Introduction
Reduced reproduction increases lifespan in many animals (Flatt, 2011; see Amdam et al. 2010 for the exception of social insects). Direct reduction in reproduction can be accomplished many ways, including ovariectomy in lubber grasshoppers and brown anole lizards (Hatle et al. 2008; Cox et al. 2010), mutations leading to halted ovarian development (Flatt et al. 2008) or irradiation (Sgró et al. 1999) in Drosophila, and surgical ablation of the germ-line stem cells in C. elegans (Arantes-Oliveira et al. 2002). In each of these manipulations, lifespan was increased over controls. The disposable soma hypothesis is the long-standing idea that seeks to explain how reduced reproduction extends lifespan (Kirkwood 1977; Kirkwood and Rose 1991). The hypothesis predicts that, upon reduced reproduction, ingested nutrients are preferentially allocated to the soma, better maintaining the tissues and hence extending lifespan. Experimental tests in the past decade have provided little support for the hypothesis, especially the nutrient allocation predictions (e.g., O’Brien et al. 2008; Grandison et al. 2009; Speakman and Krol 2010; Judd et al. 2011; Lee et al. 2014). Hence, a clear underlying mechanism has not been confirmed for this widespread trade-off between reproduction and lifespan (Flatt, 2011), though there has been recent progress on lipid metabolism and autophagy using C. elegans (Goudeau et al. 2011; Lapierre et al. 2011; McCormick et al. 2012; Ghazi 2013; and see recent paper by Labbadia and Morimoto 2015 on the heat shock response). Here, we use a technique widely used in eusocial insects, namely RNAi of the precursor to egg yolk protein (vitellogenin), to examine the relationships among reproductive investment, feeding, storage, and longevity in a non-social insect.
Testing combinations of life-extending treatments can provide insight on whether the treatments act through similar or distinct mechanisms. Gems et al. (2002) provide cautions for this approach. For example, each treatment must be complete in its own right (e.g., the ovary completely removed, not partially removed) to test whether another treatment can add to it. The vast majority of studies that have combined two means of reducing reproduction in concert have included dietary restriction as one of the means (e.g., Mair et al. 2004; Drewry et al. 2011). Dietary restriction typically both extends lifespan (Nakagawa et al. 2011) and reduces reproduction (e.g., Tatar 2011; see Lee et al. 2014 for an exception). To our knowledge, there have been no studies testing for potential additive effects of directly reducing reproduction through two distinct manipulations. Several means of directly reducing reproduction have been shown to increase lifespan, but these may affect the physiology of organisms in distinct ways (discussed in Flatt et al. 2008).
To investigate the effects of different means of reducing reproduction on lifespan, we used ovariectomy and vitellogenin-RNAi in the Eastern lubber grasshopper, Romalea microptera, which is an emerging model system for aging (Lee et al. 2015). These animals have easily determined feeding rates, are amenable to surgery, and are large enough to allow serial sampling of hemolymph. We can directly reduce reproduction by ovariectomy (Hatle et al. 2003), which increases lifespan by ~20% and reduces feeding by ~50% (Hatle et al. 2008; Drewry et al. 2011). Ovariectomy has been shown to double fat body mass and hemolymph volume (Hatle et al. 2013). Ovariectomized females still produce vitellogenin; however, there is no ovary to sequester the vitellogenin, so instead there is a 5- to 10-fold increase of vitellogenin in the hemolymph (Hatle et al. 2003, 2008). This represents a substantial investment of resources to reproduction by females despite failing to produce mature eggs (Tokar et al. 2014).
As a second, separate means of directly reducing reproduction, we can use RNA interference to decrease vitellogenin mRNA (Tokar et al. 2014). Vitellogenin is important because it may hold a key role in life extension, in part due to its role in lipid metabolism (Brandt et al. 2005). Indeed, overexpression of a vitellogenin from another species decreased lifespan of Drosophila (Ren and Hughes 2014). The vitellogenin RNAi (VgRNAi) treatment we used was characterized in young lubber grasshoppers, and it reduced vitellogenin mRNA levels 35-fold, doubled fat body mass, and prevented ovarian development (Tokar et al. 2014). Upon VgRNAi treatment vitellogenin protein was still present in the hemolymph, but it was not taken up into the ovary, and females did not produce mature eggs. The 515 bp sequence of dsRNA used for knockdown had three 11 bp regions identical to the vitellogenin receptor from Leucophaea maderae (cockroach). As a result, this treatment may partly block transport of vitellogenin from the hemolymph into the ovary (Tokar et al. 2014). Nonetheless, this VgRNAi treatment is a genetic means of reducing reproduction with all organs remaining intact, in contrast to the surgical means of reducing reproduction via ovariectomy. Because VgRNAi reduced reproduction, it may also increase longevity.
Nutrient storage, and especially lipid storage, can be important for longevity in C. elegans, Drosophila, and mice (e.g., Wang et al. 2008, reviewed in Hansen et al. 2013). For phytophagous insects fed plant diets instead of artificial diets, however, protein is essential and often limiting for reproduction (Wheeler 1996; Burmester et al. 1999). Studies using the geometric framework have shown clearly that several species of grasshopper have intake targets for protein:carbohydrate ratios of about 1:1 (reviewed in Behmer 2009). The Romaine lettuce diet used in the present study is a low protein diet for grasshoppers, as the protein to carbohydrate ratio is 0.38:1 (nutritiondata.self.com 2015). Hence, protein is likely limiting. Despite the known importance of protein storage for metamorphosis and reproduction in insects, it is unclear what role storage protein levels play in longevity (Hatle et al. 2006).
Hexameric storage proteins are well-conserved in the hemolymph of arthropods and typically provide amino acids for major developmental events including molting, holometabolous metamorphosis, and reproduction (Telfer and Kunkel 1991; Burmester 1999). Lubber grasshoppers have three hexamerins (Hex-90, Hex-270, Hex-500) that account for 80% of the non-vitellogenin proteins in the hemolymph (Hathaway et al. 2009). Ovariectomized females accumulate high levels of non-vitellogenic protein in the hemolymph in old age (Hatle et al. 2008). Knockdown of the most abundant storage protein (Hex-90) does not affect reproduction through the first clutch (Tokar et al. 2014). Because of this, we chose to use knockdown of Hex-90 as a control for general, non-specific RNAi effects. General RNAi effects can reduce lifespan 5–10% in Drosophila (Alic et al. 2012). Our Hex-90 knockdown control is superior to a ‘scramble’ RNAi control, because a fat body transcript is truly degraded, yet reproduction is not reduced.
First, we address whether VgRNAi increases lifespan in lubber grasshoppers. Next, we tested whether ovariectomy and VgRNAi treatment combined within the same individual has additive effects on lifespan, which would suggest they may act through separate mechanisms. We also quantified reproductive output, feeding rates, quantities of vitellogenin and storage proteins in the hemolymph, and anti-oxidant activities in the blood to address the physiology underlying life-extension via these two means of reduced reproduction.
