Abstract
Background
It is generally assumed that if a man does not regain urinary continence or erectile function within 12 mo of radical prostatectomy (RP), then the chance of subsequent recovery is low.
Objective
To determine the probability of achieving good urinary function (UF) or erectile function (EF) up to 48 mo postoperatively in men who reported poor UF or EF at 12 mo after RP.
Design, setting, and participants
We identified 3187 patients who underwent RP from 2007 through 2013 at a tertiary institution and had extended multidisciplinary follow-up with patient-reported UF and EF scores at ≥12 mo.
Intervention
Open or minimally invasive RP.
Outcome measurements and statistical analysis
Primary outcome was good UF as defined by a urinary score ≥17 (range: 0–21) or good EF as defined by a modified International Index of Erectile Function-6 score ≥22 (range: 1–30). The probability of functional recovery beyond 12 mo was determined by Kaplan-Meier analyses.
Results and limitations
Among patients incontinent at 12 mo, the probability of achieving good UF at 24, 36, and 48 mo was 30%, 49%, and 59%. In patients experiencing erectile dysfunction at 12 mo, the probability of recovering EF at 24, 36, and 48 mo was 22%, 32%, and 40%. On multivariable analyses, 12-mo functional score and age were associated with recovery, but only score was consistently significant.
Conclusions
Men with incontinence or erectile dysfunction at 12 mo have higher than anticipated rates of subsequent functional improvement. Probability of recovery is strongly influenced by score at 12 mo. Further research should address the impact of ongoing multidisciplinary follow-up care on our observed rates of recovery.
Patient summary
Many prostate cancer patients continue to recover urinary and erectile function after 12 mo. The level of functional recovery by 12 mo is associated with long-term recovery and should be discussed by the physician and patient when deciding on rehabilitative interventions.
Keywords: Erectile dysfunction, Patient-reported outcomes, Radical prostatectomy, Urinary incontinence
1. Introduction
An estimated 220 000 new prostate cancer (PCa) cases will be diagnosed in the United States in 2015 [1]. Radical prostatectomy (RP) remains the most common treatment option, with 52% of men ≤64 yr undergoing RP between 2009 and 2011 [2]. However, RP is associated with adverse effects [3–5], and both urinary incontinence and erectile dysfunction (ED) can impose a significant burden on patients [6–8].
The time course of improvement in urinary incontinence or ED is not fully understood. Physicians commonly tell patients based on published reports that there is little recovery in urinary function (UF) and erectile function (EF) beyond 12–24 mo after RP. While many studies characterize these outcomes, they provide average scores or recovery rates of all patients relative to baseline function and are more suitable for preoperative counseling [9,10]. There is a paucity of studies that consider how much postoperative function a patient has recovered when assessing long-term recovery, which makes it difficult to apply current evidence when counseling men with incontinence or ED following surgery.
The purpose of our study was to determine the long-term probability of achieving UF or EF for patients who reported urinary dysfunction or ED at 12 mo and to identify predictors for recovery. Based on previous studies, we hypothesized that there is very little functional recovery after 12 mo if function is not achieved by this time point.
2. Methods
2.1. Patient population
In 2007 our institution implemented routine collection of patient-reported outcomes at all follow-up visits for men treated surgically for PCa. After obtaining institutional review board approval, we retrospectively identified 3187 men who underwent open or minimally invasive RP for localized PCa from 2007 through 2013 with ≥12 mo of follow-up. We excluded patients who had previous hormonal therapy or pelvic irradiation. Our primary goal was to estimate the long-term functional recovery in patients who reported urinary dysfunction or ED at 12 mo as determined through a questionnaire completed between 10 mo and 14 mo. Therefore, our analysis further excluded patients who achieved function by 12 ± 2 mo (1825 and 553 patients for UF and EF, respectively), those with missing functional status (489 and 361 for UF and EF, respectively), and patients who had preoperative incontinence (n = 73) or ED (n = 1270) as assessed by their surgeon [11]. Our final urinary dysfunction and ED cohorts included 800 and 1003 patients, respectively.
