Abstract
When organisms are exposed to an increase in temperature, they undergo a heat shock response (HSR) regulated by the transcription factor heat shock factor 1 (HSF-1). The heat shock response includes the rapid changes in gene expression initiated by binding of HSF-1 to response elements in the promoters of heat shock genes. Heat shock proteins function as molecular chaperones to protect proteins during periods of elevated temperature and other stress. During infection, hookworm infective third stage larvae (L3) undergo a temperature shift from ambient to host temperature. This increased temperature is required for the resumption of feeding and activation of L3, but whether this increase initiates a heat shock response is unknown. To investigate the role of the heat shock in hookworm L3 activation and parasitic development, we identified and characterized the expression profile of several components of the heat shock response in the hookworm Ancylostoma caninum. We cloned DNAs encoding an hsp70 family member (Aca-hsp-1) and an hsp90 family member (Aca-daf-21). Exposure to a heat shock of 42 °C for one hour caused significant up-regulation of both genes, which slowly returned to near baseline levels following one hour attenuation at 22 °C. Neither gene was up-regulated in response to host temperature (37 °C). Conversely, levels of hsf-1 remained unchanged during heat shock, but increased in response to incubation at 37°C. During activation, both hsp-1 and daf-21 are down regulated early, although daf-21 levels increase significantly in non-activated control larvae after 12 hours, and slightly in activated larvae by 24 hours incubation. The heat shock response modulators celastrol and KNK437 were tested for their effects on gene expression during heat shock and activation. Pre-incubation with celastrol, an HSP90 inhibitor that promotes heat shock gene expression, slightly up-regulated expression of both hsp-1 and daf-21 during heat shock. KNK437, an inhibitor of heat shock protein expression, slightly down regulated both genes under similar conditions. Both modulators inhibited activation-associated feeding, but neither had an effect on hsp-1 levels in activated L3 at 16 hours. Both celastrol and KNK437 prevent the up-regulation of daf-21 and hsf-1 seen in non-activated control larvae during activation, and significantly down regulated expression of the HSF-1 negative regulator Aca-hsb-1 in activated larvae. Expression levels of heat shock response factors were examined in developing A. ceylanicum larvae recovered from infected hosts and found to differ significantly from the expression profile of activated L3, suggesting that feeding during in vitro activation is regulated differently than parasitic development. Our results indicate that a classical heat shock response is not induced at host temperature and is suppressed during larval recovery and parasitic development in the host, but a partial heat shock response is induced after extended incubation at host temperature in the absence of a developmental signal, possibly to protect against heat stress.
Keywords: hookworm, heat shock, activation, heat shock response, Ancylostoma
Graphical Abstract

1. Introduction
Soil-transmitted nematodes infect about 1.3 billion people worldwide with the morbidity predominantly attributed to roundworms, whipworms, and hookworms [1]. Hookworms of the species Ancylostoma duodenale and Necator americanus infect more than 700 million people worldwide annually, causing malnutrition and iron-deficiency anaemia leading to diminished cognitive abilities and underdevelopment in children [2, 3]. In addition to children, pregnant women and the elderly are most vulnerable for infections [1, 4]. Infections occur primarily through contact with infective larvae in contaminated soil, which penetrate the skin and develop in the small intestines to the reproductive, feeding adult stage that causes blood loss. These soil-dwelling infective third stage larvae (iL3) are non-feeding and developmentally arrested. During invasion of a permissive host, stimulating cues reinitiate feeding and development. In addition, the transition to a parasitic life is accompanied by evasion of the host’s defences including the release of excretory/secretory products and a shift from aerobic towards anaerobic energy metabolism. The signals that initiate development and the associated molecular mechanisms during the transition to parasitism are poorly understood [5]. Current treatments for hookworm disease fail to prevent re-infections, resistance to chemotherapy is beginning to emerge, and efforts to develop a vaccine have been unsuccessful [6, 7]. Improved understanding of the underlying mechanisms involved in the resumption of development is critical to development of new control strategies. 1
One of the initial events upon entry of a larva into a host is thought to be the resumption of feeding. This process can be mimicked in vitro by low pH treatment followed by incubation at host temperature in the presence of serum and glutathione. All three stimuli are required to reinitiate feeding in vitro [8–13], but development from the L3 to the fourth larval stage (L4) only occurs in a suitable host. If iL3 enter a non-suitable host, feeding can be re-initiated, but the larvae remain in a non-developing, dormant stage (paratenesis). The “package of signals” which initiates development must therefore differ from that which initiates feeding.
Recent studies investigating the obligate non-developing iL3 stage of parasitic nematodes suggest that developmental arrest is regulated by a similar mechanism as the non-developing, facultative dauer stage of the free-living nematode Caenorhabditis elegans [14–17]. In C. elegans, the forkhead transcription factor DAF-16/FOXO is one of the key regulators of dauer, and is negatively regulated by the insulin/insulin growth factor-like signalling (IIS) pathway [18, 19]. Besides DAF-16, dauer formation requires the transcription factor heat shock factor-1 (HSF-1) as shown in dauer constitutive mutants and wild type animals [20, 21]. Furthermore, the heat shock response (HSR) requires DAF-16 activity, and nuclear export of DAF-16 during recovery from heat shock involves HSF-1 and HSP70/HSP-1[22].
Hookworms encounter temperature fluctuations as free-living larval stages, but also experience a temperature shift from ambient to that of their endothermic host during infection. The elevated temperature of the host is required for in vitro activation of hookworm L3 [8], and is likely a component of the host-specific signal package that initiates development. This, together with the interaction of DAF-16 and HSF-1 in the dauer pathway, suggests a potential role for HSF-1 and HSR factors in the hookworm infectious process.
The HSR consists of four main components, which act in a highly conserved mechanism. The accumulation of unfolded proteins as a consequence of elevated temperature leads to a rapid increase of cytoprotecting proteins in order to prevent cell damage. The synthesis of these chaperones, called heat shock proteins (HSP), is regulated by HSF-1. In the absence of stress, the inactive monomeric form of HSF-1 is bound by HSP-70 and other chaperones in the cytoplasm. Accumulation of denatured proteins recruits HSP-90 and HSP-70 from the complex and allows HSF-1 to translocate into the nucleus, where it assembles into a homotrimer and is phosphorylated at activating serine residues. In its activated state, HSF-1 binds to heat shock response elements in the promoter regions of heat shock associated genes and leads to their expression. Negative feedback loops repress heat shock associated gene expression. In addition to HSP-70, HSF-1 is also negatively regulated by heat shock binding protein 1 (HSB-1). During attenuation, or recovery from heat shock, HSB-1 and HSP-70 bind to the HSF-1 trimer causing its dissociation from DNA and disassembly into the inactive state [21, 23]. In addition to regulating the HSR, HSF-1 and HSPs are required for normal development [20, 24–26], with the monomeric form of HSF regulating genes involved in development [27].
To date, the HSR has not been described for hookworms. We recently analysed the function of Aca-HSB-1[28], and herein investigate the expression of other main regulators of the HSR, HSF-1, HSP-1/HSP-70, and DAF-21/HSP-90 during heat shock. We compare the HS expression profile to the profile of in vitro activated L3 as well as developing parasitic larvae. Furthermore, we investigate the role of heat shock on activation by determining the influence of known HSR modulators on the expression of HSR factors.
2. Material and methods
2.1. Ethics statement
All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the George Washington University Medical Center Institutional Animal Care and Use Committee (protocol number: A147).
2.2. Parasites
For heat shock and in vitro larval activation experiments, the Baltimore strain of A. caninum (US National Parasite Collection No. 1000655) was maintained and harvested from dog feces as described elsewhere [28]. An Indian strain of A. ceylanicum (USNPC No. 102954) was maintained in Syrian hamsters [29] and dogs. Developing parasitic larval stages were obtained from three A. ceylanicum infected hamsters per time point and separated into different developmental stages by visual examination under a dissecting scope. A. ceylanicum adults were recovered 12d post infection (p.i.).
2.3. In vitro activation and heat shock experiments of iL3
In vitro activation of iL3 and heat shock experiments were conducted as published [28]. For activation, iL3 were decontaminated in 0.12 N HCl for 30 minutes at 22° C followed by 3 washes in RPMI1640 tissue culture medium supplemented with 25 mM HEPES (pH 7.0) and antibiotics (RPMI-c). Decontaminated iL3 (5000) were incubated in 0.5 ml of RPMI-c in individual wells of 24-well tissue culture plates at 37°C, 5% CO2 for 24 h. Larvae were activated by the inclusion of 10% (v/v) of a < 10 kDa ultrafiltrate of canine serum and 15 mM S-methyl-glutathione (GSM, Sigma Chemical, St. Louis, MO), whereas non-activated L3 were incubated in RPMI-c only. To determine the percentage feeding (i.e. activation), an aliquot of L3 were incubated as above in fluorescein isothiocyanate labeled bovine serum albumin (FITC-BSA, Sigma Chemical) for 2hr, washed in PBS, transferred to a microscope slide and examined under ultraviolet illumination as described previously [10]. Larvae that had ingested the FITC-BSA were counted and expressed as a percentage of the total number counted. Only experiments in which a minimum of 85% of activated L3 and a maximum of 10% of non-activated L3 were feeding at 24 hr were used.
For heat shock experiments, 5000 decontaminated iL3 in 0.5 ml of RPMI-c were incubated at 42°C for the desired time, followed by attenuation at 22°C for up to 60 minutes [28]. Control iL3 were incubated at 37°C followed by attenuation. Following treatment, the iL3 were immediately snap-frozen in liquid nitrogen and stored at −80°C until needed.
Heat shock response inhibitors celastrol (3-Hydroxy-24-nor-2-oxo-1(10),3,5,7-friedelatetraen-29-oic Acid) and KNK437 (N-formyl-3,4-methylenedioxy-benzylidene-γ-butyrolactam) were purchased from Calbiochem, dissolved in DMSO, and stored at −20°C according the manufacturer’s instruction. The compounds were present during activation and subsequent feeding. Heat treated L3 were pre-incubated with the compounds for 1h at room temperature followed by heat shock at 42 °C for 1h.