Methods
Animal rearing
Juvenile eastern lubber grasshoppers (Romalea microptera) were obtained from Miami, Florida, USA, raised communally, and fed an ad libitum diet of Romaine lettuce (Hatle et al. 2008). Females were separated upon molting into adults and placed individually into 500 cm3 containers in an environmental chamber (14L:10D, 35°C:27°C, 50% RH) (Hatle et al. 2001). All individuals in the study molted to adulthood within a 15 day period. Adults were fed an ad libitum diet of Romaine lettuce, weighed weekly, and feeding rates were determined every week by drying the food left uneaten and converting dry mass of lettuce to wet mass using an empirically determined relationship (Hatle et al. 2006). Individuals were not mated, as ovariectomized females are not receptive to mating.
Surgery and injection assignments
Animals were serially assigned into control operated (Sham) or ovariectomized (OVX) surgical groups. Ovariectomies were performed when grasshoppers were 1 or 2 days old as previously (e.g., Hatle et al. 2008), before females become vitellogenic (Hatle et al. 2001). Within each surgical group, individuals were serially assigned into three injection groups: RNAi treatment for vitellogenin (VgRNAi), RNAi treatment for the 90 kD hexamerin storage protein (Hex90RNAi), or injection of only Tris buffer with no dsRNA (Buffer). The injections were prepared identically to those used by Tokar et al. (2014), and the VgRNAi injection targets the only vitellogenin in lubber grasshoppers. The Hex90RNAi treatment does not reduce reproduction (Tokar et al. 2014), so it serves as a control for general RNAi effects, similar to scramble or GFP controls.
Synthesis and injection of dsRNA
Double-stranded RNA (dsRNA) for RNAi was synthesized according to the instructions of Ambion’s MEGAscript® RNAi Kit (Life Technologies, Grand Island, NY). The DNA template used was from fat body cDNA of a vitellogenic female lubber grasshopper (Tokar et al. 2014). Template or primer concentrations for PCR were reduced until pure product was obtained, as determined by melt curves and the presence of a single clear band in gel electrophoresis. dsRNA product was run on an agarose gel to ensure the product was the predicted size, and purity was tested by spectrophotometry.
Four to five days after the adult molt, individuals were injected intra-abdominally with 5 µg of dsRNA in 30 µl buffer, or only 30 µl of Tris buffer, according to injection assignments (Tokar et al. 2014). This led to a fully factorial 2 × 3 design of OVX Buffer, OVX Hex90RNAi, OVX VgRNAi, Sham Buffer, Sham Hex90RNAi, Sham VgRNAi, (n = 25 or 26 per group). Within this design, two groups have full reproduction (Sham Buffer and Sham Hex90RNAi) while the other four groups have reduced reproduction.
Survivorship, oviposition, and collection of hemolymph
Individuals were reared singly, and each was fed and checked for survival daily. Starting approximately 4 weeks after the adult molt, all lubber grasshoppers were placed on damp sand two times a week to allow for oviposition (Hatle et al. 2001). Unmated females continue to lay eggs, with an 11% delay in the timing of laying the first clutch, but no reduction in the mass of the clutch (Walker et al. 1999). When deposition of eggs occurred, the eggs were counted, measured lengthwise, and age at oviposition was recorded. Approximately one-half of the animals in the study were selected for hemolymph sampling; every 4th week, a hemolymph sample (5 µL in 250 µL of phosphate buffered saline) was taken from each individual in this subgroup (Hatle et al. 2006) and stored at −80°C until analysis. The experiment was terminated when animals were at a median age of 30.4 weeks (213 days), when the control Sham Buffer group had a survivorship of only 4%.
Measurement of vitellogenin and hexamerin proteins
Vitellogenin was quantified using a well characterized ELISA (Borst et al. 2000; Hatle et al. 2001, 2008).
Levels of storage hexamerins in the hemolymph were used as an index of protein storage (e.g., Hatle et al. 2008). These proteins were quantified by native PAGE followed by densitometry using ImageJ (Rasband 1997). Each of the three hexamerins is well isolated from other proteins by native PAGE, which has been confirmed by anion exchange liquid chromatography and amino acid sequencing (Hathaway et al. 2009). Hence, gel staining is sufficient to quantify each hexamerin.
Analysis of anti-oxidant activity
Stimulation of anti-oxidant activity has long been thought to contribute to longevity (Salmon et al. 2010), including in some insects (Williams et al. 2010). The 2, 2’ azino-bis (3-ethyldenzothiazoline-6-sulphonic acid) ABTS radical cation decolorization assay (Re et al. 1999) was used to determine total anti-oxidant activity in hemolymph samples, using Trolox standards in phosphate-buffered saline. Upon collection, samples were flash frozen, and then stored frozen until the assay. The anti-oxidant activity in a given hemolymph sample was determined per µg of hemolymph protein, and total protein was quantified using the Bradford assay with bovine serum albumin standards.
Statistics
Data were analyzed using seven two-way MANOVAs, with time as an independent variable to control for repeated sampling of each individual. Several tests were used to avoid loss of all the data from an individual if only a single sample was missing. The first MANOVA tested the effects of surgery and injection on amounts of food eaten. The second MANOVA tested the effects of surgery and injection on body masses. The third MANOVA tested the effects of injection on the age of the animal when clutches were laid, number of eggs produced per clutch, and lengths of eggs. The fourth MANOVA tested surgery and injection effects on vitellogenin levels in the hemolymph. The fifth, sixth, and seventh MANOVA tested the effects of surgery and injection on levels of each of the three hexamerins in the hemolymph. Mortality rates were tested using Cox proportional hazards models (i.e., regression). These models compare the survivorship across the entire lifespan, and they are the recommended survival analysis for the samples sizes and truncation used here (Allison 2010).
Results
Reproductive output
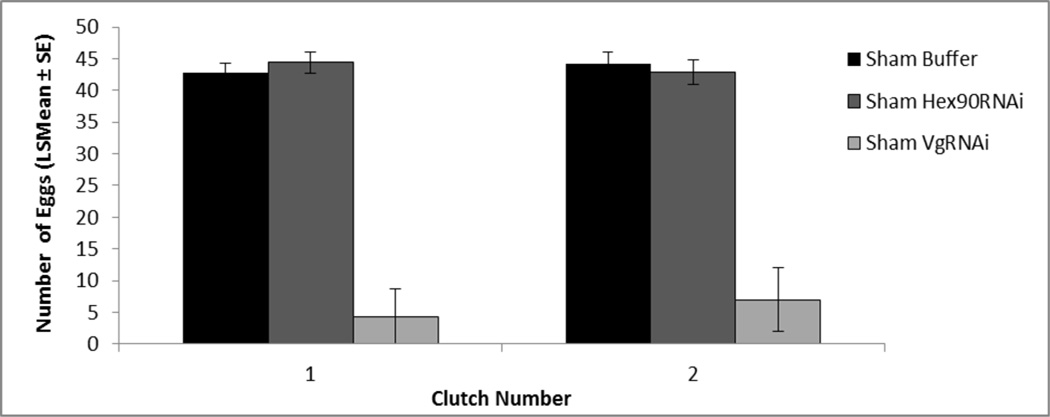
Ovariectomy resulted in no deposition of eggs. Among sham-operated groups, VgRNAi nearly eliminated reproductive output. VgRNAi reduced the number of clutches in comparison to other sham-operated groups (mean clutches: Sham Buffer = 5.60 ± 0.4, Sham Hex90RNAi = 4.52 ± 0.6, Sham VgRNAi = 0.44 ± 0.2). Because of this, the MANOVA on clutch parameters was run only through clutch 2, and included only the 3 out of 25 Sham VgRNAi individuals who laid eggs. For all clutch parameters (age at clutch, number of eggs, and length of eggs), there was a strong effect of age (Pillai’s Trace F5,40 = 489.90, P < 0.0001) and an interaction of age and injection (F10,82 = 7.56, P < 0.0001). Sham Buffer and Sham Hex90RNAi exhibited no difference in age at the laying of each clutch (all P > 0.96; data not shown), number of eggs (all P > 0.76; Fig. 1), or length of eggs in clutches 1 and 2 (all P > 0.23; data not shown). When compared to Sham Buffer and Sham Hex90RNAi, Sham VgRNAi animals laid eggs at much older ages (all P < 0.0001; LS Mean = 126 ± 4.14 days compared to Sham Buffer LSMean = 40 ± 1.46 days), produced fewer eggs (all P < 0.0001), and had smaller eggs (all P < 0.01; data not shown). The potent nature of this VgRNAi knockdown on reproductive output, and the complete blockage of reproductive output upon ovariectomy, suggest that these treatments are reasonably complete, and it is therefore valid to test whether they are additive (see Gems et al. 2002).
