA2-IIA but require even higher (10–100-fold) doses (17, 20). Where studied, differences in bacterial sensitivity to the bactericidal effects of sPLA2-IIA parallel differences in sPLA2-IIA -triggered bacterial phospholipid (PL) degradation (17, 21, 22). A point mutation (D48S) in recombinant human sPLA2-IIA that disrupts Ca2+ binding needed for catalytic activity ablates both sPLA2-IIA -induced bacterial PL degradation and killing (9, 13). Bacterial killing requires rapid and massive PL degradation (>50% of total membrane PL within 30 min) followed almost immediately by loss of the PL degradation products (free fatty acids and lyso-PL) from the bacterial membrane to extracellular albumin (21, 23–24). The ability of bacteria treated with sub-lethal doses of sPLA2-IIA (or otherwise lethal sPLA2-IIA doses in the absence of albumin) to retain viability despite substantial degradation of membrane PL suggests a capacity of these treated bacteria to replace degraded and lost membrane PL by either de novo synthesis of PL or (in the absence of albumin) recycling of PL breakdown products that remain within the bacterial membrane (24). The mechanism(s) of this reparative process and its possible role in bacterial resistance to sPLA2-IIA -mediated killing deserve further study. An autolysin-deficient mutant strain of S. aureus is as sensitive as the parent strain to the phospho-lipolytic activity of the sPLA2-IIA but requires several-fold higher sPLA2-IIA doses to be killed (23). One possible interpretation of this finding is that rapid and extensive loss of membrane phospholipids leads to premature/inappropriate activation of autolysins that help convert potentially reversible membrane phospholipid loss to irreversible cell wall damage.
Whereas differences in the bactericidal potency of sPLA2-IIA toward different species of Gram-positive bacteria correlate closely with different dose requirements to produce membrane PL degradation in intact bacteria, these differences do not reflect different substrate properties of the PL of these bacterial species, as judged either by assay of extracted PL presented as dispersions in aqueous solution or of cell wall-depleted protoplasts in which PL are presented as integral components of an intact cytoplasmic membrane (21, 22). Thus, the differences in sensitivity that are observed in various Gram-positive bacteria are a specific feature of the interactions of the intact bacteria with sPLA2-IIA and hence, by implication, distinguishing properties of the cell wall that is assembled outside of the bacterial membrane.
The most revealing insights concerning the molecular and structural determinants of the antibacterial action of sPLA2-IIA toward Gram-positive bacteria have been obtained in a series of studies of wild-type (wt) and mutant sPLA2-IIA and wt and mutant S. aureus SA113). As indicated, the most striking physical feature of the sPLA2-IIA is its remarkably high net positive charge that is manifest on virtually every exposed surface of the protein (Fig. 2). Mutational analyses of the human sPLA2-IIA have confirmed the key role of this property in its activity against both B. subtilis and S. aureus (Table 1) (21, 25). Stepwise decrements in the net (+) charge of human sPLA2-IIA achieved by substitution of basic amino acids with acidic residues produced a stepwise reduction in bactericidal potency toward both S. aureus and B. subtilis. Mutations at different sites of the enzyme had very similar effects on sPLA2-IIA antibacterial potency, demonstrating the importance of the global cationicity of the sPLA2-IIA (Fig. 2) in its antibacterial action. Activity toward S. aureus (vs. B. subtilis) appears to be somewhat more dependent on the very high net (+) charge of the sPLA2-IIA, as manifest both by the greater reduction in potency accompanying diminution of net charge from +15 to +13 and, conversely, the increase in potency accompanying an increase in net charge for +15 to + 17 (Table 1); (26).
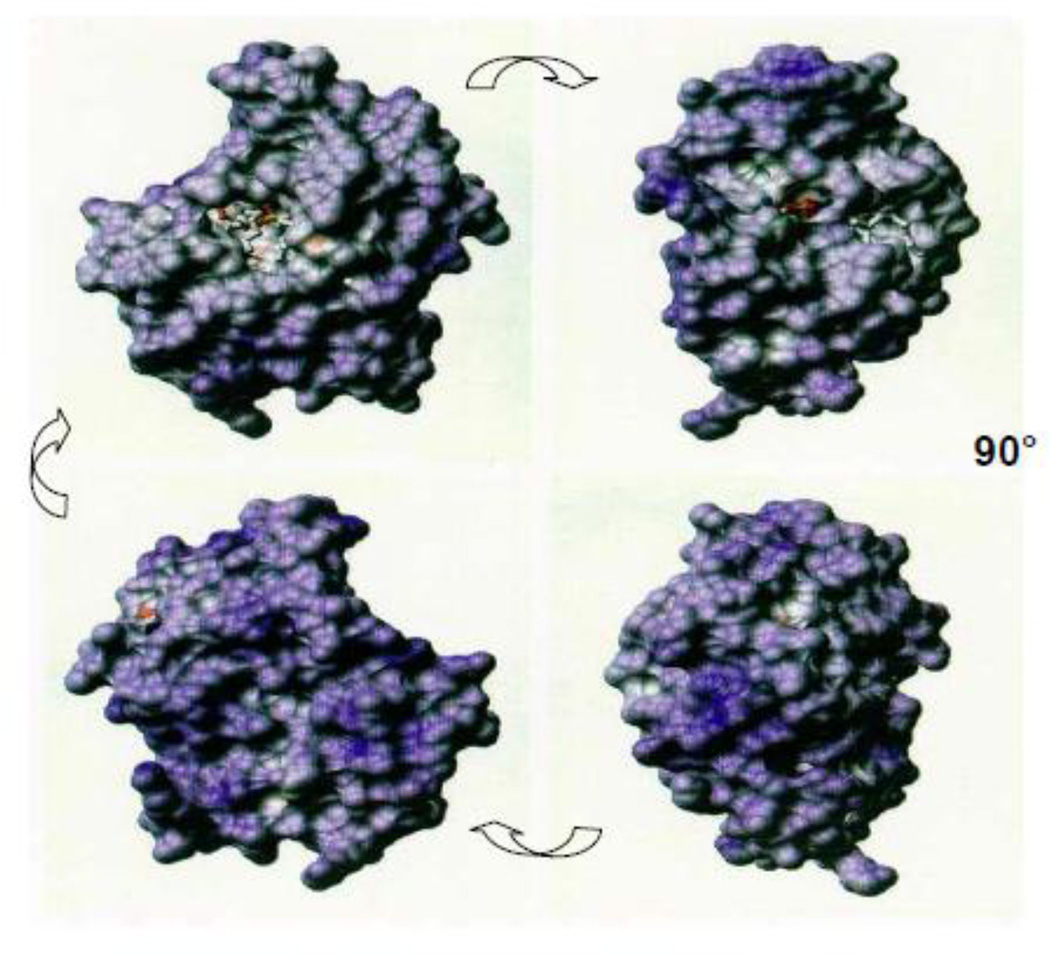
Figure 2.