2.4. RNA isolation and quantitative reverse transcriptase-PCR
RNA isolation and quantitative reverse transcriptase-PCR (qPCR) experiments were performed as described previously [28]. Data are from 2–3 runs in triplicates from 1–3 biological replicates and analysed in GraphPad InStat3 (ANOVA plus Tukey Kramer Test or unpaired t test with Welch’s correction for fewer than 3 groups). The sequences of the primers used are listed in Table 1. Amplicons were verified by sequencing.
Table 1.
Primers used for quantitative RT-PCR.
| Target | Primer name | Primer sequence |
|---|---|---|
| Aca-hsf-1 | achsf-qF1 | 5′ GCT GTG GAG CAT AGT GGA G |
| achsf-qR1 | 5′ GCT TGA AGA AAT GTG GCA GAA C | |
|
| ||
| Aca-hsp-1 | achsp70-qF1 | 5′ GAC GAT GAG AAG CTG AAG GAC |
| achsp70-qR1 | 5′ GTC TGG TTG CTG TCA AGC CA | |
|
| ||
| Aca-daf-21 | achsp90-qF2 | 5′ GTT GTT GAA GAT GAG GAT GCT G |
| achsp90-qR2 | 5′ GGA GAT GTC ATC AGG GTT GCG | |
2.5 Cloning of Aca-hsp-1 and Aca-daf-21
Analysis of expressed sequence tags (EST) encoding parts of A. caninum HSP70 member (pb06c12.y1, pb23d03.y1)[30] within the Washington University cluster group AC00920 were used to design specific forward and reverse PCR primers. The 5′ and 3′ ends of the hsp70 gene were amplified in two separate PCR reactions. In the first reaction, the nematode spliced leader (SL) primer [31, 32] containing a PstI site (italics) (SLP; 5′GGT ACT GCA GGG TTT AAT TAC CCA AG) and primer HSP70-R1 (5′TGT CTC AAT ACC AAG TGA GAG AGG AGC C) were used in a reaction containing A. caninum L3 cDNA. This reaction was diluted 1:10 and used as template in a semi-nested PCR containing SLP and HSP70-R2-XhoI (5′ ATT CTC GAG AAC GAT GTC GTG GAT CTG GCT CTT GTC C). The reaction conditions were 35 cycles at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute followed by 1 cycle of 72°C for 5 minutes. The resulting ~1000 bp fragment was cut with PstI and XhoI (New England Biolabs, Beverly, Massachusetts) and cloned into the multiple cloning site of pBluescript II SK +/− (Stratagene, La Jolla, CA) cut with the same enzymes. The 3′ end was amplified with primers HSP70F1-BamHI and T7 using a directional cDNA library constructed in Lambda Zap II as template [31]. The resulting ~1000 bp 3′ end fragment was cut with BamHI and XhoI, which cuts upstream of the T7 site in the vector used in construction of the cDNA library. This fragment was also cloned into pBluescript II SK +/−. Sequencing of the clones revealed successful amplification of the 5′ and 3′ ends of the Aca-hsp70 cDNA. The full-length hsp70 gene was amplified from cDNA using the primers HSP70-ExFX (5′TAT ACT CGA GGC TAC CAT GAC GAA AGT CAA CGC AGT CG) and HSP70-ExRH (5′AAT TAA GCT TGT CGA CTT CTT CGA TGG TTG GTC C) and cloned into XhoI/HindIII digested pcDNA3.1 (−) /myc-His (Invitrogen). The construct was verified by sequencing both strands.
A similar nested strategy was used to clone A. caninum hsp90 cDNA. A ~220 bp fragment of the hsp90 cDNA 5′end containing the SL sequence isolated inadvertently during another experiment was used to design primer HSP90-F3 (5′GAG ATA TGT CTG ACG ACA AGG GCG) internal to the SL primer and containing the last 3 bases of the SL (GAG, bold) and the methionine start codon (italicised) of Aca-HSP-90. An A. caninum EST encoding part of the hsp-90 cDNA (pa19a01.y1) was used to design a specific reverse HSP90-R1 (5′ GAA GTG ACA ATG CAG CAG GGT) and the nested primer HSP90-R2 (5′CTT CAG TTG TTG CAC GCA GTA C). In the first reaction, primer SL and R1 were used with A. caninum first strand cDNA as the template. The round 2 reaction used a 1/10 dilution of the round 1 reaction as template with primers F3 and R2. Reaction conditions were the same as above. An amplicon of 1000 bp was cloned and confirmed by sequencing to be the 5′ end.
The 3′ end was also isolated using a nested PCR strategy, using an A. caninum cDNA Lamda ZAPII library [31] as template. Forward primers HSP90-F1 (5′ TCA CTG GCG AGT CTA AGG ACG C) and HSP90-F2 (5′ GAT CCC ATT GAT GAG TAC TGC G) were designed from the A. caninum hsp90 EST, and T7prom (5′ TAA TAC GAC TCA CTA TAG) and T7nest (5′ TAC GAC TCA CTA TAG GGC G) designed from the pBluescript 3′ vector sequence flanking the library insert site. Following the second round, an amplicon of ~780 bp was cloned and confirmed by sequencing. The full length coding sequence of hsp90 was amplified from A. caninum cDNA in a single reaction using primers HSP90-FB (5′ TAT TGG ATC CAT GTC TGA CGA CAA GG) containing a 5′ BamHI site (italicised) and HSP90-RX (5′ TTT TCT AGA GTC GAC CTC CTC CAT TCG C) containing a 5′ XbaI site. The amplicon was sequentially digested with XbaI and BamHI and ligated into pcDNA3.1/V5-His digested with the same enzymes. The construct was confirmed by DNA sequencing of both strands.
Nucleotide sequence data reported in this paper are available in GenBank under the following accession numbers: Aca-daf-21(KP686395) and Aca-hsp-1 (KP686396).
2.6. Sequence analysis
Dideoxy DNA sequencing was performed commercially. Sequence analysis was performed using BioEdit Sequence Alignment Editor version 7.1.3.0 [33], and homology searches were done using BLASTP [34, 35] at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/; BLOSUM). Percentage identity and similarity was determined using the BLASTP algorithm with the BLOSUM62 matrix. The following programs at the SIB Swiss Institute of Bioinformatics ExPASy Bioformatics Resources Portal [36] were used: CLUSTAL W for multiple alignments, ScanProsite for conserved motif identification, and the compute pI/MW tool to calculate molecular weight and pI. Conserved domains were identified by searching the Conserved Domain Database at NCBI. The phylogenetic tree of Hsp70 family members was inferred using the Maximum Likelihood method based on the JTT matrix-based model in MEGA6 [37, 38]. Signal sequence analysis was performed at the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/) [39].
3. Results
3.1. Characterisation of Aca-hsp-1 and Aca-daf-21
A hemi-nested PCR strategy was used to isolate the A. caninum HSP-70 family member (hsp70) and HSP-90 cDNAs. Primers were designed from ESTs with homology to each HSP, and the 5′ and 3′ ends isolated in separate PCRs using an A. caninum L3 library or cDNA as template. For hsp70, each PCR generated fragments of approximately 1000 bp. Following DNA sequencing of the 5′ and 3′ end fragments, new primers were designed to amplify the full length hsp70 from L3 cDNA template. The ~1950 bp cDNA representing the coding region was cloned into vector pcDNA3.1 and verified by DNA sequencing.
The hsp70 cDNA 5′end contained the conserved 22 bp nematode spliced leader [32] followed by 5 untranslated nucleotides and the ATG start codon at nucleotide 28. The open reading frame encoded by the cDNA is 1929 bp, ending with a TAA termination signal at nucleotide 1957. The 3′ untranslated region is 117 bp and contains a canonical AATAAA polyadenylation signal [40] 14 nucleotides upstream of the poly (dA) tail at nucleotides 2057 to 2062. The deduced HSP70 protein is 643 amino acids with a predicted mass of 70,400 Da and a calculated pI of 5.35. Consistent with its role as an intracellular chaperone, the HSP70 deduced amino acid sequence lacks a hydrophobic signal peptide [39].
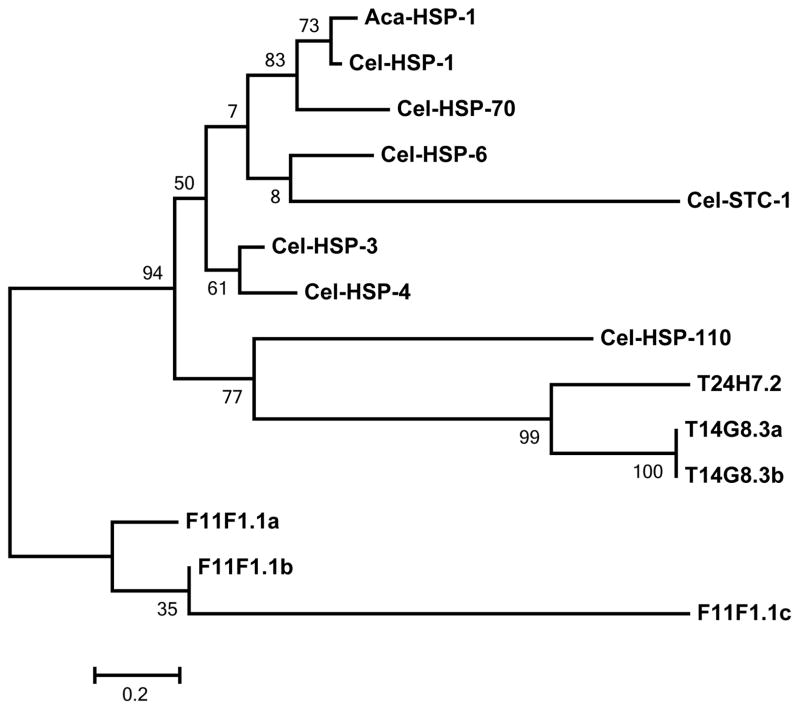
Analysis of the hookworm hsp70 cDNA confirmed that it is a member of the HSP 70 protein family. The deduced amino acid sequence contains the three Prosite HSP70 family signature consensus sequences, located at amino acids 9–16 (PS00297), 198–211(PS00329), and 335–349 (PS01036) and the EEVD motif commonly found on the C-terminus of cytoplasmic HSP70 proteins. The presence of 5 cysteines in the deduced amino acid sequence suggests that the hsp70 member is a heat inducible HSP70 [41]. When the Genbank non-redundant database was searched using the hookworm HSP70 amino acid sequence (BLASTP algorithm) [35], the best matches were to other parasitic nematode HSP-70 orthologs, including the hookworm Necator americanus (99% identical/99% similar/100% coverage), Haemonchus contortus (97/98/100), Dracunculus medinensis (94/98/95), Loa loa (93/97/95), and Wuchereria bancrofti (89/93/99). There are 13 hsp70 family members in C. elegans present in Wormbase (www.wormbase.org). A phylogenetic analysis of these proteins together with the hookworm protein (Fig. 1) indicated that the hookworm HSP70 is orthologous to C. elegans HSP-1, and therefore will be named Aca-HSP-1.