Fig. 1.
Vitellogenin RNAi greatly reduced reproductive output. Only 3 out of the 25 individuals of Sham VgRNAi laid eggs. Egg numbers per clutch in Sham operated grasshoppers are shown (mean ± SE). Sham VgRNAi individuals laid fewer eggs in clutch 1 and 2 than Sham Buffer and Sham Hex90RNAi individuals (all P < 0.0001)
Food eaten
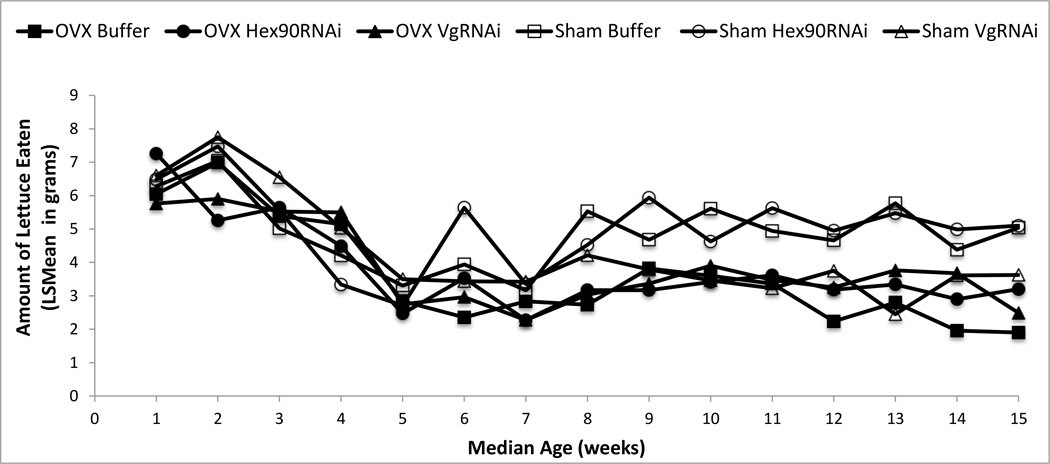
Overall, the treatments that decreased reproduction (OVX Buffer, OVX Hex90RNAi, OVX VgRNAi, and Sham VgRNAi) also resulted in reductions in feeding rates after 9 weeks, regardless of treatment method (all P < 0.025). There was a strong effect of age (MANOVA: Pillai’s Trace F14 = 11.70, P < 0.0001), no interaction of age and surgery (F14,31 = 1.85, P = 0.075), and no interaction of age and injection (F28,64 = 1.29, P = 0.20) on feeding. Ovariectomy resulted in a significant decrease in consumption of Romaine lettuce after lubber grasshoppers reached a median age of 8 weeks, continuing through median age of 15 weeks (all P < 0.04). Feeding rates tend to decrease prior to oviposition in reproductive lubber grasshoppers, likely explaining the general decrease in feeding for all treatments around 6 weeks (Fig. 2). Nonetheless, feeding rates in the two fully reproductive groups were clearly higher than the reduced reproduction groups. Feeding data were only analyzed through 15 weeks because after this point mortality was so high that sample sizes were no longer suitable for statistical analyses.
Fig. 2.
Mean amounts of Romaine lettuce eaten daily by each treatment group of adult lubber grasshoppers. The Sham VgRNAi group and all three ovariectomized groups demonstrated decreases in feeding rates relative to Sham Buffer and Sham Hex90RNAi at older ages (error bars omitted for clarity)
Body mass
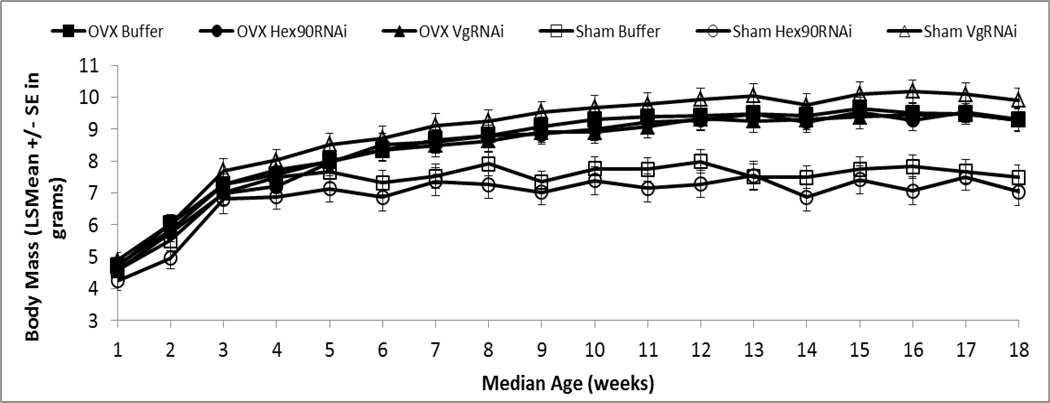
Body mass was strongly affected by age (Pillai’s Trace F17, 56 = 73.36, P < 0.0001), and there was a clear interaction between age and surgery (F17,56 = 3.29, P = 0.0004), yet no overall interaction of age and injection (i.e., Buffer, Hex90RNAi or VgRNAi) on size (F34,114 = 1.41, P = 0.09). As in previous studies (e.g., Drewry et al. 2011), body masses for all treatments increased rapidly until about 3 weeks, and continued to increase slowly until a median age of 6 weeks, a time just following the oviposition of the first clutch in fully reproductive individuals (Fig. 3). Starting at 7 weeks, non-reproductive and reduced-reproductive animals exhibited steady increases in body mass relative to fully reproductive individuals (all P < 0.0005). Ovariectomized animals exhibited steady increases in body mass relative to reproductive animals (all P < 0.05), and Sham VgRNAi individuals tended to have slightly greater masses (9.9 g ± 0.36) than ovariectomized animals (9.3 g ± 0.34) from 9 weeks on.
Fig. 3.
