Abstract
Background
Intramural ventricular septal defects (VSDs) are interventricular communications through right ventricular free wall trabeculations that can occur after repair of conotruncal anomalies. We assessed the prevalence of residual intramural VSDs and their effect on postoperative course.
Methods and Results
Children who underwent biventricular repair of a conotruncal anomaly from 1/1/06 to 6/30/13 and had a post-operative transthoracic echocardiogram were included. Images were reviewed for residual intramural or non-intramural VSDs. The primary outcome was a composite of mortality, extracorporeal membrane oxygenation (ECMO) use, and need for subsequent catheter or surgical VSD closure. The secondary outcome was post-operative hospital length of stay (PLOS). A residual VSD was present in 256 of the 442 subjects (58%), of which 231 (90%) were <2mm in size. Forty-nine (11%) had intramural VSDs and 207 (47%) had non-intramural VSDs. Patients with intramural VSDs were more likely to reach the primary composite outcome compared to those with non-intramural VSDs or no residual VSD (14/49 [29%] vs 15/207 [7%] vs 6/186 [3%], p<0.0001). In addition, those with intramural VSDs had longer PLOS compared to those with non-intramural VSDs or no residual VSD (20 days [IQR 11-42] vs 7 days [5-14] vs 6 [4-11], p=0.0001). These associations remained significant after adjusting for known risk factors for poor outcomes, including residual VSD size and operative complexity.
Conclusions
Among residual VSDs after repair of conotruncal anomalies, intramural VSDs are uniquely associated with postoperative morbidity, mortality, and longer PLOS. It is important to recognize intramural VSDs in the postoperative period.
Keywords: heart defects, congenital, pediatrics, cardiac surgery, conotruncal malformations, ventricular septal defect
Introduction
An intramural ventricular septal defect (VSD) is a residual VSD that can be seen after surgical repair of conotruncal defects that involve patch closure of a VSD from the left ventricle to a great artery. The term “intramural” VSD was coined by Preminger et al in a brief report 20 years ago1. It is defined as a tunnel-like communication that can occur when the VSD patch is not anchored to the right ventricular free wall but rather is attached to right ventricular trabeculations such that blood can flow around the VSD patch and into the right ventricular cavity (Figure 1).
Figure 1.
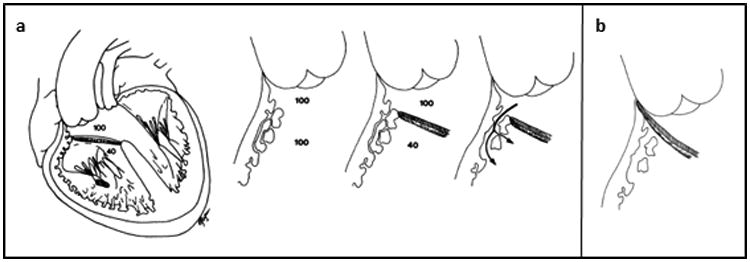
a. Left, Diagrammatic representation of “intramural” defects. Ventricular septal defect patch is anchored to right ventricular (RV) trabeculations rather than free wall; blood can pass between the trabeculae from the neo-left ventricular outflow tract to the RV cavity. Right, Magnified schematic representation of “intramural” defects. The channels might be small early after surgical repair of the conotruncal anomaly because of RV hypertrophy and enlarge to become hemodynamically significant with regression of hypertrophy after RV decompression. b. Diagrammatic representation of optimal surgical positioning of the ventricular septal defect patch required to achieve complete closure. Reproduced from: Preminger et al. Circulation. 1994; 89:236-242.
Intramural VSDs have not been well studied since their first description1, and little is known about their prevalence, associations, and impact on surgical outcome. Preminger's original case series described eight patients with intramural VSDs; all had right ventricular hypertension and underwent multiple unsuccessful reoperations. This series illustrated that intramural VSDs were distinct from other types of residual VSDs and could result in poor outcome. Another study found that four patients with no VSD or only small VSD visualized on intraoperative transesophageal echocardiogram were subsequently found to have large intramural VSDs requiring reoperation2, suggesting that intramural defects may be challenging to identify intraoperatively and may enlarge over time. Finally, there has been one other case series in which three of five children with intramural VSDs required reoperation for VSD closure3. These reports suggest that intramural VSDs are clinically important and may contribute to post-operative morbidity and mortality.