Figure 1. Maximum likelihood tree showing relationship of Aca-HSP-1 to C. elegans HSP70 family members.
The percentage of trees in which the associated taxa clustered together is shown next to the branches. Accession numbers: Cel-HSP-1, NP_503068; Cel-HSP-70, NP_492485; Cel-HSP-6, NP_504291; Cel-STC-1, NP_495808; Cel-HSP-3, NP_509019; Cel-HSP-4, NP_495536; Cel-HSP-110, NP_498868; T24H7.2, NP_495249; T14G8.3a, NP_001024913; T14G8.3b, NP_001024914; F11F1.1a, NP_001255199; F11F1.1b, NP_001255200; F11F1.1c, NP_001255201.
The A. caninum hsp90 cDNA was also isolated by PCR in 3 steps. During another experiment we fortuitously isolated a ~220 bp fragment of the 5′end of the A. caninum hsp90 cDNA. Using this sequence and that of an EST (pa19a01.y1) encoding a truncated internal portion of hsp90, we designed primers to amplify the 5′ end from first strand cDNA and the 3′ end from a cDNA library as described above. Following DNA sequencing of the resultant ends, we designed primers to amplify the hsp90 coding region, and cloned it into pcDNA3.1/V5-His vector. Both strands of the resulting construct were sequenced.
The hookworm hsp-90 cDNA also contains the trans-spliced 22 bp conserved nematode SL at the 5′ end. The ATG start codon begins at nt 25, and the open reading frame is 2121 bp, terminating with a TAA stop codon at nt 2146. The 127 bp 3′ untranslated region contains the canonical polyadenylation signal AATAA 13 bp upstream of the poly (dA) addition site. The deduced protein sequence is 707 amino acids, has a predicted molecular weight of 81,148 Da and a calculated pI of 5.03, and lacks a hydrophobic leader sequence [39]. ScanProsite detected the HSP90 protein family signature (PS00298) at amino acids 27–36 of the deduced amino acid sequence. A Conserved Domain (NCBI) search revealed an N-terminal histidine kinase-like ATPase domain characteristic of HSP90 family members, and the C-terminal tetratricopeptide repeat (TPR) interacting sequence MEEVD that interacts with TPR domain-containing cofactors [42]. A BLASTP search of the Genbank non-redundant database using the HSP90 amino acid sequence matched HSP90 molecules in other nematodes including H. contortus (98% identical/99% similar/100% coverage), N. americanus (99/99/97), and W. bancrofti (86/92/99), as well as DAF-21/HSP-90 of several Caenorhabditis species. The daf-21 designation will be used for the hookworm hsp90 gene and protein to indicate orthology with the C. elegans HSP-90 gene.
3.2. Heat shock response in iL3
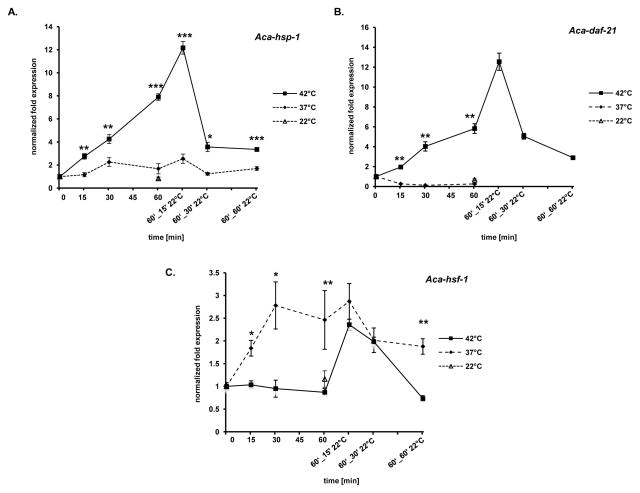
We recently showed that Aca-hsb-1 is down-regulated in iL3 following a short exposure to 42°C [28]. Here we wanted to investigate how the expression profiles of Aca-hsf-1, Aca-hsp-1 and Aca-daf-21 change during heat shock. We used the same conditions and time points of 15, 30 and 60 min at 42°C and at 37°C followed by attenuation periods for 15, 30 and 60 min at room temperature. We found impressive up-regulation of the Aca-hsp-1 (p=0.0006) and Aca-daf-21 (p=0.0035) genes when iL3 were exposed to 42°C for 60 min (Fig. 2A and B, respectively). Aca-hsp-1 transcripts increased 6-fold compared to untreated larvae at 60 min and peaked at 12-fold up-regulation after 60 min heat shock plus 15 min exposure to room temperature. Longer exposure to room temperature following heat shock led to a drop nearly to basal level. Aca-daf-21 expression showed a similar profile as Aca-hsp-1, with a 8-fold up-regulation after 60 min at 42°C, which subsequently increased further to 12-fold up-regulation after 60 min at 42°C followed by 15 min at 22°C. Aca-daf-21 transcript levels dropped almost to basal levels in the subsequent room temperature attenuation period. Transcriptional levels of both molecules were unaffected by exposure to 37°C followed by incubation at room temperature, consistent with a classic cell-protective chaperone function for Aca-HSP-1 and Aca-DAF-21 during exposure to elevated (42°C) temperature. As both molecules represent markers of a HSR, we clearly define 42°C, and not host-like temperature, as the temperature initiating heat stress. The expression level of Aca-hsf-1 remained unchanged during exposure to 42°C, but then increased about 2.3-fold during the first 15 min of room temperature attenuation, and returned to untreated control levels by 60 min at room temperature (Fig. 2C). As the HSR needs to occur immediately after heat exposure, we would not expect Aca-hsf-1 to be up-regulated, as sufficient HSF-1 protein must be present to rapidly initiate the response. The increase during attenuation might be explained by a subsequent feedback regulation due to a replacement of heat shocked damaged Aca-HSF-1. Interestingly, Aca-hsf-1 expression was up-regulated ~2.6-fold when L3 were incubated at 37°C for 30 min, where it remained at 60 min. Up to 60 minutes of attenuation at 22°C following 60 min at 37°C only caused a slight decrease in expression levels, to 2-fold above the control. As expected, Aca-hsf-1 shows the opposite response as Aca-hsb-1 [21, 43], consistent with the role of Aca-HSB-1 as negative regulator of Aca-HSF-1.
Figure 2.
Heat shock response of A. caninum iL3 occurs at 42°C, not at host like temperatures. Gene-specific mRNA levels of L3 exposed to 37° and 42°C for 15, 30 and 60 min following attenuation to room temperature (22°C) for 15, 30 and 60 min were analysed relative to the Aca-60s reference gene and untreated iL3 control at 0 h time point. A) Aca-hsp-1, B) Aca-daf-21, C) Aca-hsf-1. Asterisks indicate significant difference between activated and non-activated: * p<0.05, ** p<0.01, ***p<0.001
3.3. HSR factors in L3 activation
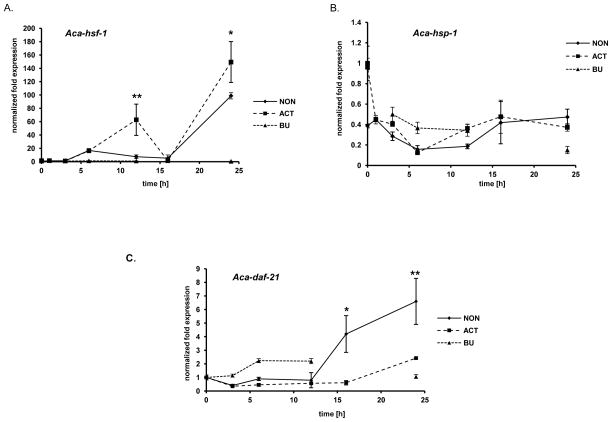
Arrested iL3 larvae resume development when exposed to permissive hosts. The initial events of recovery can be partially mimicked by incubation with serum fractions and S-methylglutathione (GSM) at host-like temperature of 37°C [44]. All three components are necessary to obtain an exsheathed, “activated” L3 population feeding at greater than 90%. After determining the expression profile of key heat shock proteins during the hookworm iL3 HSR, we wanted to determine if the response is similar when iL3 are exposed long-term to host temperature under activating (serum plus GSM) or non-activating (medium alone) conditions. After 0.5, 1, 3, 6, 12, and 24h incubation, we determined the expression levels of Aca-hsf-1, Aca-daf-21 and Aca-hsp-1 by qPCR. The expression profile for non-activated and activated L3 is clearly different from the profile during heat shock. As shown in Fig. 3A, Aca-hsf-1 begins climbing after 3h in both activated and non-activated L3 to reach a level 20-fold higher at 6 hours. Expression remained at this level in both groups until 12h, when they diverged. In non-activated L3, the level dropped to less as 10-fold, but rose again at 24h to almost a 100-fold up-regulation. In activated larvae, Aca-hsf-1 levels rose to 60-fold at 12h, then showed a decline to near baseline at 16h before rising to 150-fold up-regulation at 24h. Aca-hsf-1 levels remain unchanged when L3 were incubated in the nematode handling buffer BU [45] for 24h, indicating that Aca-hsf-1 expression is responding to differences in the medium composition, and not elevated temperature alone.
Figure 3.