Postoperative care via our multidisciplinary survivorship program involves routine follow-up and includes teaching Kegel exercises to all patients to promote continence recovery. Patients are recommended to start daily or on-demand phosphodiesterase type 5 inhibitors as soon as possible following surgery. Physical rehabilitative therapy for incontinence or ED is typically initiated after 12 mo for continued impairment.
2.2. Primary outcome
Patient-reported recovery of good UF or EF was determined through the urinary and erectile domains of the validated Prostate Quality of Life Survey (Supplement 1), which is electronically captured through our Web-based platform [12]. The urinary domain of the Prostate Quality of Life Survey scale ranges from 0 to 21; achievement of good UF is ≥17 points. As a secondary analysis, we used complete pad-free status, a more conservative alternative measure for good UF. This accounted for patients possibly adapting to urinary symptoms.
Good EF recovery was determined with a score ≥22 points on the validated International Index of Erectile Function (IIEF-6) scale ranging from 1 to 30 [13,14]. Three questions pertain to sexual intercourse and therefore depend on men having a sexual partner. Accordingly, we attempted to capture men reporting no sexual intercourse by calculating a modified score in patients who had a sum score ≥12 from the first three questions with a sum score of zero from the last three questions of the IIEF-6 and then scaling the total score to a possible 30 points. The percentage of surveys for which the modified score was calculated was 2.8%.
2.3. Statistical analysis
We used Kaplan-Meier survival analyses to determine the probabilities of regaining function at 24, 36, and 48 mo postoperatively in patients who had not achieved function by 12 mo. Survival time started at 12 mo after RP. Patients were considered to have an event if they reached our primary outcome. We censored patients receiving hormonal therapy during their follow-up course. Because androgen-deprivation or radiation therapy is common in patients who recur, patients were censored at the date of biochemical recurrence. Interval censoring was accounted for according to patients’ response to two survey questions: “When did you first achieve an erection sufficient for penetration?” and “When did you stop needing pads for urinary leakage?” Patients could answer “within the last month,” “between 1 and 2 months,” “between 2 and 3 months,” or “greater than 3 months,” and the questionnaire completion date was subtracted by 0.5, 1.5, 2.5, or 3.5 mo, respectively.
We conducted sensitivity analyses to investigate factors that may affect recovery rates. We compared Kaplan-Meier estimates before and after censoring for secondary procedures including artificial urinary sphincters, slings, or penile prostheses. Furthermore, we accounted for reporting bias by studying whether a patient’s functional status would affect subsequent survey completion. We anticipated that a 12-mo score would be associated with the probability of subsequent recovery; hence we determined whether a 12-mo score was correlated with the number of surveys completed.
Multivariable Cox proportional hazards regression models were used to identify predictors of recovery. Predictors were selected a priori and included UF or EF scores at 12 mo post-RP, age, number of comorbidities, body mass index, preoperative prostate-specific antigen (PSA), nerve-sparing status (none, unilateral, bilateral), pathologic Gleason score(≤6, 7, ≥8), and pathologic T stage. Statistical analyses were performed using Stata v.13.1 (StataCorp, College Station, TX, USA). Tests with p values <0.05 were considered significant.
3. Results
Table 1 lists the clinical characteristics of the UF and EF. Among men who reported good preoperative UF and EF, 800 patients (26%) had not reported recovery of continence, and 1003 patients (52%) had not achieved good EF by 12 mo after RP.
Table 1.