In this study, we sought to identify the prevalence of intramural VSDs in patients who have undergone biventricular repair of conotruncal defects and to determine whether intramural VSDs are associated with post-operative morbidity and mortality. We hypothesized that intramural VSDs were more likely to result in a worse outcome (re-intervention, mortality, extracorporeal membrane oxygenation [ECMO] use) and longer post-operative hospital length of stay (PLOS) compared to non-intramural (peripatch or muscular) types of residual VSD.
Methods
We performed a retrospective cohort study of all children ages 0 to 18 years at our institution who underwent biventricular repair of a conotruncal anomaly from 1/1/06 to 6/30/13. Hospital Institutional Review Board approval was obtained and waiver of consent granted.
Patient Selection
The institutional surgical database was queried for all surgical procedures during the study time period in patients with conotruncal anomalies, defined as tetralogy of Fallot, d-transposition of the great arteries, truncus arteriosus, double outlet right ventricle, l-transposition of the great arteries, anterior malalignment (Eisenmenger type) VSD, posterior malalignment VSD, double outlet left ventricle, and aorta arising from the right ventricle with pulmonary atresia. Patients were included if they were aged 0 to 18 years, underwent biventricular repair that included patch closure of a VSD from the left ventricle to a great artery (i.e. VSD baffle to the aortic valve or a truncal valve, or VSD baffle to the pulmonary valve with an arterial switch operation or Damus-Kaye-Stansel procedure), and had a post-operative transthoracic echocardiogram (TTE) that evaluated for residual VSD (adequate sweeps of the interventricular septum in 2D and color Doppler in two or more views).
Echocardiographic evaluation
The images of all first post-operative TTEs were reviewed (by MSC) for type of residual VSD. Intramural VSDs were defined as communications located anterior to the VSD patch between the great artery and the right ventricular trabeculations; to meet the definition, the VSD patch had to be seen attached to the right ventricular trabeculations rather than anchored to the right ventricular free wall adjacent to the annulus of the semilunar valve (Figure 2). Residual non-intramural VSDs were defined as peripatch VSD (defect associated with the VSD patch, with appropriate patch placement adjacent to the annulus of the semilunar valve) or muscular VSD (within the muscular septum). Patients who had both an intramural and a non-intramural VSD were classified in the intramural VSD group. VSDs were measured and categorized as ≤2 mm or >2mm, as studies have reported that most VSDs ≤2mm close spontaneously and/or do not typically require re-intervention 2, 4. Additionally, the most recent echocardiogram for all patients with residual VSD who did not require re-intervention was reviewed for presence and size of a residual VSD on follow-up.
Figure 2.
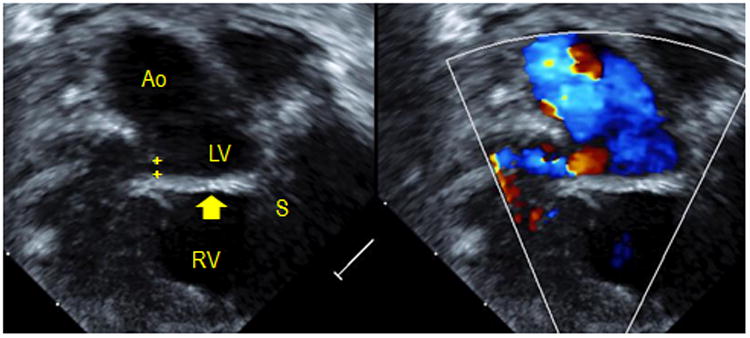
Intramural ventricular septal defect (VSD) seen by transthoracic echocardiography. Left, a 2D image demonstrates the VSD patch (arrow) connecting the crest of the ventricular septum (S) to the right ventricle (RV) free wall instead of the base of the aortic valve (Ao). A communication (crosshairs) is seen with flow in the RV free wall. Right, flow through this defect is apparent with color imaging.
Chart review
Data on hospital course were abstracted from inpatient and outpatient medical records. Parameters assessed included baseline demographics and potential clinical risk factors for postoperative morbidity and mortality 5-9 (Supplemental Table 1). The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery STAT mortality categories10 and operative times were abstracted from the institutional surgical database. Significant comorbidity was defined as a major medical condition requiring operative or medical treatment. Prematurity was defined as gestational age less than 37 weeks.