Expression levels of heat shock response components during activation of hookworm iL3. Larvae were exposed to 37°C in 1×BU, RPMI-c alone (non-activated, NON), or RPMI-c plus 15 mM glutathione-s-methionine (GSM) and 10% serum filtrate (activated, ACT) for 0.5, 1, 3, 6, 12, 16 and 24h and analysed for mRNA transcript levels. A) Aca-hsf-1, B) Aca-hsp-1, C) Aca-daf-21. Asterisks indicate significant difference between activated and non-activated: * p<0.05, ** p<0.01.
The expression patterns of Aca-hsp-1 were similar between activated and non-activated larvae over the 24 h incubation (Fig. 3B). In both cases, Aca-hsp-1 expression levels were significantly down regulated (non-activated p=0.0301; activated p=0.0310; unpaired t test) to about 0.2–0.4 fold beginning at 1h. Incubation of iL3 at 37°C for 24h in BU caused a similar decrease in Aca-hsp-1 expression as incubation in RPMI-c, indicating that host-like temperature alone is sufficient to diminish Aca-hsp-1 transcription. Aca-daf-21 expression showed a similar pattern as Aca-hsp-1 initially, with slight down regulation during the first 12h in activated larvae and non-activated larvae (Fig. 3C). In the following 12h, Aca-daf-21 level slightly increased to 2-fold up-regulation at 24 h in activated L3, whereas the level climbed to reach a 6-fold increase in non-activated L3 at 24 h. The 1-fold increase of Aca-daf-21 transcripts in BU during the first 12h that reached 2-fold at 24h was not considered significant.
Over the 24h incubation period, transcript levels of Aca-daf-21 were higher in non-activated than in activated larvae, with a significant increase in the difference after 12h. We expected Aca-daf-21 to be more highly expressed in non-activated larvae than in activated larvae as it was shown to be up-regulated in dauer larvae of C. elegans [46, 47]. However, expression is down regulated initially in both activated and non-activated L3, and only begins to increase at 12–16h in non-activated L3. This is the time when feeding reaches a plateau in activated worms. Daf-21 expression rises much more slowly in activated L3 to only 2-fold up-regulated at 24h, compared to approximately 6 fold in non-activated L3. Expression of Aca-hsf-1 also increased in both activated and non-activated larvae. However, expression of the heat shock protein Aca-daf-21 was significantly up-regulated only in non-activated L3. This might indicate that Aca-hsf-1 expression is inducing a HSR in non-activated L3, but serving a different function in activated L3.
3.4. Feeding repression by HSR modulators
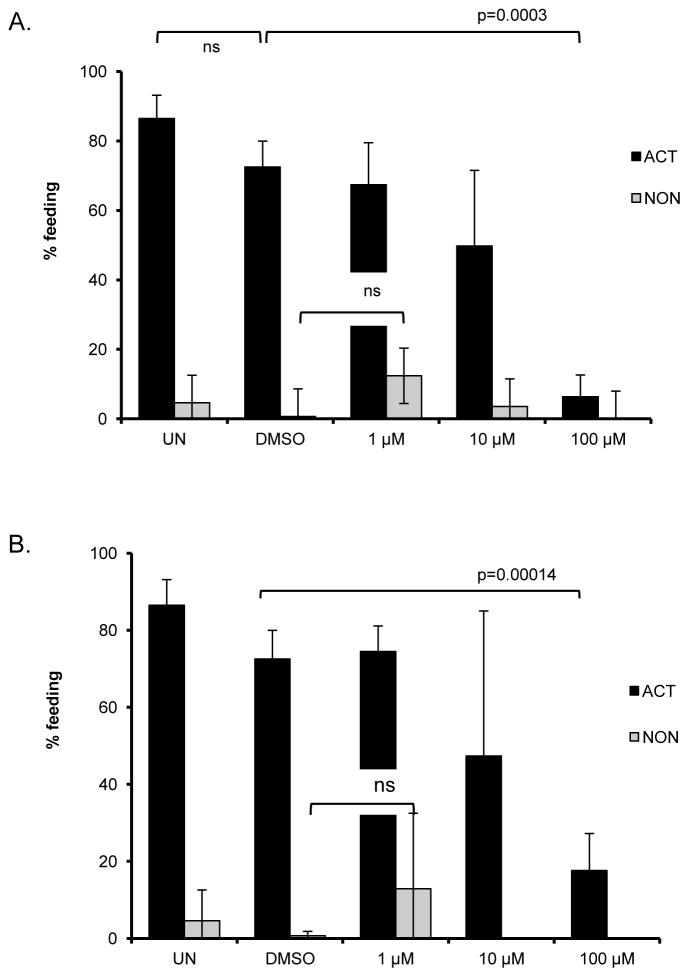
Our expression data from in vitro activated L3 suggested that HSR components play a role in the activation and feeding in arrested iL3. To better understand the role of these molecules in iL3 activation, we asked if we can affect in vitro feeding behavior with compounds known to modulate the HSR. Celastrol inhibits ATP binding activity of HSP90, induces HSF-1 binding, and promotes heat shock gene expression [48, 49], whereas KNK437 inhibits HSF-1 binding and HSP expression, including HSP70 [50, 51]. The inhibitors were used in a 10-fold dilution series of 1, 10, and 100 μM final concentrations dissolved in 1% DMSO. After 16 h incubation, L3 viability and feeding were determined. Both celastrol and KNK437 suppressed feeding in a concentration dependent manner (Fig. 4), suggesting a role for HSP-90 and HSF-1 in larval activation. Neither compound affected larval viability at any tested concentration (data not shown). For subsequent experiments, 100 μM of the compounds was used.
Figure 4.
Effect of heat shock response modulators on in vitro activation of iL3. A) KNK437, B) celastrol. Neither compound initiated feeding when GSM and serum were not included (non-activating conditions).
3.5. Expression profile of HSR factors during heat shock in the presence of HSR modulators
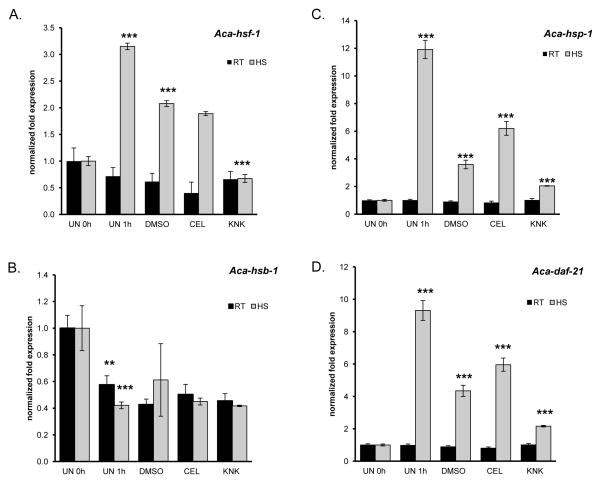
As celastrol and KNK437 both suppressed iL3 feeding under in vitro stimulating conditions, we were interested if these modulators also change the expression profile of HSR factors during heat shock. Celastrol is reported to increase the HSR at non-heat shock temperatures [49, 52], whereas KNK437 interferes with the active trimeric form of HSF-1, causing down regulation of target gene hsp70 during heat shock [48, 50]. To determine their effects on gene expression, iL3 were pre-incubated for 1h at room temperature with 100 μM KNK437 or 100 μM celastrol followed by heat shock for 1h at 42°C. The mRNA expression levels of Aca-hsp-1, Aca-daf-21, Aca-hsb-1 and Aca-hsf-1 before and after heat shock were analysed (Fig. 5). After 1h at 42°C, Aca-hsp-1 and Aca-daf-21 both showed the expected response in the untreated control, with Aca-hsp-1 up-regulated 12-fold and Aca-daf-21 up-regulated 9-fold. Interestingly, adding 1% DMSO dampened the up-regulation of both HSPs during heat shock. Aca-hsp-1 was only 3.5-fold up-regulated and Aca-daf-21 4.5-fold up-regulated, indicating a lessening of heat stress by DMSO. The 1h pre-incubation in DMSO or the compounds at room temperature had no effect on expression of any of the genes. Celastrol caused a slight but significant up-regulation of Aca-hsp-1 (2.5-fold) and Aca-daf-21 (2-fold) mRNA compared to the 1% DMSO control during heat shock, but expression failed to reach the levels seen in untreated L3 after heat shock in either case. Furthermore, celastrol had no effect on gene expression at room temperature. Celastrol had no effect on either Aca-hsf-1 or Aca-hsb-1 expression at room temperature or following heat shock.
Figure 5.
Expression of HSR factors in the presence of KNK437 and celastrol during heat shock. iL3 were incubated in RPMI-c alone (UN), supplemented with 1% DMSO (control) or 100 μM of the compound for 1h at room temperature (RT, 22°C) and heat shocked at 42°C (HS) for 1h. A) Aca-hsf-1, B) Aca-hsb-1 C) Aca-hsp-1 D) Aca-daf-21. Asterisks indicate significant difference from the appropriate control: * p<0.05, ** p<0.01, *** p<0.001.
The compound KNK437 caused an approximately 2-fold down regulation of Aca-hsp-1 and Aca-daf-21 mRNA expression compared to the 1% DMSO control (Fig 5C, D). KNK437 had no significant effect on Aca-hsf-1 mRNA levels at room temperature (Fig. 5A), but it inhibited hsf-1 expression during heat shock, consistent with KNK437 targeting the HSF-1 protein to down regulated Aca-hsp-1 and Aca-daf-21 expression during heat shock. The drop in hsf-1 transcription in response to DMSO alone is consistent with its lessening of the effect of heat stress as noted above.