After a median age of 9 weeks, ovariectomized grasshoppers had higher body masses (mean ± SE) than Sham operated groups (all P < 0.05), and Sham VgRNAi individuals showed similar body masses (mean ± SE) to OVX groups. Hence, reduced reproduction increased body mass
Life spans
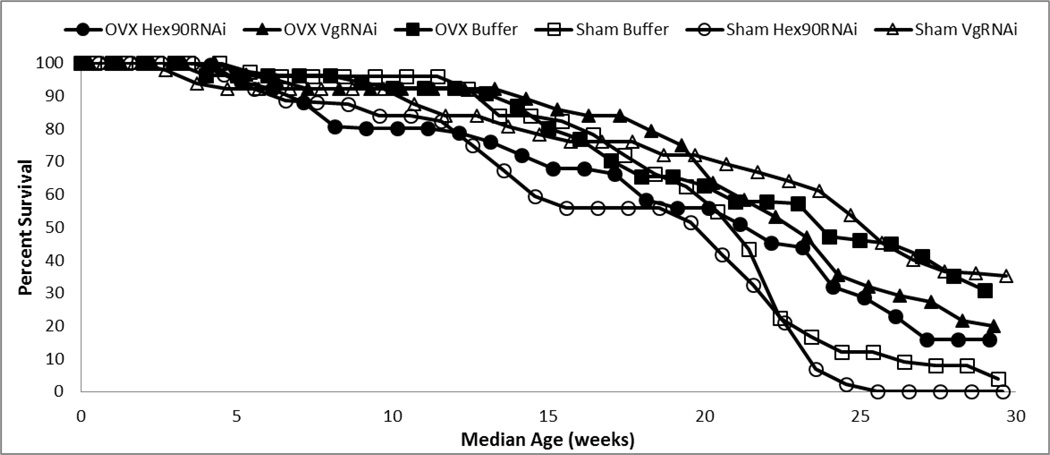
Surviving animals were terminated at a median age of 30.4 weeks (29.9–31.7 weeks). Median life spans were: OVX Buffer = 24.2 weeks (169.5 days), OVX Hex90RNAi = 21.5 weeks (150.5 days), OVX VgRNAi = 22.9 weeks (160.5 days), Sham Buffer = 21.1 weeks (147.5 days), Sham Hex90RNAi = 19.2 weeks (134.5 days), and Sham VgRNAi = 24.2 weeks (169.5 days; Fig. 4). The COX regressions revealed that ovariectomy (χ2 = 5.17, P = 0.016) and VgRNAi injection (χ2 = 8.29, P = 0.02) each increased survivorship. There was an interaction of surgery and injection (χ2 = 12.10, P = 0.002); the assumption of the statistical test is an additive interaction (i.e., an additive interaction produces a non-significant result). Hence, our results suggest that the two treatments that directly reduced reproduction did not have additive effects on lifespan.
Fig. 4.
Grasshoppers with reduced reproduction showed higher survivorship compared to fully reproductive groups (OVX: P = 0.016, VgRNAi: P = 0.02). However, the combined ovariectomy and VgRNAi treatment (OVX VgRNAi) did not result in additive survivorship
Vitellogenin levels in the hemolymph
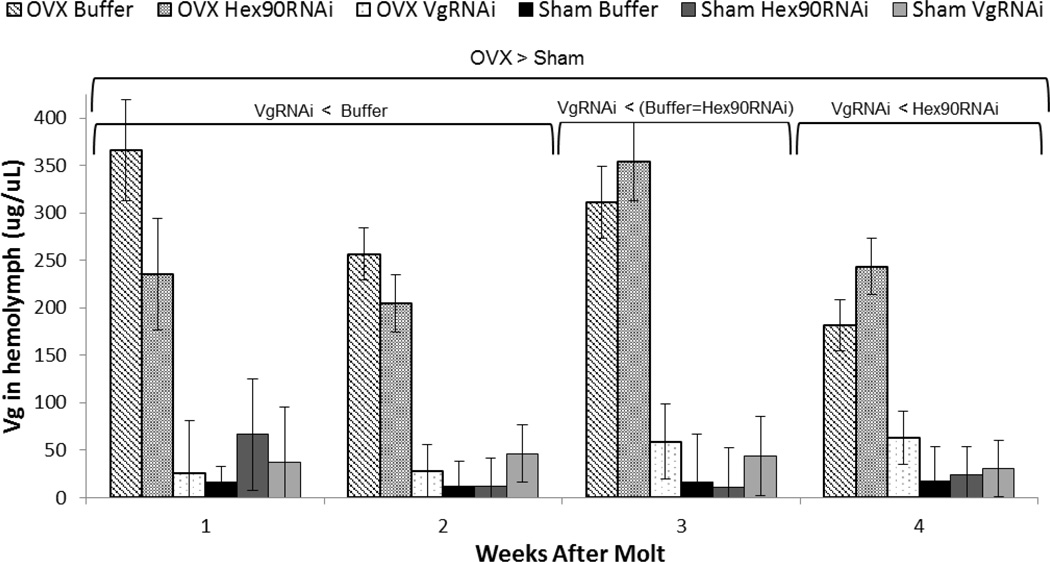
Ovariectomy resulted in a 5- to 12-fold increase in vitellogenin levels in the hemolymph through 16 weeks after the adult molt (all P < 0.001). There was no effect of age on vitellogenin concentration in the hemolymph (MANOVA, Pillai’s Trace F3,46 = 1.12, P = 0.35). There was also no interaction between age and surgery (F3,46 = 1.08, P = 0.36), and no interaction between age and injection (i.e., Buffer, Hex90RNAi or VgRNAi; F6,94 = 0.97, P = 0.45), making our treatment effects clear. The OVX VgRNAi group had decreased vitellogenin levels relative to the OVX Buffer-treated group and the OVX Hex90RNAi-treated group (all P < 0.05 starting at 4 weeks), clearly demonstrating that our VgRNAi treatment reduced vitellogenin protein levels in ovariectomized females. However, amongst females with intact ovaries, the Sham VgRNAi group did not have lower vitellogenin levels than the other fully reproductive, sham-operated groups (all P > 0.92; Fig. 5). Overall, non-reproductive animals showed higher vitellogenin levels in the hemolymph relative to reproductive groups (all P < 0.05).
Fig. 5.
Ovariectomy greatly increases hemolymph vitellogenin levels (Hatle et al. 2008). In the present study, OVX VgRNAi treatment resulted in a reduction in vitellogenin relative to the OVX Buffer and the OVX Hex90RNAi (all P < 0.001). There were no differences in vitellogenin within the sham operated groups (all P > 0.92)
Hexamerin levels in the hemolymph
Overall, reducing reproduction typically increased levels of hexameric storage proteins in the hemolymph of old adults (Fig. 6). Indeed, fully reproductive animals never had greater protein storage than animals with reduced reproduction.