Clinical characteristics of patients who did not recover urinary function or erectile function by 12 mo after radical prostatectomy
| Characteristic | Urinary dysfunction cohort (n = 800) | Erectile dysfunction cohort (n = 1003) |
|---|---|---|
| Patient age, yr | 62 (57–67) | 61 (56–65) |
|
| ||
| Preoperative PSA, ng/ml | 5.1 (3.7–7.1) | 5.1 (3.7–6.9) |
|
| ||
| Body mass index, kg/m2 | 28.1 (25.6–31.5) | 27.8 (25.5–30.7) |
|
| ||
| Comorbidities | ||
| 0 | 225 (28) | 337 (34) |
| 1 | 252 (32) | 361 (36) |
| 2 | 211 (26) | 221 (22) |
| ≥3 | 112 (14) | 84 (8.4) |
|
| ||
| Surgical approach | ||
| Open | 273 (34) | 432 (43) |
| Laparoscopic | 226 (28) | 289 (29) |
| Robot assisted | 301 (38) | 282 (28) |
|
| ||
| Nerve-sparing status | ||
| None | 96 (12) | 78 (7.8) |
| Unilateral | 137 (17) | 175 (17) |
| Bilateral | 537 (67) | 742 (74) |
| Unknown | 30 (3.8) | 8 (0.8) |
|
| ||
| Pathologic stage | ||
| T2 | 471 (59) | 610 (61) |
| T3 or higher | 285 (36) | 339 (34) |
| Unknown | 44 (5.5) | 54 (5.4) |
|
| ||
| Pathologic Gleason score | ||
| ≤6 | 154 (19) | 196 (20) |
| 7 | 586 (73) | 750 (75) |
| ≥8 | 52 (6.5) | 52 (5.2) |
| Unknown | 8 (1.0) | 5 (0.5) |
|
| ||
| Positive surgical margin (%) | 120 (15) | 131 (13) |
|
| ||
| Median UF or EF score at 12 mo† | 14.0 (11.0–15.0) | 8.0 (5.0–14.0) |
EF = erectile function; PSA = prostate-specific antigen, UF = urinary function.
All values are median (interquartile range) or frequency (proportion).
Median UF or EF score for those who did not recover function by 12 mo.
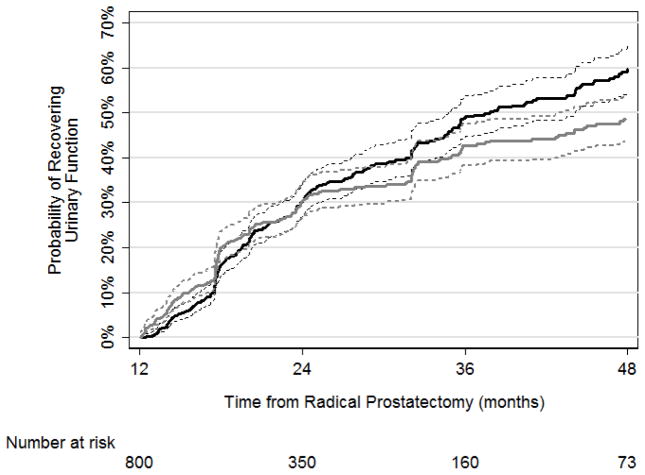
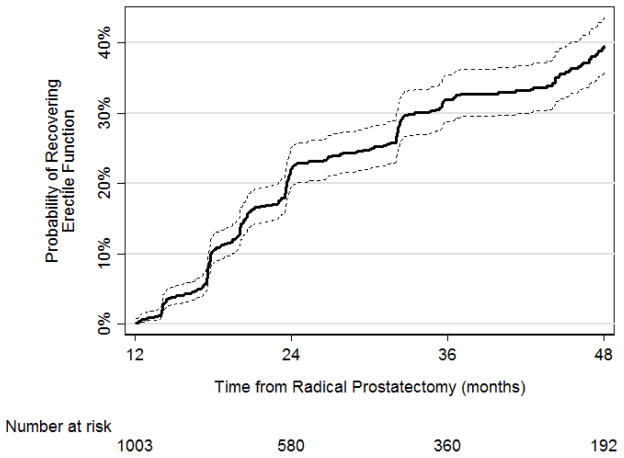
For patients who did not achieve UF by 12 mo, the probabilities of recovering UF were 30% (95% confidence interval [CI], 27–34%), 49% (95% CI, 45–54%), and 59% (95% CI, 55–65%) at 24, 36, and 48 mo, respectively (Fig. 1; Table 2). When using pad-free status to measure urinary continence, probabilities of achieving good UF at 24, 36, and 48 mo were still high, although slightly lower as time progressed at 30% (95% CI, 27–34%), 43% (95% CI, 38–48%), and 49% (95% CI, 44–54%), respectively (Fig. 1; Table 2). Median time to urinary continence was 38 mo. The rates of achieving EF at 24, 36, and 48 mo in patients who did not have good EF at 12 mo after RP were 22% (95% CI, 19–25%), 32% (95% CI, 29–35%), and 40% (95% CI, 36–44%), respectively (Fig. 2; Table 1). Only 29 men underwent artificial urethral sphincter or sling insertion; 17 underwent penile prosthesis insertion. There were no changes in Kaplan-Meier estimates after censoring these patients at the time of secondary procedures.