Surface charge distribution in human sPLA2-IIA. Cationic surfaces are represented in blue, with darker shades of blue signifying regions with higher net cationic character. Conversely, acidic surface regions are represented by shades of red. Note bound substrate analog in upper left image, just left and above center of that image. The four images were generated by 90° rotations, as indicated.
Table 1.
Role of cationic properties of human sPLA2-IIA in its activity toward intact Gram-positive bacteria and isolated membrane protoplasts.
| Activity (relative to wt sPLA2-IIA;%) | ||||
|---|---|---|---|---|
| Recombinant Human sPLA2-IIA**** | Net charge |
S. aureus* |
B.subtilis* |
B.subtilis protoplasts** |
| Wt | +15 | 100 | 100 | 100 |
| K92E;K87E;K74E;B7E | +13 | 6±3 | 65±9 | 185±30 |
| K110E.K115E; K38E.K116E; K10E.K16E;R7E;K16E;K124E.R127D |
+11 | 0.4±0.3 | 4±3 | 146±45 |
| K74E.K87E.R92E;R7E;K10E;K16E | +9 | <0.1 | <0.1 | 135±50 |
| K38E.K110E.K115E.K116E | +7 | <0.1 | <0.1 | 120 |
| K53E.R54E.R58E.K124E.R127D | +5 | <0.1 | <0.1 | 170 |
| G72K.T103K | +17 | 484±82 | 122±30 | N.T. |
| Pig sPLA2-1B | −1 | <0.1 | <0.1 | 100 |
All activities were measured as dose required to produce 90% bacterial killing (**) or 30% PL degradation (***). See references (21, 25) for description of wt and mutant enzymes and functional assays.
Mutant recombinant human sPLA2-IIA were prepared by Dr. Ning-Sheng Liang (G72K.T103K) and Drs. R. Koduri and MH Gelb (all others). Data shown were collected by Dr. Liang and represent the mean ± SEM of at least three experiments.
This dependence of the bactericidal potency of the sPLA2-IIA on its global charge properties parallels changes in PLA2 dose requirements for producing bacterial membrane PL degradation and loss. Remarkably, reduction of the net (+) charge of the sPLA2-IIA by as much as 10 charge units (+15 to +5) does not reduce sPLA2-IIA activity toward the PL of B. subtilis (Table 1) or S. aureus when the PL are presented as part of cell wall-denuded membrane protoplasts (21). These findings suggest strongly that the unique cationic properties of the sPLA2-IIA are important for initial (surface) interaction of the enzyme with intact Gram-positive bacteria and/or penetration of the enzyme through the cell wall but not for its interaction with and degradation of membrane PL once it has accessed the bacterial cytoplasmic membrane.