The primary outcome event was a composite of mortality, ECMO use, or re-intervention on the residual VSD via surgical or catheter procedure. A composite outcome was used in order to have a large enough number of outcomes to allow for adjustment of potential confounders of the primary association between residual VSD and outcome. The secondary end point was post-operative hospital length of stay (PLOS). Given that some subjects, such as premature infants, had long hospitalizations prior to their surgery, PLOS was selected as more relevant to the operation's effect on hospitalization than total hospital length of stay. Primary outcome events were ascertained for 30 days after operation or until hospital discharge if the PLOS was greater than 30 days.
Inter-rater agreement
To evaluate the inter-rater reliability for identification of intramural VSDs, another experienced echocardiography reader (SN) reviewed 10% of TTEs and inter-rater agreement was calculated by Kappa statistic.
Statistical Methods
Descriptive statistics (expressed as count [percentage] or median [interquartile range]) were employed. Chi-square test and Kruskal-Wallis equality-of-populations rank test were employed to evaluate categorical and continuous variables between patients with residual intramural VSD (including those with a residual non-intramural VSD as well), patients with only residual non-intramural VSD (peripatch or muscular defects), and patients with no residual VSD. Continuous variables were converted to dichotomous variables on the basis of standards from previous literature and from review of the distribution of our data. The decision was made to analyze the STAT mortality category as a continuous variable as the mortality rate proceeded in a fairly continuous fashion with an approximate two-fold increase in mortality rate with each incremental increase in STAT category10. Bivariable analysis was performed to identify risk factors for the primary composite outcome as well as for the logarithm of the secondary outcome of PLOS using logistic and linear regression. Multivariable regression models were then built to identify factors independently associated with outcome event and the logarithm of PLOS. Covariates with a p value <0.2 on bivariate analysis were considered for inclusion in the final multivariable model and retained if p<0.05 or if there was evidence for significant confounding or effect modification. When multiple covariates were found to be highly collinear, the variable that produced the model with the lowest Akaike Information Criterion score (post-test goodness-of-fit measure) was selected. Significant p values were determined a priori as < 0.05. All statistical analyses were performed with STATA software (version 12.1, StataCorp., College Station, Texas).
Results
Demographics
Review of the surgical database yielded 903 surgical procedures in patients with conotruncal defects over the seven-year study period. Of these, 462 were biventricular repair of conotruncal defect involving baffle closure of a VSD from the left ventricle to a great artery. Nineteen patients were excluded because they did not have post-operative TTE to adequately evaluate for residual VSD, and one was excluded who was older than 18 years, leaving 442 patients who met inclusion criteria for analysis.
Post-operative TTE was performed at a median of 4 (2-7) days after surgery. In the cohort, 256 patients (58%) had at least one residual VSD; 19 of the 256 (7%) patients had two types of residual VSD. With regard to residual VSD type, 49 (11%) subjects had intramural VSDs, 207 (47%) subjects had only non-intramural VSDs (peripatch or muscular defects) and 186 (42%) subjects had no residual VSD.
Characteristics of the cohort based on type of residual VSD are summarized in Table 1. There were differences in age at operation across residual VSD types with those with non-intramural VSD being the youngest (64 [IQR 6-120] days) and those with no residual VSD being the oldest (93 [IQR 10-139] days) at the time of operation. Residual intramural VSDs were seen after repair of all types of conotruncal defects, with a disproportionate number occurring in the less common anomalies, such as anterior malalignment (Eisenmenger type) VSD and aorta arising from the right ventricle with pulmonary atresia. The occurrence of an intramural VSD did not vary by gender or type of surgery performed. The vast majority (231/256 [90%]) of subjects with residual VSD had defects less than 2mm in diameter. Intramural VSDs were more likely to be larger (>2mm in diameter) than other types of residual VSDs (19/49 [39%] vs 6/207 [3%], p<0.0001).