Fig. 1.
Kaplan-Meier analyses for the recovery of urinary function as defined by the Prostate Quality of Life Survey urinary domain score ≥17 (black line) or pad free (gray line) with 95% confidence interval (dashed line).
Table 2.
Rates of urinary function or erectile function recovery after radical prostatectomy in patients who did not recovered function by 12 mo
| Urinary function recovery | Erectile function recovery | |||
|---|---|---|---|---|
| End point, mo | Urinary function score ≥17, % (95% CI) | Pad free, % (95% CI) | Modified IIEF-6 scoring, % (95% CI) | Original IIEF-6 scoring, % (95% CI) |
| 24 | 30 (27–34) | 30 (27–34) | 22 (19–25) | 20 (18–23) |
| 36 | 49 (45–54) | 43 (38–48) | 32 (29–35) | 31 (27–34) |
| 48 | 59 (55–65) | 49 (44–54) | 40 (36–44) | 38 (35–42) |
CI = confidence interval; IIEF-6 = International Index of Erectile Function.
Fig. 2.
Kaplan-Meier analyses for the recovery of erectile function as defined by the modified International Index of Erectile Function (IIEF)-6 score ≥22 with 95% confidence interval (dashed line).
The 12-mo UF score (hazard ratio [HR]: 1.20; 95% CI, 1.12–1.28; p < 0.0001) and age (HR: 0.96; 95% CI, 0.94–0.99; p = 0.002) were significantly predictive of long-term recovery of good UF in our multivariable Cox proportional hazards regression model (Table 3). UF score at 12 mo was the only consistent predictor that was significantly associated with long-term recovery on both univariable and multivariable analyses when using the UF questionnaire score ≥17 or complete pad-free status as the outcome. However, the value of age as a significant predictor was sensitive to the method of analysis. Results were similar for recovery of EF. Both EF score at 12 mo (HR: 1.10; 95% CI, 1.08–1.13; p < 0.0001) and age (HR: 0.98; 95% CI, 0.96–1.00; p = 0.045) were predictive of recovering EF (Table 3). However, only the 12-mo score was a consistently significant predictor in both univariable and multivariable regression models. Nonlinear modeling showed that as functional score increased, the probability of achieving function at 24, 36, and 48 mo increased (Fig. 3).
Table 3.