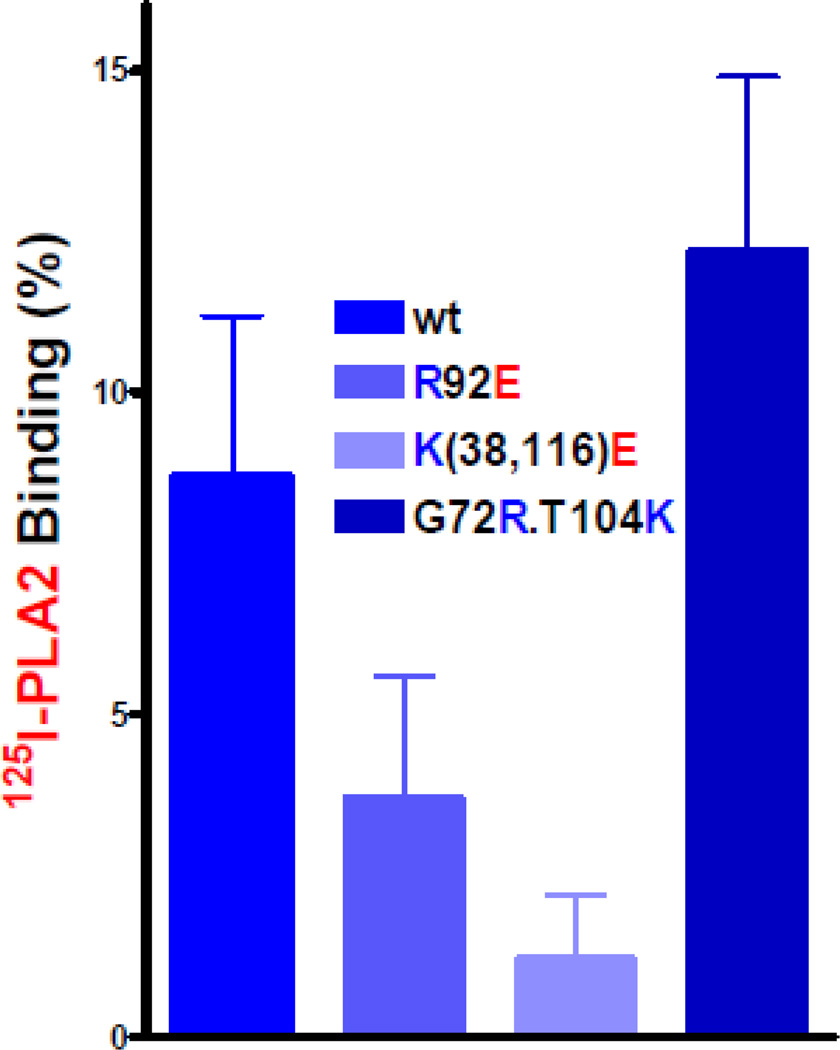
Appraising the effects of specific molecular and structural variables on initial sPLA2-IIA interactions with the Gram-positive bacterial surface and underlying cell wall could be confounded by cell wall alterations that are induced secondary to massive degradation and loss of membrane PL (23). To preclude this possibility, initial sPLA2-IIA binding has been measured in the absence of calcium to prevent calcium-dependent membrane PL degradation. That initial sPLA2-IIA binding was calcium-independent was established by demonstrating equal binding of catalytically inactive D48S human sPLA2-IIA in both the presence and absence of added 1 mM calcium chloride. Comparison of wt and charge variant [125I]PLA2 binding to S. aureus showed stepwise alterations in sPLA2-IIA binding that correlate with the net (+) charge of the enzyme (Fig. 3). However, effects of sPLA2-IIA net charge on binding are significantly less than effects on antibacterial potency (compare Table 1 and Figure 3), suggesting additional effects of the cationicity of sPLA2-IIA on cell wall penetration. To more directly test this hypothesis, doses of wt or charge variant sPLA2-IIA present during the initial incubation of sPLA2-IIA + S. aureus without calcium was adjusted to achieve roughly equal binding of each sPLA2-IIA species. When the bacteria were washed to remove unbound PLA2 and incubated with calcium, the antibacterial effects of bound sPLA2-IIA clearly correlated with the net charge of the bound enzyme (+15 > +13 > +11). In sum, the highly cationic properties of the sPLA2-IIA promote its antibacterial potency toward Gram-positive bacteria both by promoting initial surface binding and by increasing the efficiency of cell wall penetration of the bound enzyme to access membrane PL (Fig. 4). These charge-dependent non-catalytic interactions of the enzyme with the Gram-positive bacterial envelope make possible the calcium-dependent catalytic degradation of membrane PL that is required for bacterial killing. It is the ability of the wt sPLA2-IIA to access the membrane PL of intact Gram-positive bacteria and not its ability to act on the membrane PL once exposed that differentiates this sPLA2 from other sPLA2 and depends on its unique cationic properties.
Figure 3.
Comparison of binding of [125I] wt and charge variant recombinant human sPLA2-IIA to wt S. aureus SA113. Incubations contained 5 × 106 bacteria and 50 ng sPLA2-IIA in 1 ml of Hanks buffered salts solution without calcium and magnesium supplemented with 10 mM HEPES (pH 7.4) and 0.1% albumin. Results shown represent the mean ± SEM of three experiments, each done in duplicate.
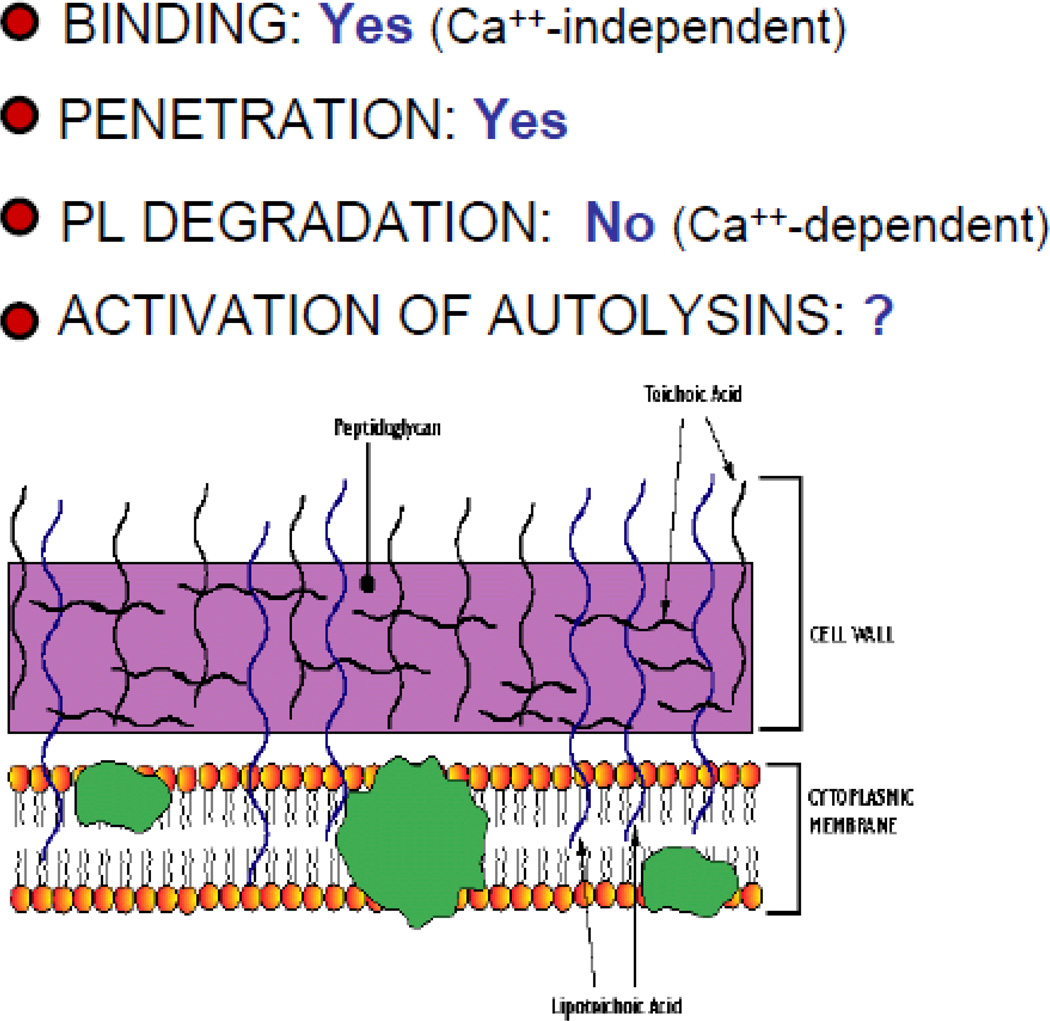
Figure 4.