3.6. Expression profile of HSR factors during in vitro activation in the presence of HSR modulators
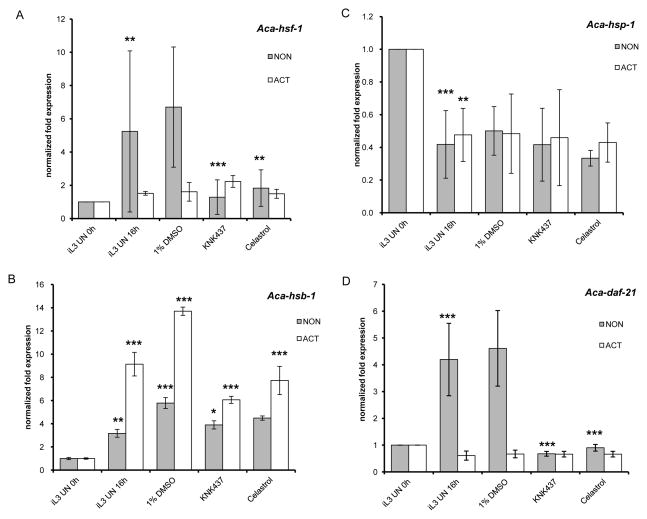
KNK437 and celastrol impacted the heat shock response and inhibited feeding, possibly due to alteration of HSP expression. To examine this possibility, we analysed the expression of the HSPs after repeating the in vitro activation assay in the presence of the compounds or the solvent DMSO alone for 16h (Fig. 6). The 16h time point was chosen because in vitro feeding reached a plateau in the iL3 population by this time [9, 10]. As seen in the previous activation experiments (Fig. 3), Aca-hsp-1 levels are down regulated in both non-activated and activated L3 at 16h. Contrary to the heat shock results, however, expression of Aca-hsp-1 was not influenced by either celastrol or KNK437 (Fig. 6C). At 16h, Aca-daf-21 mRNA levels are elevated in non-activated but not activated L3. Addition of DMSO has no effect, but both celastrol and KNK437 prevent this up-regulation in non-activated L3 (Fig. 6D). Neither compound affects Aca-daf-21 gene expression in activated L3, which is slightly down regulated at this time. Similarly, Aca-hsf-1 is up-regulated in non-activated L3 at 16 h, and this up-regulation is inhibited by the addition of both celastrol and KNK437 (Fig. 6A).
Figure 6.
Expression of HSR factors during in vitro activation in the presence of KNK437 and celastrol. A) Aca-hsf-1, B) Aca-hsb-1,C) Aca-hsp-1, D) Aca-daf-21. Asterisks indicate significant difference from the appropriate control: * p<0.05, ** p<0.01, *** p<0.001.
The expression of all three HSR components was unaffected by the long-term incubation with DMSO at 37°C, whereas expression of Aca-hsb-1 was 2.7-fold up-regulated in non-activated and 4.7-fold in activated larvae incubated with DMSO compared to the untreated control (Fig. 6B). Adding KNK437 or celastrol repressed the Aca-hsb-1 level in non-activated larvae to untreated control levels. As those changes were less than 2-fold, we considered the Aca-hsb-1 level in non-activated larvae as unchanged by the compounds. However, Aca-hsb-1 transcripts were significantly down regulated in activated larvae, namely 7.7-fold by KNK437 and 6-fold by celastrol. The Aca-hsb-1 expression profile is essentially opposite that of Aca-hsf-1 (Fig 6A), which is consistent with its role as a negative regulator of HSF-1 activity [28].
3.7. Expression of HSR factors during parasitic development
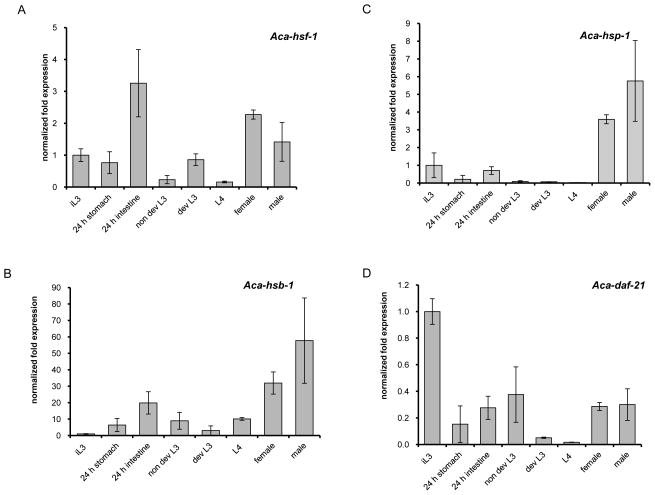
To evaluate the function of the HSR components in larval development during invasion of a permissive host, we isolated A. ceylanicum from hamsters at different stages of parasitic development and determined their expression profiles (Fig 7). We used A. ceylanicum for these experiments, as it is closely related to A. caninum, and isolating parasitic larval stages from A. caninum is logistically difficult. Following oral infection with a large dose, morphologically similar L3 were found in stomach and small intestine at 24h p.i. At 48h and 72h p.i., the vast majority (> 90%) of larvae had migrated to the intestine and started to show morphological differences, including development of a provisional buccal capsule and an increase in size. L3 larvae with still unchanged morphology were grouped as non-developing L3 larvae. By 72 h p.i., the majority of the recovered larvae were late L3 or early L4 stage, as distinguished by increased size and a complete buccal capsule in the L4.
Figure 7.
Expression of HSR factors in developing larvae and adults recovered from infected hamsters. Larvae were obtained 24h p.i. from stomach or intestine, 48h and 72h p.i. from the intestine and grouped after visible evaluation in non-developing (non dev) L3 larvae, developing (dev) L3 and L4 larvae
As shown in Fig. 7, the expression profile of the HSR components was clearly different from that of feeding L3 at 24h. Only Aca-hsp-1 expression was similar to feeding L3, as it was down-regulated over the course of development, with the lowest expression in L4s (0.04-fold). Expression increased in adult worms, where the expression is about 2-fold higher in males (5.8-fold) than females (3.6-fold) (Fig. 7C). This further supports the role of host temperature as stimulus of the Aca-hsp-1 down-regulation, at least in the larval stages, as was seen in the in vitro activation assay. Aca-daf-21 transcripts diminished dramatically in L3 found in the stomach at 24h, increased slightly in L3 residing in the small intestine at 24h and older non-developing larvae. Expression fell again in developing L3 larvae and bottomed at the minimum of 0.02-fold in L4 larvae. An increase to 0.3-fold occurs in females and males, but expression remains well below control levels (Fig. 7D). We found that Aca-daf-21 is down regulated in in vitro stimulated L3 within the first 12h, but then increases in the latter half of the incubation to reach nearly 6-fold up-regulation in non-activated and ~2-fold up-regulation in activated L3 (Fig. 3C). The fact that Aca-daf-21 levels remain down regulated throughout the parasitic life cycle suggests that development negatively regulates daf-21 expression, and that down regulation of daf-21 represents a marker for the resumption of development.
Aca-hsf-1 and Aca-hsb-1 expression showed a distinctive profile also (Fig 7A and B). Aca-hsf-1 was slightly down regulated in 24h stomach L3 (0.8-fold), but is somewhat surprisingly up-regulated 3-fold in 24h intestinal L3. This may be associated with the beginning of development, which occurs when the L3 are exposed to a host cue in the small intestine. After this spike in hsf-1 expression, levels are subsequently down regulated during the L3 and L4 stages. Expression increases again approximately 1.4 – 2.2-fold in the adults, levels that are probably not significant. Not unexpectedly, the down regulation of Aca-hsf-1 is associated with a concomitant up-regulation of Aca-hsb-1. Expression levels of Aca-hsb-1 are at least 10-fold higher than those of Aca-hsf-1 throughout parasitic development. In L3 recovered from the stomach at 24h p.i., Aca-hsb-1 was expressed at a level similar to that of 24 h in vitro activated L3. Aca-hsb-1 peaked in 24h intestinal L3 (20-fold) at the same time that Aca-hsf-1 reached its maximum level (3-fold up-regulated). Aca-hsb-1 levels remained higher than hsf-1 levels throughout development, suggesting that the HSR needs to be down regulated during development. Aca-hsb-1 levels rose higher in adults (32- and 58-fold in males and females, respectively). This profile differs from in vitro activation at 24h, where Aca-hsb-1 was 7-fold up-regulated [28] and Aca-hsf-1 150-fold (Fig. 3A). Taken together, these data suggest that feeding during in vitro activation is regulated differently than resumption of development in vivo.
4. Discussion
Sensation of ambient temperature leads to molecular processes that are essential for an organism to survive in case of heat stress or to resume development where temperature shifts provide a trigger for exit from arrest. Both events are linked by the involvement of HSPs and their main regulator HSF. We described here, for the first time, a heat shock response in hookworms. We identified two heat shock proteins, one a member of the HSP70 family (Aca-HSP-1), and the other a member of the HSP90 family (Aca-DAF-21). We provide gene expression data for both molecules during heat shock, feeding, and the transition from the free-living iL3 to parasitic stages in the host. In addition, we include gene expression data for the key transcription factor Aca-HSF-1 and its negative regulator Aca-HSB-1 during the life cycle.
The HSR is evolutionarily conserved from bacteria to humans and prevents cellular damage by orchestrating rapid increases in the expression of chaperone proteins. A classical marker commonly used to profile changes in HSR is the inducible HSP70 (HSP-1). We identified a cDNA encoding a 643 amino acid protein with homology to HSP70 family proteins. Comparison to the 13 members of the C. elegans HSP70s indicated that the hookworm molecule was most closely related to Cel-HSP-1. The hsp-1 gene is expressed throughout development in C. elegans, and is up-regulated 2–6 fold in response to heat shock in mixed stages and dauer larvae [46, 53, 54]. The hookworm HSP70 member was also up-regulated to similar levels during heat shock. Based on the observed induction by heat shock and sequence homology, we conclude that the hookworm HSP70 we have identified is an orthologue of C. elegans HSP-1.
HSP90 is a molecular chaperone that, together with several co-chaperones, stabilizes, activates and regulates more than 200 client proteins [42, 55]. In addition to regulating the heat shock response (HSR) through its interaction with HSF-1, HSP90 functions in muscle and germline development [42], proteostasis [56], regulation of signalling pathways [42], and dauer development in C. elegans [57]. HSP90 represses HSF-1 by binding to the monomeric form and preventing formation of the active DNA binding trimer [58–61]. We identified a cDNA encoding a 707 amino acid protein with significant homology to HSP90 family proteins. The hookworm sequence contained all of the hallmarks of the HSP90 family including signature sequences, an N-terminal ATPase domain, and the C-terminal MEEVD sequence. The high homology to HSP90 molecules from other nematodes, including C. elegans, confirms its identity as the ortholog of Cel-DAF-21.