Multivariable Cox proportional hazards regression models predicting for urinary functional and erectile functional recovery
| Predictor | Urinary function
|
Erectile function
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
|
|
|
|||||
| Functional domain score at 12 mo† | 1.20 | 1.12–1.28 | <0.0001 | 1.10 | 1.08–1.13 | <0.0001 |
| Patient age | 0.96 | 0.94–0.99 | 0.002 | 0.98 | 0.96–1.00 | 0.045 |
| Preoperative PSA | 1.00 | 0.96–1.04 | 0.9 | 1.01 | 0.98–1.04 | 0.5 |
| Body mass index | 1.02 | 0.98–1.06 | 0.3 | 0.97 | 0.94–1.00 | 0.079 |
| Comorbidities | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | 1.26 | 0.83–1.92 | 0.3 | 0.93 | 0.67–1.29 | 0.7 |
| 2 | 0.94 | 0.59–1.49 | 0.8 | 0.71 | 0.46–1.10 | 0.12 |
| ≥3 | 1.13 | 0.63–2.03 | 0.7 | 1.19 | 0.71–2.02 | 0.5 |
| Nerve-sparing status | ||||||
| None | Ref. | |||||
| Unilateral | 0.98 | 0.53–1.83 | 1.0 | 1.91 | 0.74–4.96 | 0.2 |
| Bilateral | 1.06 | 0.62–1.83 | 0.8 | 2.32 | 0.93–5.77 | 0.070 |
| Pathologic Gleason score | ||||||
| ≤6 | Ref. | Ref. | ||||
| 7 | 1.19 | 0.77–1.83 | 0.4 | 1.27 | 0.87–1.86 | 0.2 |
| ≥8 | 1.22 | 0.55–2.74 | 0.6 | 1.23 | 0.57–2.68 | 0.6 |
| Pathologic T stage | ||||||
| T2 | Ref. | Ref. | ||||
| T3 or higher | 0.91 | 0.64–1.30 | 0.6 | 1.05 | 0.75–1.46 | 0.8 |
CI = confidence interval; HR = hazard ratio; IIEF-6 = International Index of Erectile Function; PSA = prostate-specific antigen; Ref. = reference.
In patients who have urinary incontinence or erectile dysfunction at 12 mo after radical prostatectomy.
For erectile function, the modified IIEF-6 scoring was applied to the functional domain score at 12 mo.
Fig. 3. (a) Probability of achieving urinary function at 24, 36, and 48 mo based on the Prostate Quality of Life Survey urinary domain score at 12 mo; (b) probability of achieving erectile function at 24, 36, and 48 mo based on the modified International Index of Erectile Function score. Gray-shaded curve represents frequency distribution of survey scores.
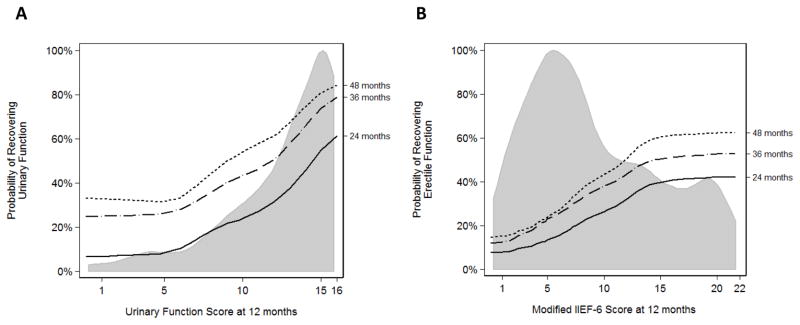
IIEF-6 = International Index of Erectile Function.
Multiple sensitivity analyses were conducted to assess the presence of bias. Reporting bias may explain some of the observed recovery rates because there was a statistically significant association between EF score at 12 mo and total surveys completed (coefficient = 0.04; 95% CI, 0.01–0.07; p = 0.015), however, the effect size was small. There was no statistically significant correlation between UF score at 12 mo and total number of surveys completed (coefficient = 0.01; 95% CI, −0.01 to 0.07; p = 0.7). As expected, there were slightly lower rates of achieving good EF using the original IIEF-6 scoring versus the modified IIEF-6 scoring. The rates were 20% (95% CI, 18–23%), 31% (95% CI, 27–34%), and 38% (95% CI, 35–42%) at 24, 36, and 48 mo, respectively (Table 2).