Summary of required steps in bactericidal action of sPLA2-IIA vs. Gram-positive bacteria (e.g., S. aureus) and role of cationic properties of the sPLA2-IIA in each step of the process.
Unpublished observations of ours have revealed only slightly greater calciumin-dependent sPLA2-IIA binding to B. subtilis than to S. aureus, strongly suggesting that the remarkable sensitivity of B. subtilis to wt sPLA2-IIA corresponds to much more facile penetration of the cell wall by sPLA2-IIA in these bacteria.
Complementing the insights gained from the mutational and mechanistic studies of sPLA2-IIA have been studies of the PLA2 sensitivity of a variety of envelope mutants of S. aureus derived from SA113. As charge modification of the sPLA2-IIA has little impact on its ability to degrade membrane PL once the enzyme has access to the bacterial cytoplasmic membrane, charge modification of phosphatidylglycerol (PG), the major PL species of many Gram-positive bacteria including S. aureus and B. subtilis, has little effect on the sensitivity to sPLA2-IIA of either intact S. aureus or cell-wall depleted membrane protoplasts (21). This has been demonstrated by comparing the sensitivity of wt and mprF S. aureus. MprF is a Mr 97,000 integral membrane protein that mediates: 1) modification of membrane PG with the cationic amino acid lysine at the cytoplasmic surface of the cytoplasmic membrane to convert PG to lysyl PG; and 2) translocation of the newly formed lysyl-PG to the outer leaflet of the membrane (27); i.e., the membrane leaflet that is accessed by sPLA2 (28). Whereas conversion of PG to lysyl PG has been linked to increased bacterial (e.g., S. aureus) resistance to several different relatively small cationic antimicrobial peptides (CAMPs; 29) and also to daptomycin (30), mprF and wt S. aureus show little difference in sensitivity to sPLA2-IIA and derived membrane protoplasts are virtually identical in sensitivity to sPLA2-IIA (21).
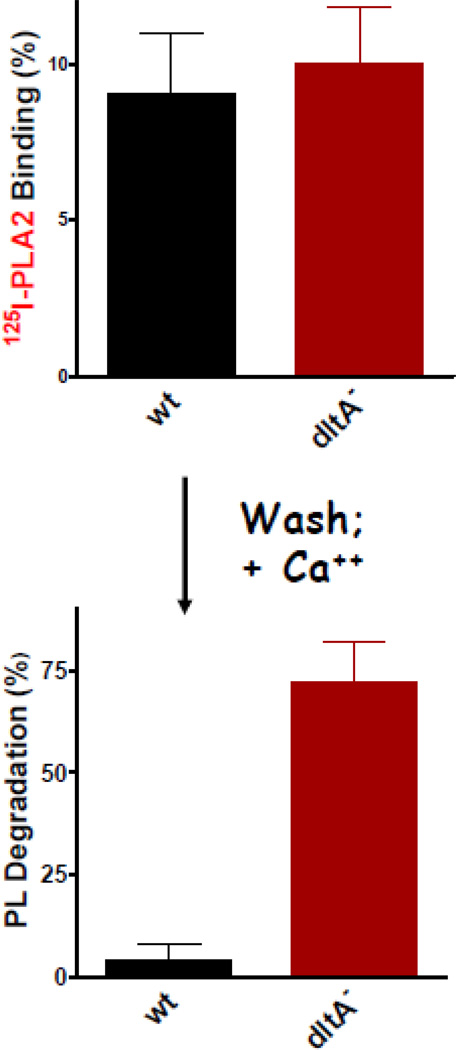
In marked contrast, D-alanylation of cell envelope teichoic acids (TA), both peptidoglycan-linked wall teichoic acids (WTA) and membrane-inserted lipoteichoic acids (LTA) has a profound impact on the sensitivity of S. aureus (SA113) (intact bacteria but not membrane protoplasts) to sPLA2-IIA (14, 21). DltA S. aureus are nearly 100-fold more sensitive to both the phospho-lipolytic and bactericidal actions of sPLA2-IIA, in comparison to the wt parent strain. Together with the abundant peptidoglycan polymers that make up the matrix of the cell wall, WTA and LTA represent the major determinants of the net (-) charge of the Gram-positive bacterial surface and cell wall (31). In S. aureus, the WTA and LTA polymers are comprised of repeating units of ribitol (WTA) or glycerol (LTA) phosphate (32, 33). Together, WTA and LTA form a negatively charged lattice bridging the cell membrane through the cell wall (31). As such, it is conceivable that they provide an anionic “ladder” down which the highly cationic sPLA2-IIA could advance, displacing as it proceeds less cationic autolysins that are normally constrained by electrostatic interactions with WTA and LTA (31, 34). Substitution of WTA and LTA by D-alanine leaves unshielded the (partial) positive charge of the free α-NH2 group of D-alanine and thus partially reduces the net (−) charge of the substituted WTA and LTA polymers (31, 35). The reduced sensitivity of wt vs. dltA S. aureus to a variety of small CAMPs including Magainin II amide and human β-defensin 3(HBD-3) has been attributed to the charge-neutralizing effects of D-alanine substitution (36). However, these lead to only 3-fold changes in sensitivity of wt and dltA S. aureus under the same experimental conditions in which sensitivity to sPLA2-IIA is changed 100-fold (21). Similar comparative analyses of sPLA2 binding, cell wall penetration, and activity as described above for wt and mutant sPLA2-IIA revealed no effect of D-alanylation of TA on either initial calcium-independent sPLA2-IIA binding (Figure 5) or activity against membrane protoplasts (21). Remarkably, the marked increase in sensitivity of dltA S. aureus was equally manifest with wt and mutant human sPLA2-IIA of lower net (+) charge (+13 or +11 vs. +15 of wt enzyme) (21). These findings suggest that the principal effect of D-alanylation of TA (WTA and LTA) vis a vis sPLA2-IIA action against S. aureus is on penetration of