Table 1. Patient characteristics by presence and type of residual ventricular septal defect.
| Intramural VSD | Non-Intramural VSD | No Residual VSD | Total | p | |
|---|---|---|---|---|---|
| Number of Subjects | 49* (11%) | 207† (47%) | 186 (42%) | 442 | |
| Gender (female) | 17 (35%) | 90 (43%) | 87 (47%) | 194 (43%) | 0.3 |
| Age at operation (d) | 83 (16-127) | 64 (6-120) | 93 (10-139) | 79 (7-131) | 0.03 |
| Age at operation <30 days | 16 (33%) | 84 (41%) | 53 (28%) | 153 (35%) | 0.04 |
| Weight at operation (kg) | 4.4 (2.9-5.9) | 4.2 (3.3-6.0) | 4.9 (3.4-6.0) | 4.6 (3.3-6.0) | 0.07 |
| Birth weight (kg) | 2.8 (2.4-3.3) | 3.1 (2.6-3.5) | 3.0 (2.5-3.5) | 3.1 (2.5-3.5) | 0.2 |
| Prematurity | 12 (24%) | 40 (20%) | 44 (24%) | 96 (22%) | 0.6 |
| Additional congenital abnormality or comorbidity | 10 (20%) | 33 (16%) | 21 (11%) | 64 (14%) | 0.19 |
| Genetic syndrome | 13 (27%) | 31 (15%) | 32 (17%) | 76 (17%) | 0.14 |
| Diagnosis: | |||||
| - TOF | 33 (67%) | 154 (74%) | 132 (71%) | 319 (72%) | 0.003 |
| - Truncus Arteriosus | 2 (4%) | 15 (7%) | 21 (11%) | 38 (9%) | |
| - DORV | 3 (6%) | 14 (7%) | 3 (2%) | 20 (5%) | |
| - D-TGA with VSD | 4 (8%) | 20 (10%) | 21 (11%) | 45 (10%) | |
| - Other | 7 (14%) | 4 (2%) | 9 (5%) | 20 (5%) | |
| - Anterior malalignment (Eisenmenger type) VSD | 2 (4%) | 0 | 2 (1%) | 4 (1%) | |
| - Aorta from RV with PA | 2 (4%) | 1 (0.5%) | 1 (0.5%) | 4 (1%) | |
| - L-TGA with VSD | 1 (2%) | 0 | 4 (2%) | 5 (1%) | |
| - DOLV | 1 (2%) | 0 | 0 | 1 (0.2%) | |
| - Posterior malalignment VSD‡ | 1 (2%) | 3 (1.5%) | 2 (1%) | 6 (1%) | |
| History of prior surgical palliation | 3 (6%) | 3 (1%) | 9 (5%) | 15 (3%) | 0.3 |
| Operation | 0.15 | ||||
| - VSD closure, transannular patch | 28 (57%) | 101 (49%) | 84 (45%) | 213 (48%) | |
| - VSD Closure§ | 8 (16%) | 45 (22%) | 42 (23%) | 95 (22%) | |
| - Rastelli procedure | 8 (16%) | 37 (18%) | 34 (18%) | 79 (18%) | |
| - ASO and VSD closure | 2 (4%) | 19 (9%) | 17 (9%) | 38 (9%) | |
| - Yasui procedure | 1 (2%) | 3 (1%) | 2 (1%) | 6 (1%) | |
| - Nikaidoh procedure | 0 | 2 (1%) | 2 (1%) | 4 (1%) | |
| - Atrial and arterial switch, VSD closure | 0 | 0 | 4 (3%) | 4 (1%) | |
| - Atrial switch and Rastelli procedure | 2 (4%) | 0 | 1 (0.5%) | 3 (1%) | |
| Bypass time (min) | 80 (53-131) | 65 (42-85) | 68 (41-97) | 67 (42-93) | 0.02 |
| STAT Mortality Category | 0.11 | ||||
| - Category 1 | 8 (16%) | 42 (20%) | 39 (21%) | 89 (20%) | |
| - Category 2 | 25 (51%) | 106 (51%) | 86 (46%) | 217 (49%) | |
| - Category 3 | 4 (8%) | 3 (1%) | 3 (2%) | 10 (2%) | |
| - Category 4 | 12 (24%) | 56 (27%) | 58 (31%) | 126 (29%) | |
| VSD size > 2mm | 19 (39%) | 6 (3%) | NA | 25 (6%) | <0.0001 |
| Post-operative day TTE performed | 4 (2-7) | 4 (2-7) | 4 (3-7) | 4 (2-7) | 0.5 |
Data listed as count (percent) or median (interquartile range). Abbreviations: VSD (Ventricular septal defect), TOF (Tetralogy of Fallot), DORV (Double outlet right ventricle), TGA (Transposition of the great arteries), RV (Right Ventricle), PA (pulmonary atresia), DOLV (Double outlet left ventricle), Arterial switch operation (ASO).