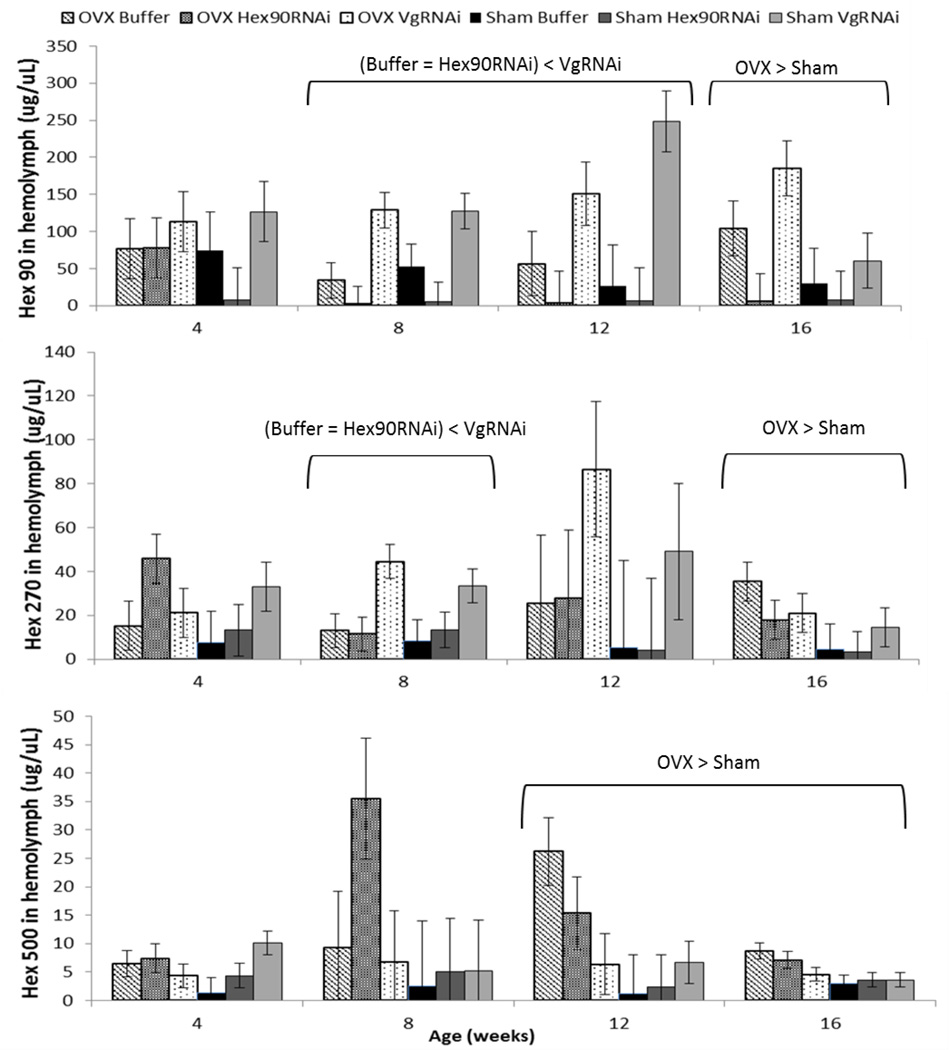
Fig. 6.
Reducing reproduction tended to increase storage protein in old adult grasshoppers. All hexamerins increased upon ovariectomy after 16 weeks (Hex-90: P = 0.05, Hex-270: P = 0.03, Hex-500: P = 0.01). VgRNAi treatment resulted in increases in hexamerin-90, which is the most abundant storage protein (P < 0.01). Levels of hexamerin-270 (P = 0.003), but not in hexamerin-500, were also increased by VgRNAi treatment
All three hexamerin proteins increased upon ovariectomy at 16 weeks. VgRNAi treatment resulted in increases in hexamerin-90 and hexamerin-270 at 8 weeks, but did not result in increases in hexamerin-500. The three MANOVAs on hexamerins showed no interaction effects on age, age and surgery, and age and injection (all P > 0.05).
Hexamerin-90 was 3-fold greater upon ovariectomy at 16 weeks (P = 0.05), and this storage protein increased with VgRNAi at 8 weeks (2-fold, P = 0.01) and 12 weeks (4.5-fold, P = 0.004). Hexamerin90 RNAi treatment decreased hexamerin-90 protein levels in the hemolymph with detectably less hexamerin-90 protein in the blood of Hex90RNAi-treated individuals than VgRNAi-treated individuals (after 4 weeks all P < 0.01). Hexamerin-90 protein levels in the blood did not differ between the Hex90RNAi and the Buffer injection groups after 4 weeks (all P > 0.31). However, Hex90RNAi treatment was successful in suppressing hexamerin-90 protein levels in the blood after 8 weeks and up through 16 weeks of age. Hexamerins are present during all life stages, and they have long half-lives estimated at ~5 d (DW Borst, unpublished data). Hence, reducing the protein levels may require several weeks after initiation of transcript degradation. It may be that the turnover of hexamerin-90 was altered by OVX, but the direct comparison of OVX Hex90RNAi and Sham Hex90RNAi was not significant early.
When considering levels of the other two hexamerin proteins, hexamerin-270 increased 3-fold upon ovariectomy at 16 weeks (P = 0.03) and increased 3.5-fold with VgRNAi at 8 weeks (P = 0.003). Similarly, Hexamerin-500 increased upon ovariectomy at 12 (3-fold; P = 0.01) and 16 weeks (2-fold; P = 0.01) but did not change due to Vg-RNAi.
Anti-oxidant activity
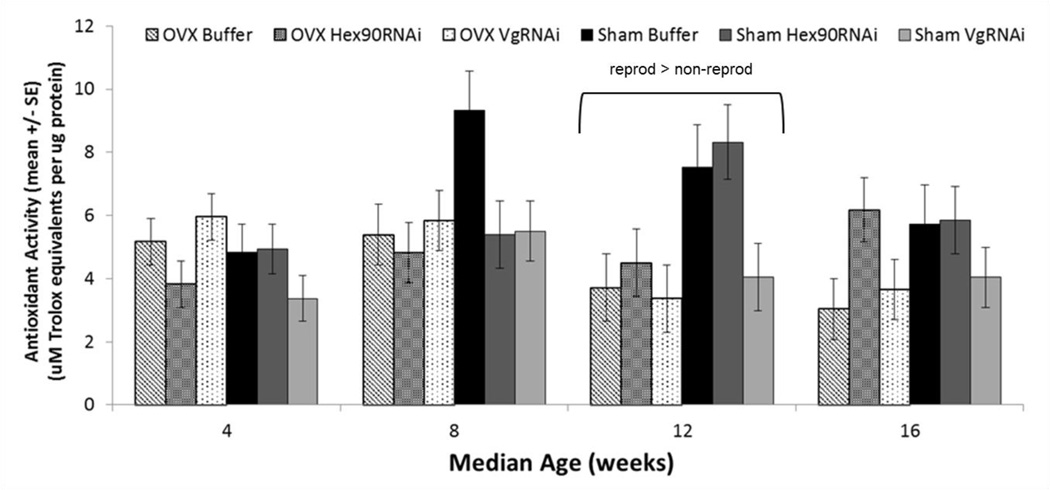
When all reproductive groups were compared with all reduced-reproduction groups, reproduction increased total anti-oxidant activity in the hemolymph at 12 weeks (MANOVA, F1,52 = 15.70, P = 0.0002), but not at the other times of sampling. There was a slight effect of age on anti-oxidant activity (Fig. 7; MANOVA, Pillai’s Trace F3,46 = 3.16, P = 0.034), probably due to somewhat higher levels at week 8 than at week 4. There was also a slight interaction of age and surgery (F3,46 = 3.19, P = 0. 032), due to higher antioxidant activities in sham than in ovariectomized lubber grasshoppers at 12 weeks. There was no interaction between age and injection on antioxidant activity (i.e., Buffer, Hex90RNAi or VgRNAi; F6,94 = 1.50, P = 0.185).