bound PLA2 to the cell membrane. That this effect of D-alanylation is manifest irrespective of the net charge of the sPLA2-IIA may mean that the effect of D-alanine substitution is more a consequence of steric hindrance of cell wall penetration caused by the bulkier presence of the substituted D-alanine residue (vs. associated divalent cations) than one based on reduced electrostatic interactions although other possible pleiotropic consequences of altered dltA function can not be excluded. It should be emphasized that despite the far greater effect of D-alanylation of TA on the potency sPLA2-IIA (vs. CAMPs) toward wt S. aureus, the molar potency of sPLA2-IIA toward wt S. aureus is still nearly 100× greater than that of the various non-catalytic CAMPs. Whether or not this reflects the greater efficiency, even in wt S. aureus, of binding and cell wall penetration of the wt sPLA2-IIA (vs. CAMPs) or the ability of even a very small number of molecules of sPLA2-IIA reaching the bacterial cytoplasmic membrane to produce lethal damage by virtue of its catalytic properties is not yet known.
Figure 5.
D-alanylation of teichoic acids of S. aureus (SA113) markedly inhibits ability of bound human sPLA2-IIA to degrade membrane PL of intact bacteria. Binding of [125I] wt sPLA2-IIA was measured after incubation of bacteria without calcium. Unbound PLA2 was removed by washing and bacteria with bound PLA2 was incubated with calcium to monitor calcium-dependent PL degradation. Initial incubations contained 5 × 106 bacteria and 1 ng PLA2 (lower dose of sPLA2-IIA (vs. that use in experiments shown in Fig. 3) reflects exquisite sensitivity of the dltA S. aureus.
Several other genotypic and phenotypic modifiers of sPLA2-IIA potency toward S. aureus have been identified that support the notion that the efficiency of cell wall penetration is a key determinant of sPLA2-IIA potency. This includes the greater sensitivity of: 1) logarithmic vs. stationary phase bacteria (or bacteria pre-treated with a bacteriostatic antibiotic) (23); 2) bacteria pre-treated with a sub-inhibitory dose of a β-lactam antibiotic to reduce cell wall peptidoglycan cross-linking (23); and 3) wt vs. tag O S. aureus (lacking WTA) (22). In each instance, initial PLA2 binding and activity vs. isolated membrane protoplasts are essentially the same, strongly suggesting selective effects of these bacterial modifications on sPLA2-IIA cell wall penetration. Bound PLA2 could be nearly fully displaced from the bacteria with 1M NaCl, confirming that in each instance bacterial binding of PLA2 was mediated by initial electrostatic interactions between the cationic protein and anionic sites exposed on the bacterial surface. sPLA2-IIA binding to tagO S. aureus demonstrated unequivocally that this binding was not dependent on interactions with WTA, a somewhat surprising result. However, exchange of substituted D-alanine between WTA and LTA have suggested close physical proximity of these abundant polyanionic polymers (31, 37; Figure 1). If so, initial sPLA2-IIA binding to WTA may provide the most favorable route for translocation of bound PLA2 from the bacterial surface to the cell membrane. This migration of bound PLA2 may – en route – lead to localized displacement of autolysin(s) bound to TA, promoting localized autolysin activity (e.g., severing of peptidoglycan cross-links) and more efficient sPLA2-IIA penetration of the cell wall.
Similar detailed studies have not yet been carried out in other Gram-negative bacterial species leaving open the question of whether or not similar mechanistic concepts apply to sPLA2-IIA action against other Gram-positive bacteria. An important exception has been provided by recent studies seeking to better understand the basis of the generally much lower sensitivity of Group A vs. Group B streptococci to sPLA2-IIA (20). These studies have revealed an important role of proteins covalently tethered to the cell wall via specific linkages dependent on sortase A. Increased sensitivity of SrtA S. pyogenes to the bactericidal action of sPLA2-IIA was paralleled by increased sPLA2-IIA -triggered bacterial PL degradation despite greater PLA2 binding to wt bacteria. These findings suggest that in S. pyogenes (Group A streptococci), sortase A-dependent cell wall proteins provide a significant impediment to the access of bacterial bound PLA2 to the bacterial cytoplasmic membrane.