7 with peripatch VSDs and 2 with muscular VSDs as well
181 with peripatch VSDs, 16 with muscular VSDs, 10 with both
undergoing Yasui procedure
includes those with muscle bundle resection or non-transannular patch placement as well
Primary Outcome Events
The composite outcome event (death, ECMO use, or VSD re-intervention) occurred more frequently in subjects with intramural VSDs compared to those with only non-intramural VSDs or no residual VSD (14/49 [29%] vs 15/207 [7%] vs 6/186 [3%], p<0.0001) (Table 2). Additionally, all the individual component events of the composite outcome occurred more frequently in subjects with intramural VSD compared to the other subjects.
Table 2.
Outcome events during hospitalization or within 30 days of operation.
| Intramural VSD (n=49) | Non-Intramural VSD (n=207) | No Residual VSD (n=186) | Total (n=442) | p | |
|---|---|---|---|---|---|
| Primary Outcome Events: | |||||
| Composite | 14 (29%) | 15 (7%) | 6 (3%) | 35 (8%) | <0.0001 |
| Catheter VSD closure | 3 (6%) | 2 (1%) | 0 | 5 (1%) | 0.001 |
| Surgical VSD closure | 7 (14%) | 7 (3%) | 0 | 14 (3%) | <0.0001 |
| ECMO | 8 (16%) | 7 (3%) | 3 (2%) | 18 (4%) | <0.0001 |
| Mortality | 5 (10%) | 4 (2%) | 4 (2%) | 13 (3%) | 0.006 |
| Secondary Outcome Events: | |||||
| Post-operative LOS (days) | 20 (11-42) | 7 (5-14) | 6 (4-11) | 7(5-16) | 0.0001 |
| Cardiac ICU LOS (days) | 11 (5-28) | 7 (4-13) | 6 (3-10) | 7 (4-12) | 0.001 |
| Cardiac arrest | 8 (16%) | 9 (4%) | 10 (5%) | 27 (6%) | 0.007 |
| Arrhythmia requiring therapy | 23 (47%) | 67 (33%) | 51 (28%) | 141 (32%) | 0.03 |
| Days intubated | 7 (1-23) | 1 (0-4) | 1 (0-3) | 1 (0-4) | 0.0001 |
| Days on inotropic agent | 3 (0-7) | 1 (0-3) | 1 (0-2) | 1 (0-3) | 0.003 |
| Days on milrinone | 6 (2-17) | 3 (2-5) | 2 (2-4) | 3 (2-5) | 0.0001 |
| Days with chest tube | 3 (2-9) | 2 (2-3) | 2 (2-3) | 2 (2-3) | 0.002 |
Data expressed as count (percentage) or median (IQR). Abbreviations: VSD (Ventricular septal defect), ECMO (Extra-corporeal membrane oxygenation), Length of stay (LOS), Intensive Care Unit (ICU).
Bivariable and multivariable models of potential risk factors for composite outcome event were created (Table 3). In the unadjusted analysis, subjects with a residual intramural VSD and non-intramural VSD were more likely to have an outcome event compared to those with no residual VSD (OR 12.0 [6.3-33.4], p<0.0001 and 2.3 [0.89-6.2], p=0.09). A multivariable model demonstrated that the presence of an intramural VSD (OR 4.3 [1.2-15.6], p=0.03) was a significant independent risk factor for the outcome event, after controlling for VSD size, additional congenital abnormality or comorbidity, STAT mortality category, and birth weight.
Table 3.