HSP90 was originally identified as a classic heat shock protein [42, 62, 63], but levels of daf-21 transcripts during nematode development and during heat shock vary considerably. For example, Cel-daf-21 was only slightly up-regulated by heat exposure in mixed stage worms [64], whereas Williams et al found that daf-21 mRNA was up-regulated by heat shock at all developmental stages except the L3 [65]. Hsp-1 is expressed throughout the life cycle, but is up-regulated 2–6 fold in mixed stages of C. elegans subjected to heat shock [53, 54]. We did not measure chaperone expression during heat shock in other stages, but transcription of both Aca-hsp-1 and Aca-daf-21 was significantly heat responsive in iL3, showing 6- and 8-fold up-regulation following a heat shock of 1h. The hsp90 and hsp70 of the rat nematode Nippostrongylus brasiliensis iL3 were up-regulated similarly in response to heat shock [66]. In hookworm iL3, the levels of both transcripts continued to climb for the first 15 minutes of a room temperature attenuation period, but fell precipitously over the next 15 minutes, and ending at levels slightly higher than baseline at 1h attenuation. This represents a classic HSR, in which expression of heat shock proteins, or chaperones, increases to maintain proteostasis during thermal stress. This response is mediated by binding of HSF-1 as a homotrimer on heat shock response elements in the promoters of genes encoding heat shock proteins [26]. Interestingly, the transcript levels of hookworm hsf-1 remained at baseline for the entire 60 min of heat shock, indicating that new transcription of hsf-1 is not required for the HSR to occur. However, transcription of hsf-1 increases 2-fold during the first 15 minutes of room temperature attenuation, and remains near this level for the remainder of the 60 min attenuation period. This suggests a feedback mechanism is operating to replace HSF-1 protein damaged or destroyed by the elevated temperature during heat shock.
Once we established molecular markers for a HSR, we asked what role elevated temperature plays in infection, specifically during larval activation and the resumption of development that occurs during infection. The shift from ambient to host temperatures during hookworm infection may initiate a HSR that induces developmental pathways. For example, in Brugia malayi microfilaria and adult males, hsp-1 is not significantly heat inducible, but undergoes a 5-fold induction when L3 undergo the shift from the vector temperature (22°C) to that of the host (37°C)[67], suggesting a possibly function for HSP-1 in development during infection. To investigate whether a shift to host temperature initiated a HSR, we incubated iL3 at 37°C for 1h to mimic the shift to the host, followed by a room temperature attenuation period of 1h. These conditions failed to mimic the HSP up-regulation seen during heat shock. Transcripts of hsp-1 were down regulated during the temperature shift, and those of daf-21 rose only slightly. However, transcription of hsf-1 increased nearly 3-fold over the first 30 min, where it remained until 60 min of attenuation. This suggests that hsf-1 transcription may play a role other than initiating a HSR and chaperone expression at host-like temperature.
Next we asked whether L3 activation elicited a heat shock response. When hookworm iL3 are incubated in vitro with serum components and glutathione analogs at 37°C, a significant portion of the larval population “activates”, as determined by the resumption of feeding [5, 8]. Activation is regulated by IIS, and involves expression of an activation gene program and release of excretory/secretory products associated with infection [5, 17, 68–72]. To determine the transcriptional response of heat shock components during activation, we measured transcript levels at several times during the 24 h activation period. Expression of hsf-1 increased significantly in both non-activated and activated L3 at 6h. This corresponds to the time in which L3 exposed to activating stimuli begin to feed [8, 10]. Transcription returned to near baseline in non-activated L3 by 16h, but continued to climb in activated L3, reaching a peak of approximately 60-fold up-regulation at 12h, the time at which feeding reaches a maximum and begins to plateau in stimulated L3 [8, 10]. This suggests that hsf-1 expression is required for activation, perhaps controlling expression of activation-associated genes. However, activating conditions failed to induce a classic HSR, as expression of both hsp-1 and daf-21 are down regulated over the first 12h, indicating that hsf-1 functions independently of the HSR in activation. HSF-1 is known to regulate development and aging in C. elegans, and has transcriptional targets that are key components of signal transduction pathways in addition to those with chaperone functions [20, 21, 25, 27, 73]. Recently Morton et al hypothesized that these targets are not transcribed by trimeric HSF-1 in heat shock granules, as occurs during stress, but rather by monomeric HSF-1 [27]. A similar mechanism may control the expression of genes involved in L3 activation.
Despite resuming feeding and releasing infection-associated molecules, in vitro activated worms fail to resume development due to the absence of the appropriate stimulus from a permissive host. While the increase in hsf-1 expression levels in activated L3 over the first 12h is likely regulating expression of activation-associated genes, the subsequent increase in both non-activated (after 16h) and activated L3 (after 24h) may represent a classic HSR to protect against extended incubation at elevated temperatures in the absence of the developmental signal. If this is true, we would predict an increase in expression of heat shock genes associated with the increase in hsf-1. While hsp-1 expression remains depressed throughout incubation, there is an increase of daf-21 expression in the second half of the activation period, to approximately 6-fold in non-activated and 2-fold in activated L3. The absence of hsp-1 induction suggests that the increased hsf-1 levels are not mediating a classical HSR, but rather a modified, partial HSR necessary to protect the L3 and maintain viability at host-like temperatures for extended periods in the absence of the developmental signal, as would occur during paratenesis or hypobiosis. We have previously proposed that during the period that feeding is increasing (6–16 h incubation), the HSR is repressed when activation stimuli are present, but not in L3 exposed to host temperature alone [28]. Once the feeding level in the population reaches its maximum, a similar, although delayed, HSR is induced in activated L3, in which hsf-1 and daf-21 are up-regulated, hsb-1 is down regulated, and hsp-1 is unchanged. HSP90 is known to stabilize receptors in an active conformation [26] and hence may be maintaining the unbound host signal receptor(s) or other proteins in situations where L3 exist at host-like temperature without developing. Support for this comes from the rapid down regulation of daf-21 and hsp-1 expression seen in developing parasitic L3 (pL3) recovered early in infection (Fig. 7).
In order to further dissect the heat shock pathway, we used two modulators of the HSR that work through different mechanisms. KNK437 inhibits HSF-1 activity, resulting in repression of hsp expression and reduced accumulation of HS proteins [50]. As expected, KNK437 abrogated the increased expression of both hsp-1 and daf-21 in heat shocked iL3. Similarly, the elevated Aca-daf-21 expression seen at 16h incubation in non-activated L3 was completely inhibited. KNK437 also inhibited the increased hsf-1 expression in 16h non-activated L3, but had no additional inhibitory effect on the already decreased Aca-hsp-1 levels (Fig 6C). These data suggest that the increased DAF-21 expression in non-activated larvae is regulated by HSF-1, and that HSF-1 regulates its own transcription as well under non-activating conditions.
Celastrol promotes heat shock gene expression by inducing HSF-1 DNA binding and hyperphosphorylation, and by inhibiting the HSF-1 repressor HSP90 [48, 49, 52]. During heat shock, celastrol increased hsp-1 and daf-21 expression above the DMSO control, as would be expected from its activation of HSF-1. Celastrol has no effect on heat shock protein expression during activation, but inhibits the elevated levels of hsf-1 and daf-21 expression seen in non-activated L3 at 16h. This further supports the idea that the increase in daf-21 expression in extended incubations at host temperature represents a non-traditional heat shock response.
Interestingly, both HSR modulators inhibited activation-associated feeding, leading us to question how two modulators that have opposite effects on the HSR can exhibit the same effect on L3 activation. Our data suggest that the HSR must be repressed for activation to occur, but that HSF-1 functions in a non-heat shock response role to express genes necessary for activation to occur. Celastrol most likely has a direct inhibitory effect through its increase of the HSR and HS protein expression. On the other hand, KNK437 decreases the HSR through its inhibitory effect on HSF-1 activity, and therefore might be expected to promote or have no effect on feeding. However, if monomeric HSF-1, rather than trimeric HSF-1, is required for activation, then KNK437 might act by preventing monomer binding and initiation of activation. Further investigation of the effects of the modulators on HSP protein levels is required to support this hypothesis.
As in activation, hsf-1 expression increases in pL3 recovered from the host intestine 24h post-infection. However, the concomitant increase in daf-21 expression seen in activated L3 beginning at 24h is absent from 24 h pL3. High expression of hsb-1 at this time may inhibit formation of active trimeric HSF-1, thereby preventing a heat shock response and daf-21 expression during development. Similarly, elevated hsb-1 levels at 16h in activated, but not non-activated L3 (Fig. 6B), may suppress the HSR and allow activation to occur. The HSR, as measured by hsp-1 and daf-21 expression, is repressed throughout early parasitic development, most likely mediated by the negative regulation of HSF-1 by the elevated levels of hsb-1 during this period (Fig. 7). However, HSF-1 is probably active during parasitic development, perhaps in monomeric form [27], as HSF-1 is required for normal development in C. elegans [20, 21]. The spike in hsf-1 expression at 24h in pL3 may provide sufficient HSF-1 protein to initiate or regulate gene expression required for development. Recently, HSF-1 was shown to stimulate DAF-9/cytochrome P450 activity to repress dauer formation and allow development [73]. DAF-9 synthesizes dafachronic acid, a steroid ligand of DAF-12 that promotes reproductive development in C. elegans and activation in hookworms [74–76]. This suggests that the HSR must be supressed while HSF-1 regulates the genes required for activation and development, and further supports a role for HSF-1 outside of the stress response.