4. Discussion
Based on longitudinal patient-reported outcomes, we found that men who undergo open or minimally invasive RP and have incontinence or ED at 12 mo have a higher than anticipated probability of future recovery of function, with 59% and 40% achieving good UF and EF at 48 mo after surgery. We also found that the level of function achieved at 12 mo was highly predictive for UF and EF recovery at longer term follow-up.
Prior studies have suggested little to no improvement in function beyond 12–24 mo. For example, Prabhu et al [15] reported that mean UF score decreased from 2 to 10 yr, and Sivarajan et al [16] showed that EF was stable between the same time points. In an analysis of 1288 patients as part of the prospective Prostate Cancer Outcomes Study, the frequency of incontinence, number of urinary pads, and sexual function summary scores remained relatively stable from years 2 to 5 [10]. There are two possible reasons for the difference between prior research and our findings. First, the estimates provided by previous authors represent mean functional scores or recovery rates of all men from the time of their RP onward rather than focusing specifically on men reporting dysfunction at 12 mo. It is also possible that differences in postoperative follow-up care at our institution, with ongoing multidisciplinary support, lead to better long-term outcomes.
Few studies to date consider postoperative status when investigating functional outcomes. Abdollah et al [17] performed a conditional survival analysis to examine the probability of recovery based on function at 6-mo interval time points. Although the study did not provide long-term recovery rates of men who had incontinence or ED at 12 mo, they showed a continued improvement up to 42 mo and 36 mo for UF and EF, respectively. In another study of 73 patients incontinent at 12 mo, continence rate at 4 yr post-RP was 60%, which is similar to our findings [18]. Furthermore, Glickman et al [19] showed that some men report global improvement between 24 and 48 mo despite dysfunction at 24 mo. Our study expands on this clinically relevant question of what a man’s chances of recovering function are if he has incontinence or ED after RP.
Many studies report predictors of long-term recovery that are determined primarily from baseline patient characteristics or perioperative treatment details and thus are more suitable for preoperative counseling. Alemozaffar et al [20] showed that the probability of erectile recovery increases with preoperative sexual function score. In addition, they found that age, nerve sparing, and PSA were associated with erections at 24 mo. Other reported predictors of functional recovery include age, comorbidities, Gleason score, laparoscopic robot-assisted RP, and nerve-sparing status [21–26]. Our institution previously showed that functional scores between 3 and 12 mo were predictive of recovery at 12 and 24 mo [27]. This current study further establishes that postoperative functional score at 12 mo is highly predictive for recovery of UF and EF between 12 and 48 mo. Interestingly, neither age nor nerve sparing were consistently significant in our models, which may be explained by their effect being most contributory for recovery within the first year after RP.
We believe our results are robust based on multiple sensitivity analyses to investigate whether other factors affected our observed recovery rates. We used a modified IIEF-6 score to determine if having a sexual partner had an impact on long-term EF rates. By considering only questions related to erectile quality, we observed slightly higher recovery rates; this may be due to misclassification of men as having ED based on the absence of a sexual partner when using the original IIEF-6 questionnaire. In addition, we addressed potential response biases by using pad-free usage as a secondary outcome. Although it is possible that men adapted to urinary symptoms with time, using pad-free status as the outcome did not result in substantial differences in recovery. Last, there was no difference in functional recovery when rehabilitative procedures were factored into our analysis.
Our retrospective study is not without limitations. We had response rates of 64%, 65%, 53%, and 41% at 12, 24, 36, and 48 mo, respectively, based on the number of patients eligible for follow-up at those time points. Compared with other studies [28–30], we incorporated the collection of patient-reported outcomes as part of routine clinical practice rather than a prospective research protocol. Our sensitivity analyses showed no sizable association between the number of completed surveys and functional score. This suggests a low likelihood of nonrandom missingness, that our respondents are representative, and observed recovery rates reliable. In addition, these outcomes are from a high-volume referral center that limits generalizability, and also patients may have received surgical rehabilitative procedures at outside institutions. However, we obtain follow-up for patients as part of our survivorship program, and secondary procedures would be captured. Moreover, the observed improvement may reflect patients with survey scores near the cut point between poor and good function, which should be taken into consideration when interpreting our results. Last, although the modified scoring system is nonvalidated, the results are comparable when using the validated original IIEF-6 as a sensitivity analysis.