Risk factors for composite primary outcome event.
| Bivariable | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Type of VSD: | ||||
| No VSD | 1.0 | -- | 1.0 | -- |
| Intramural | 12.0 (6.3-33.4) | <0.0001 | 4.9 (1.1-21) | 0.03 |
| Non-Intramural | 2.3 (0.89-6.2) | 0.09 | 2.4 (0.84-6.7) | 0.1 |
| VSD >2mm | 10.4 (4.3-25.6) | <0.0001 | 5.4 (1.3-23) | 0.02 |
| Additional congenital abnormality or comorbidity | 3.6 (1.7-7.6) | 0.001 | 3.6(1.5-9.1) | 0.006 |
| Prematurity | 2.5 (1.2-5.2) | 0.01 | ||
| STAT Mortality Category* | 1.7 (1.3-2.4) | 0.001 | 2.3 (1.5-3.5) | <0.0001 |
| Bypass time (10 minutes) | 1.2 (1.1-1.3) | <0.0001 | ||
| Weight at operation (1.0 kg) | 0.84 (0.69-1.0) | 0.09 | ||
| Birth weight (1.0 kg) | 0.48 (0.30-0.76) | 0.002 | 0.39 (0.22-0.68) | 0.001 |
Gender, age at operation, history of prior operation, and presence of genetic syndrome were not associated with outcome event (p>0.2).
Abbreviations: Ventricular septal defect (VSD)
As described in O'Brien et al. JTCVS 2009
There were 7 surgical and 3 catheter based re-interventions on a total of 9 of the 49 patients (18%) with residual intramural VSDs. The VSD size was greater than 2mm on the first post-operative TTE for 7 of the 9 (78%) patients who subsequently underwent re-intervention and 12 of the 40 (30%) of patients who did not (p=0.008). Catheterization was performed post-operatively on 8 of the children who had re-intervention and 13 of those who did not have re-intervention. Children who underwent re-intervention had a higher ratio of pulmonary to systemic blood flow (2.1 [1.6-2.9] vs 1.3 [1.0-1.6], p=0.03). Additionally, children who underwent re-intervention had a longer time on inotropic agents (10 days [7-11] vs 2 days [0-5], p=0.0005) and on ventilator support (30 days [14-47] vs 4 days [0-11], p=0.001) during their hospital stay.
Post-operative Length of Stay and Hospital Course
The PLOS was significantly longer in subjects with residual intramural VSDs compared to those with residual non-intramural VSDs or no residual VSD (20 days [IQR 11-42] vs 7 days [5-14] vs 6 [4-11], p=0.0001). Bivariable and multivariable analyses were performed to evaluate the association of potential risk factors with PLOS (Table 4). The multivariable model demonstrated that presence of intramural residual VSD remained significantly associated with longer PLOS, after controlling for VSD size, STAT mortality category, birth weight, and the presence of a genetic syndrome.
Table 4.
Risk factors for post-operative hospital length of stay (PLOS).
| Bivariable | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| Variable | Coefficient (95% CI) | p | Coefficient (95% CI) | p |
| Type of VSD: | ||||
| No VSD | Reference | -- | Reference | -- |
| Intramural | 0.93 (0.64-1.2) | <0.0001 | 0.64 (0.32-0.95) | <0.0001 |
| Non-Intramural | 0.12 (-0.066-0.30) | 0.21 | 0.16 (-0.0069-0.32) | 0.06 |
| VSD >2mm | 1.0 (0.64-1.4) | <0.0001 | 0.43 (0.018-0.84) | 0.04 |
| Additional congenital abnormality or comorbidity | 0.47 (0.22-0.72) | <0.0001 | ||
| Prematurity | 0.46 (0.24-0.67) | <0.0001 | ||
| Genetic syndrome | 0.48(0.25-0.72) | <0.0001 | 0.38 (0.18-0.58) | <0.0001 |
| STAT mortality category* | 0.37 (0.29-0.44) | <0.0001 | 0.36 (0.29-0.43) | <0.0001 |
| Bypass time (10 minutes) | 0.13 (0.11-0.15) | <0.0001 | ||
| Weight at operation (kg) | -0.082 (-0.12- -0.048) | <0.0001 | ||
| Birth weight (kg) | -0. 34 (-0.46- -0.21) | <0.0001 | -0.34 (-0.44- -0.23) | <0.0001 |
Gender, age of operation, history of prior operation and right ventricular outflow tract obstruction on post-operative TTE not associated with PLOS (p>0.2)
Abbreviations: Ventricular septal defect (VSD)
As described in O'Brien et al. JTCVS 2009
In addition to longer PLOS, subjects with residual intramural VSD also had more complicated hospital courses (Table 2). They were more likely to have cardiac arrest or arrhythmia requiring therapy. They also had more days requiring intubation, chest tubes, inotropic agents, and milrinone therapy.