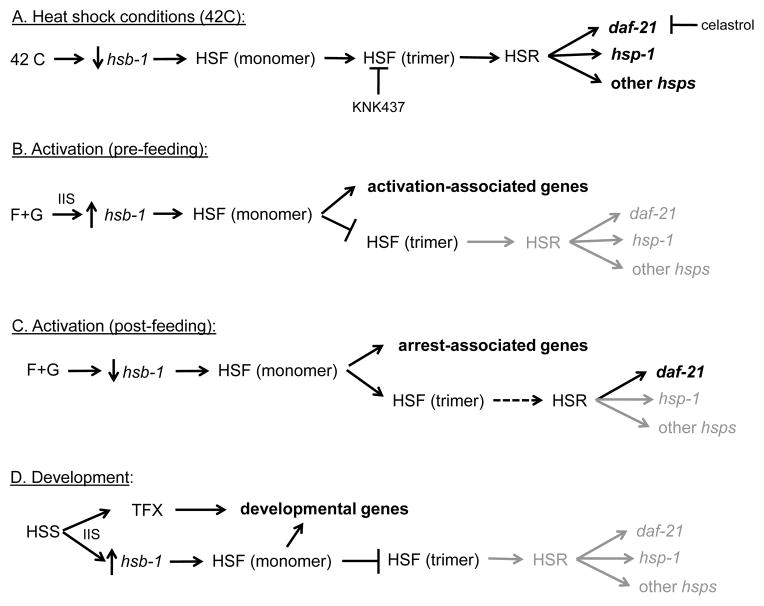
Previously we proposed a model for the interaction of HSF-1 and DAF-16, together with an unknown transcription factor (TFX) which we suggested was the nuclear hormone receptor DAF-12, in regulating L3 activation and the transition to parasitism during hookworm infection [28]. In this model, we proposed that DAF-16 and HSF-1 negatively regulated development, while TFX activity was required for development. DAF-16 and HSF-1 positively regulate genes that maintain developmental arrest and repressed developmental genes, and therefore were active in arrested iL3. During infection, the developmental signal and the elevated temperature encountered in a permissive host negatively regulate DAF-16 and HSF-1, repressing their target genes. Concurrently the developmental signal activates TFX, perhaps by a parallel pathway, to initiate the developmental program. Under activating conditions, temperature and IIS initiated by the in vitro stimulus negatively regulate HSF-1 and DAF-16, but the permissive host signal is absent and TFX is not active. Consequently, the L3 activate but do not develop, allowing them to survive at elevated temperature while awaiting the developmental signal. After approximately 16h, the elevated temperature causes HSB-1 to be down regulated, allowing HSF-1 to bind and express genes that maintain arrest and protect against heat stress. The data reported here, together with recent research using C. elegans, generally support this model, and allow us to refine the role of HSF-1 and the HSR in these processes.
One prediction of this model was that, based on increased expression of hsb-1, the initiation of activation and feeding required the repression of HSF-1 and a HSR. Two results reported here support his prediction. First, we found that hsf-1 expression levels remained low early in activation, but began to rise between 4 and 6h incubation to reach a peak nearly 60-fold greater than untreated L3 at 12h. A rise in hsb-1 expression over this time was reported previously [28], but it reached a maximum of only 15 fold up-regulation. Despite high levels of hsf-1, expression of heat shock proteins daf-21 and hsp-1 were down regulated significantly over the first 12h of incubation, indicating that a HSR was not initiated despite high levels of hsf-1 expression, perhaps because of the relatively high hsb-1 levels. Second, the HSR modulator celastrol, which increases HSF-1 activity and heat shock protein expression [49, 52], completely inhibited feeding. Together, these results suggest that the HSR component of HSF-1 is suppressed by the activation stimulus, but HSF-1 remains active to regulate the genes associated with activation. In light of these results we propose that, rather than a requirement for HSF-1 to be “off” in order for feeding to initiate, it binds in monomeric form and induces expression of genes necessary for the feeding response during activation (Figure 8). In C. elegans, IIS signalling negatively regulates HSF-1 by promoting the formation of an inhibitory complex composed of HSF-1, HSB-1, and two daf-16-dependent longevity gene products DDL-1 and DDL-2 [77]. IIS during hookworm L3 activation [72] may negatively regulate trimeric HSF by a similar mechanism to repress the HSR, but allow monomeric HSF-1 to bind and express activation-associated genes. In non-activated L3, the absence of a developmental or activation signal initiates binding of trimeric HSF-1 and initiation of a protective partial HSR resulting in daf-21 (and possibly other HSP) expression at 12–16 h (Figure 8). This response is delayed and only slightly elevated in activated L3, perhaps because activation is partially protective against heat stress. In either case, this partial HSR would protect the larvae from stress damage while they are in a non-permissive habitat, such as a paratenic host.
Figure 8.
Proposed interactions between heat shock factors during heat shock, activation and development. Dark arrows are positive effects, gray arrows and text indicated down regulation, bold text indicates expression and bars indicate inhibitory effects. A. During heat shock, decreasing levels of hsb-1 expression allow the formation of HSF trimers, which initiates the heat shock response (HSR) by binding to the promoters of heat shock genes, including hsp-1 and daf-21, causing expression. B. During early activation, the activation stimulus (filtrate and glutathione, F+G) increases hsb-1 expression, preventing trimerization and inhibiting the HSR. Monomeric HSF-1 binds to promoters of activation-associated genes, resulting in expression and activation. C. In the absence of a host specific signal (HSS), hsb-1 expression begins increasing later in activation, after feeding has reached its maximum in the larval population. This allows the trimerization of some HSF monomers, resulting in a partial HSR (dashed arrow), as demonstrated by increased daf-21, but not hsp-1, expression. D. In response to the HSS, hsb-1 expression increases, blocking the HSR and allowing monomeric HSF, together with an unknown transcription factor (TFX), stimulate expression of genes associated with development.
As activated worms fail to develop, a component of the developmental signal is absent from the in vitro activation conditions. Our model and the data presented here suggest that both DAF-16 and HSF-1 must be negatively regulated for expression of TFX regulated genes and development to occur. We propose that the host-specific signal initiates IIS to negatively regulate DAF-16 and HSF-1 to repress arrest-associated genes, and positively regulates a second pathway, possibly signaling through DAF-11 and DAF-7 [73], to activate TFX and express development-associated genes.
In conclusion, we report the molecular cloning and characterization of two heat shock proteins, HSP70 family member Aca-HSP-1 and HSP90 family member Aca-DAF-21 from the hookworm A. caninum. Expression of both heat shock proteins was increased in response to a one hour heat shock at 42°C, but not at 37°C, suggesting that a heat shock response is not induced in response to the elevated temperature encountered during infection of a host, and that the expression profile of both genes makes them useful as markers for the HSR in hookworm L3. We also present evidence that in addition to mediating a HSR, HSF-1 functions independently of the stress pathway to regulate aspects of development, including activation, possibly by binding to target genes as a monomer. Furthermore, extended incubation at host temperature in the absence of the developmental signal induces daf-21, but not hsp-1, expression, perhaps as protection against heat stress. Conditions that activate worms to begin feeding delay and dampen this partial heat shock response. Finally, we report that the HSR is suppressed in developing larvae recovered from the host, suggesting that the role of hsp-1 and daf-21 during infection is limited to alternative developmental pathways such as activation and paratenesis.
Highlights.
We cloned hsp-1 and daf-21 cDNAs from the hookworm Ancylostoma caninum.
Heat shock up-regulates expression of both genes, but host temperature does not.
Heat shock response is suppressed during parasitic development.
Partial HSR is induced at host-like conditions without developmental stimulus
Acknowledgments
The authors thank Devora Champa, Camille Dowling, and Kevin Goggin for assistance with parasite maintenance. The project was supported by grant R01AI069293 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
Abbreviations: iL3, third stage larva; IIS, insulin/insulin growth factor-like signalling; HSF, heat shock factor; HSR, heat shock response; HSP, heat shock protein; HSB, heat shock binding protein; P.i., post-infection.