Actions of sPLA2-IIA and other related sPLA2 against Gram-negative bacteria
In contrast to the ability of nM concentrations of purified sPLA2-IIA to act against a variety of Gram-positive bacteria, independent antibacterial activity of sPLA toward the Gram-negative bacteria thus far tested including Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, and Neiseria meningitidis requires enzyme concnetrations that greatly exceed sPLA2-IIA levels at most or perhaps all body sites even during inflammation (9, 38). However, redistribution of phospholipids from the inner to outer leaflet of the Gram-negative bacterial outer membrane (Fig. 1), as likely induced by sublethal actions of the neutrophil bactericidal/permeability-increasing protein (BPI) and the complement membrane-attack complex (1, 10) or during shedding of outer membrane vesicles (“blebs”; 39) render some of bacterial phospholipids susceptible to sPLA2-IIA. The rate and extent of membrane phospholipid degradation by sPLA2-IIA in concert with BPI or the membrane-attack complex depends on the extent of envelope alterations produced by BPI and the membrane-attack complex and the concentration of sPLA2-IIA. These requirements for sPLA2-IIA action toward the Gram-negative bacteria tested to date are consistent with the restricted access of sPLA2 to phospholipids in the outer leaflet of an intact bilayer structure (28) and the asymmetry in distribution of lipopolysaccharides (LPS) and phospholipids in the outer membrane of these bacteria (Fig. 1; 40). The extent to which these properties apply to other Gram-negative bacteria requires further study.
The activity of sPLA2-IIA toward BPI- or membrane-attack complex-treated Gram-negative bacteria (e.g., Escherichia coli) is also dependent on the cationic properties of these enzymes that promote non-catalytic interactions of the enzyme with the altered bacterial surface (41, 42). However, in contrast to the requirements for sPLA2 action against Gram-positive bacteria, certain functionally related sPLA2 with much lower global cationic properties (e.g., basic sPLA2 isoform pf Agkistrodon halys blomhoffii venom (net charge of +7); human pancreatic sPLA2-IB (net charge of +3) that have <0.1% the activity of human sPLA2-IIA toward S. aureus and B. subtilis (Liang NS and Weiss JP; unpublished observations) display substantial activity toward BPI-treated E. coli (41, 42). For those two sPLA2, BPI-dependent binding and degradation of phospholipids of E. coli depend on a cluster of basic residues along the polar face of an alpha-helix near the NH2-terminus that represents a variable surface region among all sPLA2 (42, 43). Mutational analyses in recombinant human sPLA2-IIA also support a key role of basic amino acids in this region in the action of the sPLA2-IIA on BPI- and complement-treated E. coli, as well as on E. coli ingested by neutrophils (9).
Antibacterial actions of sPLA2-IIA in biological fluids, ex vivo
sPLA2-IIA -rich biological fluids, both those that are constitutively sPLA2-IIA -rich (e.g., tear fluid, seminal plasma; 16, 44) and those in which the presence of sPLA2-IIA is triggered by either sterile or infectious inflammation (6, 11, 12, 18, 45), display potent antibacterial activity against Staphylococcus aureus and a number of other Grampositive bacterial species. sPLA2-IIA levels in inflammatory fluids can be as high as 500 nM and in tear fluids up to 5 µM, sufficient to account for most if not nearly all of the bactericidal activity of these fluids toward, for example, S. aureus. Blood plasma and many tissue fluids under resting conditions, by contrast, contain <1 nM sPLA2-IIA and no measurable activity toward the same Gram-positive bacteria. sPLA2-IIA is fully active in fluids containing physiologic (extracellular) levels of monovalent and divalent cations.
Studies of sPLA2-IIA-rich inflammatory fluids have been instrumental in showing the potential contribution of mobilized extracellular sPLA2-IIA to digestion and disassembly of bacteria ingested by neutrophils and, in so doing, the integration of mobilized cellular and extracellular host defenses (1, 2, 9, 13, 14). This role has been demonstrated toward both Gram-negative (e.g., Escherichia coli) and Gram-positive (e.g., S. aureus) bacteria under biological conditions in which the extracellular sPLA2-IIA alone or even together with the whole inflammatory fluid produced much less bacterial PL degradation because of limiting levels of extracellular sPLA2-IIA and/or the intrinsic resistance of the bacterial target. In each circumstance, the contribution of extracellular sPLA2-IIA could not be substituted by other human sPLA2, including those (Group V and Group X sPLA2) present natively in the neutrophil (46), nor by mutants of sPLA2-IIA that are not active when assayed alone vs. S. aureus or together with host defense proteins (e.g., neutrophil-derived bactericidal/permeability-increasing protein, membrane-attack complex of complement) that perturb the Gram-negative bacterial outer membrane, rendering Gram-negative bacteria more susceptible to the sPLA2-IIA. Many more synergistic interactions involving the sPLA2-IIA, for example with peptidoglycan-interacting and degrading proteins (e.g., lysozyme) present in both the cellular and extracellular compartments of neutrophil-rich exudates, seem likely and deserving much further study.