Our study has important clinical implications. This is one of the first studies specifically to address functional recovery beyond 12–24 mo after RP. Because we studied men with incontinence or ED at 12 mo after RP, the estimates provided in this study are relevant to postoperative counseling. We have shown that in a heterogeneous cohort of men with varying degrees of postoperative function, a patient’s current level of function is highly predictive of recovery. In other words, men with high functional scores at 12 mo are more likely to improve and may continue careful monitoring within a survivorship program. In those with very low function, the low probability of recovery may be incorporated into decision making for early surgical intervention. Our observed recovery rates may be reflective of other therapies, such as erectogenic agents, or to a wider extent our survivorship program, and therefore further research into the role of these interventions are warranted.
5. Conclusions
In a large cohort of men with varying degrees of incontinence and impotence at 12 mo after RP, a considerable proportion of men continue to have functional improvement. Contrary to previous studies, we observed increasing rates of continence and EF as time progresses beyond 12 mo. The degree of dysfunction is a useful predictor for postoperative counseling and should be considered when making informed decisions regarding continued supportive care versus early secondary rehabilitative procedures.
Supplementary Material
Take-home message.
In this large cohort with urinary incontinence or erectile dysfunction at 12 mo after radical prostatectomy, we observed increasing probability of functional recovery with time. Functional score is a useful predictor for postoperative counseling and making decisions regarding rehabilitative interventions.
Acknowledgments
Funding/Support and role of the sponsor: Supported in part by funds from David H. Koch provided through the Prostate Cancer Foundation, the Sidney Kimmel Center for Prostate and Urologic Cancers, a P50-CA92629 SPORE grant from the National Cancer Institute to H.I. Scher, and a P30-CA008748 NIH/NCI Cancer Center Support Grant to the Memorial Sloan Kettering Cancer Center. These sponsors did not have any role in the design/conduct of the study, collection/management of the data, analysis, interpretation of the data, or preparation, review, or approval of the manuscript.
Footnotes
Author contributions: Justin K. Lee had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lee, Vickers, Ehdaie.
Acquisition of data: Lee.
Analysis and interpretation of data: Lee, Thong, Vickers, Ehdaie.
Drafting of the manuscript: Lee.
Critical revision of the manuscript for important intellectual content: Lee, Assel, Sjoberg, Thong, Mulhall, Sandhu, Vickers, Ehdaie.
Statistical analysis: Lee, Assel, Sjoberg, Vickers.
Obtaining funding: None.
Administrative, technical, or material support: Vickers, Ehdaie.
Supervision: Vickers, Ehdaie.
Other (specify): None.