References
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Lozoff B. Iron and learning potential in childhood. Bull NY Acad Med. 1989;65:1050–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. New Engl J Med. 1991;235:687–94. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 4.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;12:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hawdon JM, Hotez PJ. Hookworm: developmental biology of the infectious process. Curr Opin Genet Dev. 1996;6:618–23. doi: 10.1016/s0959-437x(96)80092-x. [DOI] [PubMed] [Google Scholar]
- 6.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–76. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Hawdon JM. Controlling Soil Transmitted Helminths: Time to Think inside the Box? J Parasitol. 2014;100:166–88. doi: 10.1645/13-412.1. [DOI] [PubMed] [Google Scholar]
- 8.Hawdon JM, Schad GA. Serum stimulated feeding in vitro by third stage infective larvae of the canine hookworm Ancylostoma caninum. J Parasitol. 1990;76:394–8. [PubMed] [Google Scholar]
- 9.Hawdon JM, Schad GA. Albumin and a dialyzable serum factor stimulate feeding in vitro by third-stage larvae of the canine hookworm Ancylostoma caninum. J Parasitol. 1991;77:587–91. [PubMed] [Google Scholar]
- 10.Hawdon JM, Schad GA. Ancylostoma caninum: reduced glutathione stimulates feeding by third-stage infective larvae. Exp Parasitol. 1992;75:40–6. doi: 10.1016/0014-4894(92)90120-y. [DOI] [PubMed] [Google Scholar]
- 11.Hawdon JM, Volk SW, Pritchard DI, Schad GA. Resumption of feeding in vitro by hookworm third-stage larvae: a comparative study. J Parasitol. 1992;78:1036–40. [PubMed] [Google Scholar]
- 12.Hawdon JM, Schad GA. Ancylostoma caninum: glutathione stimulates feeding in third-stage larvae by a sulfhydryl-independent mechanism. Exp Parasitol. 1993;77:489–91. doi: 10.1006/expr.1993.1110. [DOI] [PubMed] [Google Scholar]
- 13.Hawdon JM, Volk SW, Rose R, Pritchard DI, Behnke JM, Schad GA. Observations on the feeding behaviour of parasitic third-stage hookworm larvae. Parasitology. 1993;106 (Pt 2):163–9. doi: 10.1017/s0031182000074953. [DOI] [PubMed] [Google Scholar]
- 14.Massey JHC, Bhopale MK, Li X, Castelletto M, Lok JB. The fork head transcription factor FKTF-1b from Strongyloides stercoralis restores DAF-16 developmental function to mutant Caenorhabditis elegans. Int J Parasitol. 2006;36:347–52. doi: 10.1016/j.ijpara.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castelletto ML, Massey HC, Jr, Lok JB. Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathog. 2009;5:e1000370. doi: 10.1371/journal.ppat.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu M, Lok JB, Ranjit N, Massey HC, Jr, Sternberg PW, Gasser RB. Structural and functional characterisation of the fork head transcription factor-encoding gene, Hc-daf-16, from the parasitic nematode Haemonchus contortus (Strongylida) Int J Parasitol. 2010;40:405–15. doi: 10.1016/j.ijpara.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelmedin V, Brodigan T, Gao X, Krause M, Wang Z, Hawdon JM. Transgenic C. elegans dauer larvae expressing hookworm phospho null DAF-16/FoxO exit dauer. PLoS ONE. 2011;6:e25996. doi: 10.1371/journal.pone.0025996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brownawell AM, Kops GJ, Macara IG, Burgering BM. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol Cell Biol. 2001;21:3534–46. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill CM, Tzivion G, Nasrin N, Ogg S, Dore J, Ruvkun G, et al. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent pathways. J Biol Chem. 2001;276:13402–10. doi: 10.1074/jbc.M010042200. [DOI] [PubMed] [Google Scholar]
- 20.Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. FASEB J. 2003 doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- 21.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–64. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh V, Aballay A. Regulation of DAF-16-mediated Innate Immunity in Caenorhabditis elegans. J Biol Chem. 2009;284:35580–7. doi: 10.1074/jbc.M109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays in biochemistry. 1997;32:17–29. [PubMed] [Google Scholar]
- 24.Walker GA, White TM, McColl G, Jenkins NL, Babich S, Candido EPM, et al. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2001;56:B281–7. doi: 10.1093/gerona/56.7.b281. [DOI] [PubMed] [Google Scholar]
- 25.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–9. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 26.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–55. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton EA, Lamitina T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 2013;12:112–20. doi: 10.1111/acel.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krepp J, Gelmedin V, Hawdon JM. Characterisation of hookworm heat shock factor binding protein (HSB-1) during heat shock and larval activation. Int J Parasitol. 2011;41:533–43. doi: 10.1016/j.ijpara.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garside P, Behnke JM. Ancylostoma ceylanicum in the hamster: observations on the host-parasite relationship during primary infection. Parasitology. 1989;98:283–9. doi: 10.1017/s003118200006220x. [DOI] [PubMed] [Google Scholar]
- 30.Wylie T, Martin JC, Dante M, Mitreva MD, Clifton SW, Chinwalla A, et al. Nematode.net: a tool for navigating sequences from parasitic and free-living nematodes. Nucleic Acids Res. 2004;32:D423–6. doi: 10.1093/nar/gkh010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawdon JM, Jones BF, Hotez PJ. Cloning and characterization of a cDNA encoding the catalytic subunit of a cAMP-dependent protein kinase from Ancylostoma caninum third-stage infective larvae. Mol Biochem Parasitol. 1995;69:127–30. doi: 10.1016/0166-6851(94)00203-y. [DOI] [PubMed] [Google Scholar]
- 32.Bektesh SL, Hirsh DI. C. elegans mRNAs acquire a spliced leader through a trans-splicing mechanism. Nuc Acids Res. 1988;16:5692. doi: 10.1093/nar/16.12.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–8. [Google Scholar]
- 34.Altschul SF, Madden TL, Schaeffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic Local Alignment Search Tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 36.Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–82. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen TN, Brunak S, von Heijne Gr, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth. 2011;8:785–6. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal T, Steward K. RNA processing and gene structure. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Plainview, New York, USA: Cold Spring Harbor Laboratory Press; 1997. pp. 117–45. [PubMed] [Google Scholar]
- 41.Menoret A, Chaillot D, Callahan M, Jacquin C. Hsp70, an immunological actor playing with the intracellular self under oxidative stress. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2002;18:490–505. doi: 10.1080/02656730210146926. [DOI] [PubMed] [Google Scholar]
- 42.Haslbeck V, Kaiser CJ, Richter K. Hsp90 in non-mammalian metazoan model systems. Biochim Biophys Acta. 2012;1823:712–21. doi: 10.1016/j.bbamcr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Kiss J, Gao X, Krepp J, Hawdon J. Interaction of hookworm 14-3-3 with the forkhead transcription factor DAF-16 requires intact Akt phosphorylation sites. Parasit Vectors. 2009;2:21. doi: 10.1186/1756-3305-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int J Parasitol. 2003;33:787–93. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 45.Hawdon JM, Schad GA. Long-term storage of hookworm infective larvae in buffered saline solution maintains larval responsiveness to host signals. J Helm Soc Wash. 1991;58:140–2. [Google Scholar]
- 46.Dalley BK, Golomb M. Gene expression in the Caenorhabditis elegans dauer larva: developmental regulation of Hsp90 and other genes. Dev Biol. 1992;151:80–90. doi: 10.1016/0012-1606(92)90215-3. [DOI] [PubMed] [Google Scholar]
- 47.Jones SJ, Riddle DL, Pouzyrev AT, Velculescu VE, Hillier L, Eddy SR, et al. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Genome Res. 2001;11:1346–52. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- 48.Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–30. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Trott A, West JD, Klaic L, Westerheide SD, Silverman RB, Morimoto RI, et al. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–12. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manwell LA, Heikkila JJ. Examination of KNK437- and quercetin-mediated inhibition of heat shock-induced heat shock protein gene expression in Xenopus laevis cultured cells. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2007;148:521–30. doi: 10.1016/j.cbpa.2007.06.422. [DOI] [PubMed] [Google Scholar]
- 51.Ohnishi K, Takahashi A, Yokota S, Ohnishi T. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. International journal of radiation biology. 2004;80:607–14. doi: 10.1080/09553000412331283470. [DOI] [PubMed] [Google Scholar]
- 52.Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–60. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 53.Snutch TP, Heschl MFP, Baillie DL. The Caenorhabditis elegans hsp70 gene family: a molecular genetic characterization. Gene. 1988;64:241–55. doi: 10.1016/0378-1119(88)90339-3. [DOI] [PubMed] [Google Scholar]
- 54.Heschl MFP, Baillie DL. The HSP70 multigene family of Caenorhabditis elegans. Comparative Biochemistry and Physiology Part B. 1990;96:633–7. doi: 10.1016/0305-0491(90)90206-9. [DOI] [PubMed] [Google Scholar]
- 55.Neckers L. Heat shock protein 90: the cancer chaperone. Journal of biosciences. 2007;32:517–30. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 56.van Oosten-Hawle P, Porter RS, Morimoto RI. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell. 2013;153:1366–78. doi: 10.1016/j.cell.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 59.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–66. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci. 2002;115:2809–16. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- 61.Voellmy R, Kivie M. Transcriptional Regulation of the Metazoan Stress Protein Response. Prog Nucleic Acid Res Mol Biol. 2004;78:143–85. doi: 10.1016/S0079-6603(04)78004-6. [DOI] [PubMed] [Google Scholar]
- 62.Ashburner M, Bonner JJ. The induction of gene activity in Drosophilia by heat shock. Cell. 1979;17:241–54. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- 63.Morcillo G, Diez JL, Carbajal ME, Tanguay RM. HSP90 associates with specific heat shock puffs (hsr omega) in polytene chromosomes of Drosophila and Chironomus. Chromosoma. 1993;102:648–59. doi: 10.1007/BF00352313. [DOI] [PubMed] [Google Scholar]
- 64.Gaiser AM, Kaiser CJ, Haslbeck V, Richter K. Downregulation of the Hsp90 system causes defects in muscle cells of Caenorhabditis elegans. PLoS ONE. 2011;6:e25485. doi: 10.1371/journal.pone.0025485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams TR, Lee TM, Johnson CM. Glaucoma studies in the eyeless worm: stress responsiveness and temporal expression of the Caenorhabditis elegans myocilin-like gene, cof-2. Cell Mol Biol. 2004;50:723–31. [PubMed] [Google Scholar]
- 66.Arizono N, Yamada M, Tegoshi T, Takaoka Y, Ohta M, Sakaeda T. Hsp12.6 expression is inducible by host immunity in adult worms of the parasitic nematode Nippostrongylus brasiliensis. PLoS One. 2011;6:e18141. doi: 10.1371/journal.pone.0018141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothstein N, Rajan TV. Characterization of an hsp70 gene from the human filarial parasite, Brugia malayi (Nematoda) Mol Biochem Parasitol. 1991;49:229–37. doi: 10.1016/0166-6851(91)90066-f. [DOI] [PubMed] [Google Scholar]
- 68.Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271:6672–8. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- 69.Hawdon JM, Jones BF, Perregaux MA, Hotez PJ. Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Exp Parasitol. 1995;80:205–11. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 70.Hawdon JM, Narasimhan S, Hotez PJ. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol Biochem Parasitol. 1999;99:149–65. doi: 10.1016/s0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 71.Datu BJ, Gasser RB, Nagaraj SH, Ong EK, O’Donoghue P, McInnes R, et al. Transcriptional changes in the hookworm, Ancylostoma caninum, during the transition from a free-living to a parasitic larva. PLoS Negl Trop Dis. 2008;2:e130. doi: 10.1371/journal.pntd.0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol. 2004;34:909–14. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 73.Barna J, Princz A, Kosztelnik M, Hargitai B, Takacs-Vellai K, Vellai T. Heat shock factor-1 intertwines insulin/IGF-1, TGF-beta and cGMP signaling to control development and aging. BMC Dev Biol. 2012;12:32. doi: 10.1186/1471-213X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, et al. Identification of Ligands for DAF-12 that Govern Dauer Formation and Reproduction in C. elegans. Cell. 2006;124:1209–23. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Zhou XE, Motola DL, Gao X, Suino-Powell K, Conneely A, et al. Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Natl Acad Sci U S A. 2009;106:9138–43. doi: 10.1073/pnas.0904064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhi X, Zhou XE, Melcher K, Motola DL, Gelmedin V, Hawdon J, et al. Structural Conservation of Ligand Binding Reveals a Bile Acid-like Signaling Pathway in Nematodes. J Biol Chem. 2012;287:4894–903. doi: 10.1074/jbc.M111.315242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–34. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]