Fig. 7.
Total anti-oxidant activity (in uM Trolox equivalents per ug of protein) in hemolymph of fully reproductive (Sham Buffer and Sham Hex90RNAi) and reduced reproductive (OVX Buffer, OVX Hex90RNAi, OVX VgRNAi, and Sham VgRNAi) grasshoppers measured at 4 week intervals after adult molting. When all reproductive groups were compared with all reduced-reproduction groups, reproduction increased total anti-oxidant activity in the hemolymph at 12 weeks (MANOVA, F1,52 = 15.70, P = 0.0002)
Discussion
The goal of this study was to determine if reduction of vitellogenin mRNA extends lifespan, and if simultaneous ovariectomy and vitellogenin mRNA knockdown results in additive effects on lifespan extension. We hypothesized that the lubber grasshopper might exhibit additive effects on lifespan with a combination of ovariectomy and VgRNAi (i.e., OVX VgRNAi), implying that these two distinct manipulations for reducing reproduction would extend lifespan through different pathways. When used separately from each other, VgRNAi treatments and ovariectomy each result in similar increases in lifespan, body mass, and storage protein levels, and a similar decrease in feeding rate. At the same time, we show that vitellogenin protein levels in the hemolymph were much greater in ovariectomized animals than in VgRNAi treated animals, and VgRNAi treated animals have an intact ovary, which together demonstrate that the treatments are not identical. Despite the fact that they are different methods to reduce reproduction, both manipulations result in similar physiological responses that are not additive.
Lifespan was increased upon VgRNAi
Lubber grasshopper lifespan was markedly increased by VgRNAi. Reproduction is widely thought to have a costly effect on energy and maintenance in non-eusocial organisms, ultimately shortening lifespans (Hansen et al. 2013). Our VgRNAi treatment reduces reproduction by decreasing levels of vitellogenin mRNA and halting ovarian development (Tokar et al. 2014). We saw that Sham VgRNAi animals lived 13–21% longer than either of the sham-operated controls, similar to the extension of lifespan upon ovariectomy. Murphy et al. (2003) have shown that knockdown of each of two vitellogenins increased lifespan in C. elegans; thus vitellogenin knockdown may generally increase lifespan in non-eusocial animals.
Feeding rates decreased similarly upon ovariectomy and VgRNAi
Sham VgRNAi and ovariectomized animals each exhibited decreases in feeding rates relative to fully reproductive controls (i.e., Sham Buffer, and Sham Hex90RNAi). Animals with both ovariectomy and VgRNAi treatment maintained the same feeding levels as the other reduced reproduction groups, indicating that the changes to feeding were not additive. Drewry et al. (2011) show that ovariectomy reduces feeding (~40%) and proposed that other ways to reduce reproduction may also decrease feeding as well.
Despite the fact that dietary restriction may be the best studied means of life-extension across taxa, studies that achieve longevity via other treatments rarely report measures of feeding rates across the lifespan, perhaps because it is challenging in C. elegans and Drosophila (e.g., Min et al. 2007; Piper et al. 2007) and expensive in mice. Lubber grasshoppers offer the advantage of easy, repeatable measuring of feeding for each individual.
We expect that reducing reproduction may reduce feeding through hormones involved in feeding regulation. In several insects, including Drosophilia and honey bees, neuropeptide F increases forging behavior and feeding (e.g., Van Wielendaele et al. 2013). It is possible that ovariectomy and VgRNAi both decrease neuropeptide F or other similar endocrine signals, leading to reductions in feeding.
Judd et al. (2011) demonstrate that although the body mass of lubber grasshoppers increases upon ovariectomy, and there are increases in somatic storage, there is no increased allocation of ingested nutrients to somatic tissues. Our data shows increases in body mass upon VgRNAi, but OVX VgRNAi treatments did not show larger body masses than any other reduced reproductive group, suggesting that these pathways extending lifespan may overlap mechanistically.
Protein storage is increased upon VgRNAi
Late in life, all three hexameric storage proteins were increased upon ovariectomy, and similarly two of the three hexamerins (including hexamerin-90, which makes up ~80% of total storage protein) were increased upon VgRNAi treatment. Reduced reproduction and longevity are often linked with lipid storage, as is the case in C. elegans, mice, and lubber grasshoppers (e.g., Judd et al. 2011; Hatle et al. 2013; reviewed in Hansen et al. 2013). In addition to the ability to store lipids, insects (and crustaceans) are able to store amino acids. Hexamerins store amino acids for use in metamorphosis, reproduction, and development (Telfer and Kunkel, 1991; Wheeler et al. 1995, Burmester 1999). Proteins are vital for reproduction, especially on plant diets (e.g., Chapman 1998; Hatle et al. 2005), and in grasshoppers storage proteins are known to peak about two weeks before oviposition, then decline as proteins are invested in reproduction (Hatle et al. 2001). The increased protein storage observed in the present study may be an outcome of feeding ad libitum while blocking reproduction.
How might increase protein storage affect lifespan? In Drosophila, the abundance of dietary amino acids has a greater effect on lifespan than calories alone (Lee et al. 2008). In particular, restriction of dietary methionine extends lifespan in Drosophila that do not receive high levels of dietary amino acids (compare Grandison et al. 2009 and Lee et al. 2014). In contrast, our results suggest that any reduction in reproduction, regardless of method, may increase hexamerins in the hemolymph, and ultimately methionine availability. In particular, the hexamerin-270 of lubber grasshoppers contains approximately 4% methionine and therefore is classified as a methionine-rich hexamerin (Telfer and Kunkel 1991; Hathaway et al. 2009). In the present study, levels of hexamerin-270 in the hemolymph were increased at 8 weeks by VgRNAi and at 16 weeks by ovariectomy. Hence, in Drosophila dietary methionine restriction extends lifespan, while in grasshoppers a reduced reproduction-induced increase in methionine storage occurs during life extension. These seemingly contradictory roles of methionine in life extension in insects are of interest. A recent report showed that restriction of sulfur amino acids (i.e., methionine and cysteine) in mice results in increased hydrogen sulfide production rates, which contributes to longevity, and an increased production of hydrogen sulfide upon life extension was also observed in Drosophila (Hine et al. 2015). The hypothesis that methionine storage directly affects lifespan by increasing the production of cellular hydrogen sulfide remains to be tested.
Late life effects of early life treatments
Many of the effects of VgRNAi did not appear until later in life in our study, despite the fact that treatments were applied by the 5th day of adulthood. Increased hexamerin-90 protein levels, increased anti-oxidant activity, and decreased feeding, all became detectable between 8 weeks and 12 weeks of age. Together, this suggests that the alterations occur not as an immediate, direct result of vitellogenin mRNA reduction, but instead as a result of long-term effects of reducing reproduction. This also serves to clarify results from our earlier paper examining the reproduction and storage trade-off in young lubber grasshoppers upon VgRNAi (Tokar et al. 2014). In that paper, we observed no trade-off between reproductive protein and storage protein, however, we did not track protein storage beyond the first clutch (~5 weeks of age). In the present study, evidence of the trade-off becomes apparent in older individuals, as hemolymph storage proteins levels increase.