Concluding remarks
The defined nature of the biochemical action of the sPLA2-IIA has made characterization of its specific interactions and actions on bacterial targets more amenable than for most antimicrobial compounds that, by contrast, exert their efforts by non-catalytic mechanisms that are often difficult to clearly distinguish one from the other. What also stands out in the antibacterial action of the sPLA2-IIA is the integration of both initial non-catalytic interactions with the bacterial envelope that subsequently make possible the catalytic events (membrane phospholipid degradation) that are ultimately linked to bacterial injury and death. These distinct actions of the enzyme, all required for its potent antibacterial action, have made possible identification of different structural and functional attributes of the protein that are needed at different stages of the enzyme’s antibacterial interactions. This, in turn, has provided an unusually well-defined context in which to determine the mechanisms by which specific bacterial properties and components affect sensitivity and resistance to the sPLA2-IIA. Finally, by virtue of the specificity of the mechanistic insights gained, clearly different determinants of bacterial sensitivity and resistance to the PLA2 vs. many of the smaller and non-catalytic cationic antimicrobial peptides have been able to be appreciated (47), an insight that should be exploited in future efforts for new drug development.
Supplementary Material
Highlights.
Humans express ten closely related secreted phospholipases A2 (sPLA2)
Unique features of Group IIA sPLA2 : strong cationicity; nM antibacterial activity
Cationic properties of sPLA2-IIA promote bacterial binding, cell wall penetration
Bacterial death follows membrane phospholipid degradation, activation of autolysins
Antibacterial actions of sPLA2-IIA enhanced by other host defense systems
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elsbach P, Weiss J, Levy O. Integration of antimicrobial host defenses: Role of the bactericidal/permeability-increasing protein. Trends in Microbiology. 1994;2:324–328. doi: 10.1016/0966-842x(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 2.Weiss J, Bayer AS, Yeaman M. Cellular and extracellular defenses against staphylococcal infections. In: Novick R, Fischetti VE, editors. Gram-positive pathogens. 2nd edition. Washington D.C.: ASM Press; 2006. pp. 544–559. [Google Scholar]
- 3.Areschoug T, Pluddemann A, Gordon S. Innate immunity against bacteria. In: Kaufmann SHE, Rouse B, Sacks D, editors. Immune Response to Infection. 2nd Ed. Washington DC: ASM Press; 2011. pp. 209–223. [Google Scholar]
- 4.Nevalainen TJ, Graham GG, Scott KF. Antibacterial actions of secreted phospholipases A2. Review. Biochim Biophys. Acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Birts CN, Barton CH, Wilton DC. Catalytic and non-catlaytic function of human IIA phospholipase A2. Trends Biochem Sci. 2010;35:28–35. doi: 10.1016/j.tibs.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Raymond B, Goossens PL, Njamkepo E, Guiso N, Paya M, Touqui L. Type-IIA secreted phospholipase A2 is an endogenous antibiotic-like protein of the host. Biochimie. 2010;92:561–582. doi: 10.1016/j.biochi.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Forst S, Weiss J, Elsbach P, Maraganore JM, Reardon I, Heinrikson RL. Structural and functional properties of a phospholipase A2 purified from an inflammatory exudate. Biochemistry. 1986;25:8381–8385. doi: 10.1021/bi00374a008. [DOI] [PubMed] [Google Scholar]
- 8.Wright G, Ooi CE, Weiss J, Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J. Biol. Chem. 1990;265:6675–6681. [PubMed] [Google Scholar]
- 9.Weiss J, Inada M, Elsbach P, Crowl RM. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J. Biol. Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]
- 10.Madsen L, Inada M, Weiss J. Determinants of activation by Complement of (type II) phospholipase A2 acting against. Escherichia coli. Infect. Immun. 1996;64:2425–2430. doi: 10.1128/iai.64.7.2425-2430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinrauch Y, Elsbach P, Madsen LM, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14 KDa phospholipase A2. J. Clin. Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of Group IIA phospholipase A2. J. Clin. Invest. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Femling J, Nauseef WM, Weiss J. Synergy between extracellular Group IIA phospholipase A2 and phagocyte NADPH oxidase in digestion of phospholipids of Staphylococcus aureus ingested by human neutrophils. J Immunol. 2005;175:4653–4661. doi: 10.4049/jimmunol.175.7.4653. [DOI] [PubMed] [Google Scholar]
- 14.Hunt CL, Nauseef WM, Weiss JP. Effect of D-alanylation of (Lipo) Teichoic acids of Staphylococcus aureus on host secretory phospholipase A2 action before and after phagocytosis by human neutrophils. J Immunol. 2006;176:4987–4994. doi: 10.4049/jimmunol.176.8.4987. [DOI] [PubMed] [Google Scholar]
- 15.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 16.Qu X-D, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foreman-Wykert AK. Microbiology (Ph.D. thesis) New York: New York University; 1999. Determinants of the Bactericidal Action of Mammalian 14 kDa Group IIA Phospholipase A2 Against Gram-Positive Bacteria. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gronroos JO, Laine VJO, Nevalainen TJ. Bactericidal Group IIA Phospholipase A2 in serum of patients with bacterial infections. J. Infect. Dis. 2002;185:1767–1772. doi: 10.1086/340821. [DOI] [PubMed] [Google Scholar]