Financial disclosures: Justin K. Lee certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu is a consultant for American Medical Systems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer treatment and survivorship facts and figures 2014–2015. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 3.Matthew AG, Alibhai SM, Davidson T, et al. Health-related quality of life following radical prostatectomy: long-term outcomes. Qual Life Res. 2014;23:2309–17. doi: 10.1007/s11136-014-0664-1. [DOI] [PubMed] [Google Scholar]
- 4.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 5.Schiavina R, Borghesi M, Dababneh H, et al. Survival, Continence and Potency (SCP) recovery after radical retropubic prostatectomy: a long-term combined evaluation of surgical outcomes. Eur J Surg Oncol. 2014;40:1716–23. doi: 10.1016/j.ejso.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–9. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 7.Le JD, Cooperberg MR, Sadetsky N, et al. Changes in specific domains of sexual function and sexual bother after radical prostatectomy. BJU Int. 2010;106:1022–9. doi: 10.1111/j.1464-410X.2010.09231.x. [DOI] [PubMed] [Google Scholar]
- 8.Alivizatos G, Skolarikos A. Incontinence and erectile dysfunction following radical prostatectomy: a review. Scientific World Journal. 2005;5:747–58. doi: 10.1100/tsw.2005.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101:888–92. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 10.Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701–5. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 11.Saranchuk JW, Kattan MW, Elkin E, Touijer AK, Scardino PT, Eastham JA. Achieving optimal outcomes after radical prostatectomy. J Clin Oncol. 2005;23:4146–51. doi: 10.1200/JCO.2005.12.922. [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, Savage CJ, Shouery M, Eastham JA, Scardino PT, Basch EM. Validation study of a Web-based assessment of functional recovery after radical prostatectomy. Health Qual Life Outcomes. 2010;8:82. doi: 10.1186/1477-7525-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulhall JP, Bivalacqua TJ, Becher EF. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med. 2013;10:195–203. doi: 10.1111/j.1743-6109.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 14.Briganti A, Gallina A, Suardi N, et al. What is the definition of a satisfactory erectile function after bilateral nerve sparing radical prostatectomy? J Sex Med. 2011;8:1210–7. doi: 10.1111/j.1743-6109.2010.02179.x. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu V, Sivarajan G, Taksler GB, Laze J, Lepor H. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:52–7. doi: 10.1016/j.eururo.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivarajan G, Prabhu V, Taksler GB, Laze J, Lepor H. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. Eur Urol. 2014;65:58–65. doi: 10.1016/j.eururo.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Abdollah F, Sun M, Suardi N, et al. Prediction of functional outcomes after nerve-sparing radical prostatectomy: results of conditional survival analyses. Eur Urol. 2012;62:42–52. doi: 10.1016/j.eururo.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 18.Jeong SJ, Kim HJ, Kim JH, et al. Urinary continence after radical prostatectomy: predictive factors of recovery after 1 year of surgery. Int J Urol. 2012;19:1091–8. doi: 10.1111/j.1442-2042.2012.03106.x. [DOI] [PubMed] [Google Scholar]
- 19.Glickman L, Godoy G, Lepor H. Changes in continence and erectile function between 2 and 4 years after radical prostatectomy. J Urol. 2009;181:731–5. doi: 10.1016/j.juro.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei JT, Dunn RL, Marcovich R, Montie JE, Sanda MG. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164:744–8. doi: 10.1097/00005392-200009010-00029. [DOI] [PubMed] [Google Scholar]
- 22.Nandipati KC, Raina R, Agarwal A, Zippe CD. Nerve-sparing surgery significantly affects long-term continence after radical prostatectomy. Urology. 2007;70:1127–30. doi: 10.1016/j.urology.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 23.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term followup. J Urol. 2010;183:2206–12. doi: 10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 25.Suardi N, Moschini M, Gallina A, et al. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int. 2013;111:717–22. doi: 10.1111/j.1464-410X.2012.11315.x. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui KM, Billia M, Mazzola CR, et al. Three-year outcomes of recovery of erectile function after open radical prostatectomy with sural nerve grafting. J Sex Med. 2014;11:2119–24. doi: 10.1111/jsm.12600. [DOI] [PubMed] [Google Scholar]
- 27.Vickers AJ, Kent M, Mulhall J, Sandhu J. Counseling the post-radical prostatectomy patients about functional recovery: high predictiveness of current status. Urology. 2014;84:158–63. doi: 10.1016/j.urology.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92:1582–92. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 29.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepor H, Kaci L. The impact of open radical retropubic prostatectomy on continence and lower urinary tract symptoms: a prospective assessment using validated self-administered outcome instruments. J Urol. 2004;171:1216–9. doi: 10.1097/01.ju.0000113964.68020.a7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.