Vitellogenin may be regulated by pathways that also affect lifespan
The trade-off between reproduction and longevity is common in non-eusocial insects (Flatt 2011). Vitellogenin levels typically are negatively correlated with longevity; for example, C. elegans with reduced activity of vitellogenin encoding genes vit-2 and vit-5 have increased lifespan (Murphy et al., 2003). Vitellogenesis is regulated by juvenile hormone (JH) in non-dipteran insects, and JH is in turn regulated by nutritional signals that are controlled through the target of rapamycin (TOR; Maestro et al. 2009) and insulin-like signaling pathways (ILS; Seo et al. 2013). Both TOR and ILS are involved in lifespan extension (e.g., Johnson et al. 2013; Kaletsky and Murphy 2010). These two pathways share transcription factors (e.g., DAF-16/FOXO and HSF-1) and may be important in investing resources from somatic preservation to reproduction (DePina et al. 2011; Tullet 2015).
It has been hypothesized that vitellogenin has a vital role in lifespan regulation across species (Brandt et al. 2005; Ren and Hughes 2014). When methods to reduce reproduction were combined (OVX and VgRNAi), no additive physiological responses were observed, suggesting ovariectomy and VgRNAi each may extend lifespan by overlapping or convergent pathways. It has been documented that vitellogenesis is a product of the TOR and ILS longevity pathways in other invertebrates (e.g., Maestro et al. 2009). Therefore, it may be that VgRNAi affects these TOR and ILS pathways as well, with the pathways converging on DAF-16/FOXO. While manipulation of TOR or ILS signaling has not been tested with ovariectomy, we propose that ovariectomy may act on these pathways as well. These two treatments may affect different transcription factors that ultimately converge on these pathways.
Acknowledgements
We thank James Gelsleichter for use of his qPCR machine, Michael Lentz for sharing electrophoresis equipment, Tom Jackson for shipping lubber grasshoppers, Matt Williams for help feeding lubber grasshoppers, and members of the Hatle lab for discussion. Funded by NIA award 2R15AG028512-02A1 to JDH and NSF awards IOS-1051890 and IOS-1257298 to DAH.
Contributor Information
Alicia G. Tetlak, Email: alicia@tetlak.com, 8130 Baymeadows Way W Suite 200 Jacksonville, FL 33256, USA.
Jacob B. Burnett, Email: burnett@uga.edu, University of Georgia, Department of Genetics, 120 Green Street, Athens, GA 30602, USA.
Daniel A. Hahn, Email: dahahn@ufl.edu, University of Florida, Department of Entomology and Nematology, P.O. Box 110620 Gainesville, FL 32611, USA.
John D. Hatle, Email: jhatle@unf.edu, University of North Florida, Department of Biology, 1 UNF Drive, Jacksonville FL 32224, USA.
References
- Alic N, Hoddinott MP, Foley A, Slack C, Piper MDW, Partridge L. Detrimental Effects of RNAi: A Cautionary Note on Its Use in Drosophila Ageing Studies. PLOS One. 2012;(7):e45367. doi: 10.1371/journal.pone.0045367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD. Survival Analysis Using SAS®: A Practical Guide. second ed. Cary, NC: SAS Institute Inc.; 2010. [Google Scholar]
- Amdam GV, Simões ZLP, Hagen A, Norberg K, Schrøder K, Mikkelsen Ø, Kirkwood TB, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp Gerontol. 2004;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin, Kenyon C. Regulation of life-span by germ-line stem cells in C. elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Behmer ST. Insect Herbivore Nutrient Regulation. Ann Rev Entomol. 2009;54:165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- Borst DW, Eskew MR, Wagner SJ, Shores K, Hunter J, Luker L, Hatle JD, Hecht LB. Quantification of juvenile hormone III, vitellogenin, and vitellogenin-mRNA during the oviposition cycle of the lubber grasshopper. Insect Biochem Mol Biol. 2000;30:813–819. doi: 10.1016/s0965-1748(00)00053-9. [DOI] [PubMed] [Google Scholar]
- Brandt BW, Zwaan BJ, Beekman M, Westendorp RGJ, Slangboom PE. Shuttling between species for pathways of lifespan regulation: a central role for the vitellogenin gene family? BioEssays. 2005;27:339–346. doi: 10.1002/bies.20161. [DOI] [PubMed] [Google Scholar]
- Burmester T. Evolution and function of the insect hexamerins. Eur J Entomol. 1999;96:213–225. [Google Scholar]
- Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell. 2007;6:715–721. doi: 10.1111/j.1474-9726.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Chapman RF. The Insects: Structure and Function. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Cox RM, Parker EU, Cheney DM, Liebl AL, Martin LB, Calsbeek R. Experimental evidence for physiological costs underlying the trade-off between reproduction and survival. Functional Ecology. 2010;24:1–8. [Google Scholar]
- DeLoof A. Longevity and aging in insects: Is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J Insect Physiol. 2011;57:1–11. doi: 10.1016/j.jinsphys.2010.08.018. [DOI] [PubMed] [Google Scholar]
- DePina AS, Iser WB, Park S, Maudsley S, Wilson MA, Wolkow CA. Regulation of Caenorhabditis elegans vitellogenesis by DAF-2/IIS through separable transcriptional and posttranscriptional mechanisms. BMC Physiol. 2011;11:1–12. doi: 10.1186/1472-6793-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillen S, Zels S, Verlinden H, Spit J, Van Wielendaele P, Broeck J. Functional Characterization of the Short Neuropeptide F Receptor in the Desert Locust, Schistocerca gregaria. PLOS One. 2013;8:1–9. doi: 10.1371/journal.pone.0053604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry MD, Williams JM, Hatle JD. Life-extending dietary restriction and ovariectomy result in similar feeding rates but different physiologic responses in grasshoppers. Exp Gerontol. 2011;46:781–786. doi: 10.1016/j.exger.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tartar M. Drosophila germ-line modulation of insulin signaling and lifespan. PNAS. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T. Survival costs of reproduction in Drosophila. Exper. Geront. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Flatt T, Amdam GV, Kirkwood TB, Omholt SW. Life-history evolution and the polyphenic regulation of somatic maintenance and survival. Quart Rev Biol. 2013;88:185–218. doi: 10.1086/671484. [DOI] [PubMed] [Google Scholar]
- Gems D, Pletcher S, Partridge L. Interpreting interactions between treatments that slow aging. Aging Cell. 2002;1:1–9. doi: 10.1046/j.1474-9728.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- Ghazi A. Transcriptional networks that mediate signals from reproductive tissues to influence lifespan. Genesis. 2012;51:1–15. doi: 10.1002/dvg.22345. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:24–31. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudeau J, Bellemin S, Toselli-Mollereau E, Shamalnasab S, Chen Y, Aguilaniu H. Fatty acid desaturation links germ cell loss to longevity through NHR•80/HNF4 in C. elegans. PLOS Biol. 2011;9:1–16. doi: 10.1371/journal.pbio.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: what Is the connection? Cell Metab. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway M, Hatle JD, Li S, Ding X, Barry T, Hong F, Wood H, Borst D. Characterization of hexamerin proteins and their mRNAs in the adult lubbergrasshopper: The effects of nutrition and juvenile hormone on their levels. Comp Biochem Physiol A. 2009;154:323–332. doi: 10.1016/j.cbpa.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Eskew MR, Juliano SA. Maximum titers of vitellogenin and total hemolymph protein occur during the canalized phase of grasshopper egg production. Physiol Biochem Zool. 2001;74:885–893. doi: 10.1086/324475. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Julanio SA. Hemolymph ecdysteroids do not affect vitellogenesis in the lubber grasshopper. Arch of Insect Biochem and Physio. 2003;52:45–57. doi: 10.1002/arch.10067. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Waskey T, Jr, Juliano SA. Plasticity of grasshopper vitellogenin production in response to diet is primarily a result of changes in fat body mass. J Comp Physiol B. 2005;176:27–34. doi: 10.1007/s00360-005-0028-9. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Wells SM, Fuller E, Allen IC, Gordy LJ, Melnyk S, Quattrochi J. Calorie restriction and late-onset calorie restriction extend lifespan but do not alter protein storage in female grasshoppers. Mech Aging Devel. 2006;127:883–891. doi: 10.1016/j.mad.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatle JD, Paterson CS, Jawaid I, Lentz C, Wells SM, Fronstin RB. Protein accumulation underlying lifespan extension via ovariectomy in grasshoppers is consistent with the disposable soma hypothesis but is not due to dietary restriction. Exp Gerontol. 2008;43:900–908. doi: 10.1016/j.exger.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatle JD, Kellenberger JW, Viray E, Smith AM, Hahn D. Life-extending ovariectomy in grasshoppers increases somatic storage, but dietary restriction with an equivalent feeding rate does not. Exp Gerontol. 2013;48:966–972. doi: 10.1016/j.exger.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C, Harputugil E, Zhang Y, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR as a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd ET, Wessels FJ, Drewry MD, Grove M, Wright K, Hahn DA, Hatle JD. Ovariectomy in grasshoppers increases somatic storage, but proportional allocation of ingested nutrients to somatic tissues is unchanged. Aging Cell. 2011;10:972–979. doi: 10.1111/j.1474-9726.2011.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R, Murphy CT. The role of IFG/insulin-like signaling in C. elegans longevity and aging. Disease Models Mech. 2010;3:415–419. doi: 10.1242/dmm.001040. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Nature. 1977;24:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of senescence - late survival sacrificed for reproduction. Phil Trans R Soc Lond B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. Repression of the heat shock response is a programmed event at the onset of reproduction. Molecular Cell. 2015 doi: 10.1016/j.molcel.2015.06.027. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kaya, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino acid status. Nat Comm. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signaling. Nature Cell Biol. 2008a;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Lee H-Y, Lee S-H, Min K-J. Insects as model systems for aging studies. Entomol Res. 2015;45:1–8. [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Nat Acad Sci. 2008b;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Gelion S, Melendez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro JL, Cobo J, Bellés X. Target of Rapamycin (TOR) Mediates the Transduction of Nutritional Signals into Juvenile Hormone Production. J Biol Chem. 2009;284:5506–5513. doi: 10.1074/jbc.M807042200. [DOI] [PubMed] [Google Scholar]
- Mair W, Sgro CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exper Geront. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Maier T, Güell M, Serrano J. Correlation of mRNA and protein in complex biological samples. FEBS Letters. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- McCormick M, Chen K, Ramaswamy P, Kenyon C. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell. 2012;11:192–202. doi: 10.1111/j.1474-9726.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Bush S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarrol SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- O’Brien DM, Min K-J, Larsen TL, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr Biol. 2008;18:R155–R156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Piper M, Mair W, Partridge L. Comment by Matthew Piper, William Mair, Linda Partridge on Min KJ., Flatt T., Kulaots I., Tatar M., 2007 “Counting calories in Drosophila dietary restriction”. Exp Gerontol. 2007;42:253–255. doi: 10.1016/j.exger.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ, U.S. National Institutes of Health. Bethesda, Maryland, USA: 1997–2014. http://imagej.nih.gov/ij/ [Google Scholar]
- Ren Y, Hughes KA. Vitellogenin family gene expression does not increase Drosophila lifespan or fecundity [v1; ref status: indexed, http://f1000r.es/3ac] F1000Research. 2014;3:125. doi: 10.12688/f1000research.3975.1. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rion S, Kawecki TJ. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol. 2007;20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- Rose MR, Charlesworth B. A test of evolutionary theories of senescence. Nature. 1980;287:141–142. doi: 10.1038/287141a0. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radi Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehuus S, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. PNAS. 2006;103(4):962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K, Choi E, Lee D, Jeong D, Jang SK, Lee S. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans. Aging Cell. 2013;12:1073–1081. doi: 10.1111/acel.12140. [DOI] [PubMed] [Google Scholar]
- Sgró CM, Partridge L. A Delayed Wave of Death from Reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Król E. The heat dissipation limit theory and evolution of life histories in endotherms—time to dispose of the disposable soma theory? Integ Comp Biol. 2010;50:793–807. doi: 10.1093/icb/icq049. [DOI] [PubMed] [Google Scholar]
- Tang B, Wang S, Zhang F. Two storage hexamerins from the beet armyworm Spodoptera exigua: cloning, characterization and the effect of gene silencing on survival. BMC Mol Biol. 2010;11:1–13. doi: 10.1186/1471-2199-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. The plate half-full: status of research on the mechanisms of dietary restriction in Drosophila melanogaster. Exp Geron. 2011;46:363–368. doi: 10.1016/j.exger.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer WH, Kunkel JG. The function and evolution of insect storage hexamerins. Ann Rev Entomol. 1991;36:205–228. doi: 10.1146/annurev.en.36.010191.001225. [DOI] [PubMed] [Google Scholar]
- Tokar DR, Veleta KV, Canzano J, Hahn DA, Hatle JD. Vitellogenin RNAi halts ovarian growth and diverts reproductive proteins and lipids in young grasshoppers. Integ Comp Biol. 2014;54:931–941. doi: 10.1093/icb/icu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JMA. DAF-16 target identification in C. elegans: past, present and future. Biogerontology. 2015;16:221–234. doi: 10.1007/s10522-014-9527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wielendaele P, Dillen S, Zels S, Badisco L, Vanden Broeck J. Regulation of feeding by Neuropeptide F in the desert locust, Schistocerca gregaria. Insect Biochem Mol Bio. 2013;43:102–114. doi: 10.1016/j.ibmb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Walker MP, Lewis CJ, Whitman DW. Effects of males on the fecundity and fertility of female Romalea microptera grasshoppers. J Orthoptera Res. 1999;8:277–283. [Google Scholar]
- Wang M, O'Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DE, Martínez T. Storage proteins in ants (Hymenoptera:Formicidae) Comp Biochem Physiol. 1995;112:15–19. doi: 10.1016/0305-0491(95)00035-7. [DOI] [PubMed] [Google Scholar]
- Wheeler DE. The role of nourishment in oogenesis. Ann Rev Entomol. 1996;41:407–431. doi: 10.1146/annurev.en.41.010196.002203. [DOI] [PubMed] [Google Scholar]
- Williams JB, Roberts SP, Elekonich M. Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Exp Gerontol. 2010;43:538–549. doi: 10.1016/j.exger.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Yanagi S, Miyatake T. Costs of mating and egg production in female Callosobruchus chinensis. J Insect Physiol. 2003;49:823–827. doi: 10.1016/S0022-1910(03)00119-7. [DOI] [PubMed] [Google Scholar]