- 19.Gimenez AP, Wu YZ, Paya M, et al. High bactericidal efficiency of type iia phospholipase A2 against Bacillus anthracis and inhibition of its secretion by the lethal toxin. J Immunol. 2004;173:521–530. doi: 10.4049/jimmunol.173.1.521. [DOI] [PubMed] [Google Scholar]
- 20.Movert E, Wu Y, Lambeau G, Touqui L, Areschoug T. A novel bacterial resistance mechanism against human Group IIA-secreted phospholipase A2: Role of Streptococcus pyogenes sortase A. J Immunol. 2011;187:6437–6446. doi: 10.4049/jimmunol.1100499. [DOI] [PubMed] [Google Scholar]
- 21.Koprivnjak T, Peschel A, Gelb MH, Liang NS, Weiss JP. Role of the charge properties of bacterial envelope in bactericidal action of human Group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 2002;277:47636–47644. doi: 10.1074/jbc.M205104200. [DOI] [PubMed] [Google Scholar]
- 22.Koprivnjak T, Weidenmaier C, Peschel A, Weiss JP. Wall teichoic acid deficiency in Staphylococcus aureus confers selective resistance to mammalian group IIA phospholipase A2 and human β defensin-3. Infect Immun. 2008;76:2169–2176. doi: 10.1128/IAI.01705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foreman AK, Weinrauch Y, Elsbach P, Weiss J. Cell wall determinants of the bactericidal action of group IIA phospholipase A2 against Gram-positive bacteria. J. Clin. Invest. 1999;103:715–721. doi: 10.1172/JCI5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foreman-Wykert AK, Weiss J, Elsbach P. Increased phospholipid synthesis by S. aureus during (sub) lethal attack by mammalian 14 kDa group IIA phospholipase A2. Infect. Immun. 2000;68:1259–1264. doi: 10.1128/iai.68.3.1259-1264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, Wilton DC. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277:1788–1793. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JP, Elsbach P, Weinrauch Y. Recombinant antibacterial Group IIA phospholipase A2 and methods of use thereof. U.S. Patent #6,767,584. 2004
- 27.Ernst CM, Kuhn S, Slavetinsky CJ, Krismer B, Heilbronner S, Gekeler C, Kraus D, Wagner S, Peschel A. The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits. MBio. 2015;27:1–9. doi: 10.1128/mBio.02340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain MK, Gelg MH, Rogers J, Berg OG. Kinetic basis for interfacial catalysis by phospholipase A2. Methods Enzymol. 1995;249:567–614. doi: 10.1016/0076-6879(95)49049-3. [DOI] [PubMed] [Google Scholar]
- 29.Ernst CM, Peschel A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol Microbiol. 2011;80:290–299. doi: 10.1111/j.1365-2958.2011.07576.x. [DOI] [PubMed] [Google Scholar]
- 30.Bayer AS, Schneider T, Sahl HG. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann. NY. Acad. Sci. 2013 doi: 10.1111/j.1749-6632.2012.06819.x. 1277-139–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidenmaier C, Kokai-Kun JF, Kulauzovic E, Kohler T, Thumm G, Stoll H, Gotz F, Peschel A. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Micriobiol. 2008;298:505–513. doi: 10.1016/j.ijmm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Baddiley J, Buchanan JG, Hardy FE, Martin RO, Rajbhandary UL, Sanderson AR. The structure of the ribitol teichoic acid of Staphylococcus aureus. Biochim Biophys Acta. 1961;52:406–407. doi: 10.1016/0006-3002(61)90699-0. [DOI] [PubMed] [Google Scholar]
- 34.Biswas R, Martinez RE, Gohring N, Schlag M, Josten M, Xia G, Hegler F, Gekeler C, Gleske AK, Gotz F, Sahl HG, Kappler A, Peschel A. Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS One. 2012;7:e41415. doi: 10.1371/journal.pone.0041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Micriobiol. 2009;300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 37.Haas R, Koch HU, Fischer W. Alanyl turnover from lipoteichoic acid to teichoic acid in Staphylococcus aureus. FEMS Microbiol Lett. 1984;21:27–31. [Google Scholar]
- 38.Harwig SS, Tan L, Qu XD, Cho Y, Eisenhauer PB, Lehrer RI. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem. 2005;280:32383–32394. doi: 10.1074/jbc.M508063200. [DOI] [PubMed] [Google Scholar]
- 40.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lidpid A modification systems in Gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forst S, Weiss J, Maraganore JM, Heinrikson RL, Elsbach P. Relation between binding and athe action of phospholipases A2 on Escherichia coli exposed to the bactericidal/permeability-increasing protein of neutrophils. Biochimica et Biophysica Acta. 1987;920:221–225. doi: 10.1016/0005-2760(87)90098-1. [DOI] [PubMed] [Google Scholar]
- 42.Weiss J, Wright G, Bekkers ACAPA, van den Bergh CJ, Verheij HM. J Biol Chem. 1991:4162–4167. [PubMed] [Google Scholar]
- 43.Forst S, Weiss J, Blackburn P, Frangione B, Goni F, Elsbach P. Amino acid sequence of a basic Agkistrodon halys blomhoffii phospholipase A2. Possible role of NH2-terminal lysines in action on phospholipids of Escherichia coli. Biochemistry. 1986;25:4309–4314. doi: 10.1021/bi00363a020. [DOI] [PubMed] [Google Scholar]
- 44.Nevalainen TJ, Meri KM, Niemi M. Synovial-type (group II) phospholipase A2 human seminal plasma. Andrologia. 1993;25:355–358. doi: 10.1111/j.1439-0272.1993.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 45.Pernet E, Guillemot L, Burgel PR, Martin C, Lambeau G, Sermet-Gaudelus I, Sands D, Leduc D, Morand PC, Jeammet L, Chignard M, Wu Y, Touqui L. Pseudomonas aeruginosa eradicates Staphylocccus aureus by manipulating the host immunity. Nat Commun. 2014;5:5105. doi: 10.1038/ncomms6105. [DOI] [PubMed] [Google Scholar]
- 46.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB. Groups IV, V, X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 47.Koprivnjak T, Peschel A. Bacterial resistance mechanisms against host defense peptides. Cell Mol Life Sci. 2011;68:2243–2254. doi: 10.1007/s00018-011-0716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.