Follow up of residual defects
Of the 237 subjects with a residual VSD on post-operative TTE who did not have early surgical or catheter re-intervention, 183 had a follow-up TTE performed a median of 1.6 (0.5-4.0) years after the operation. On follow-up TTE, a residual VSD was present in 10 of 33 (30%) of subjects with previously visualized intramural VSDs and 43 of 150 (29%) of those with non-intramural VSDs (p = 0.9). There were no subjects in the intramural VSD group and two subjects in the non-intramural VSD group who had VSD >2mm on follow up echocardiogram (p = 0.5). During follow-up, one subject underwent reoperation for VSD patch dehiscence, and six subjects had small residual VSDs closed as part of another procedure (i.e. right ventricle to pulmonary artery conduit replacement or right ventricular outflow tract pseudoaneurysm resection).
Inter-rater Agreement
In the identification of intramural VSDs based on post-operative TTE, there was high inter-rater agreement with a Kappa statistic of 0.884 (95% CI 0.662-1.000).
Discussion
To our knowledge, this study represents the first comprehensive evaluation of the prevalence, risk factors, and outcomes in patients with conotruncal malformations who have residual intramural VSDs after surgery. It is likely that these defects have generally not been recognized as a distinct clinical entity from other residual VSDs in conotruncal repairs.
In this study, we evaluated 442 patients with conotruncal defects undergoing repair. Operations reflected the modern surgical strategy at our institution with the majority of surgical repairs occurring in the first few months of life. Our rate of residual VSD detected by post-operative TTE was 68%; this is similar to a prior study reporting a rate of 52%11 and higher than another study with a rate of 38%12. The vast majority (90%) of subjects in our study with residual VSD had small defects (<2mm).
In our cohort, intramural VSDs occurred in 11% of children after repair of conotruncal anomalies and comprised 19% of all residual VSDs. There was no association between early age at repair and intramural VSD suggesting that these defects are not occurring because of small patient size.
Case reports have described intramural VSDs after surgery for d-transposition of the great arteries, tetralogy of Fallot, double outlet right ventricle, and truncus arteriosus1-3; but whether they occur in other types of conotruncal defects has not been previously reported. We found that intramural VSDs occurred in subjects after biventricular repair of any type of conotruncal defect that involved baffle closure of the VSD from the left ventricle to a great vessel. While the majority (33/49, 67%) of intramural VSDs were seen in patients with tetralogy of Fallot (the most common conotruncal defect) the occurrence rate of intramural VSDs was higher in patients with less common forms of conotruncal defects, such as double outlet left ventricle or aorta arising from the right ventricle with pulmonary atresia. We hypothesize that this increased occurrence rate may be due to the challenge of optimal VSD patch placement in these anatomically complex conotruncal defects. While we did not identify an association between occurrence of intramural VSD and type of operation performed, we did observe that the rate of intramural VSDs was higher in complex operations such as the combined atrial switch and Rastelli procedures.
We found that intramural VSDs were more likely to be larger than non-intramural VSDs, which may in part explain their associated morbidity and mortality. However, intramural VSDs were still associated with worse outcomes even after adjusting for residual VSD size, suggesting that there are other characteristics of these defects that may affect surgical outcome. We surmise that some of the intramural VSDs that were small on the first post-operative TTE may have subsequently become larger. They may also be larger than they appear by TTE because of their complex serpiginous course; thus, TTE may underestimate their size. From an anatomic standpoint, we believe these VSDs may enlarge over time because the defects are not between stitches of a patch but rather through right ventricular free wall trabeculations. There may be more impetus for flow across an intramural VSD as the patch pulls further away from the right ventricular free wall. Preminger et al hypothesized that intramural VSDs can be initially small and enlarge post-operatively as the decompressed right ventricle's hypertrophy resolves1. This finding has also been supported by Yang et al who reported that four subjects with no or small VSDs seen on intraoperative transesophageal echocardiogram were subsequently found to have large intramural VSDs on TTE that eventually required reoperation2. Belli et al subsequently suggested a transaortic surgical approach for closure of intramural VSDs because of their unusual location and difficulty in surgical visualization of these defects; they reported successful reoperations in three cases3.
The outcome of patients with intramural VSDs was previously unknown. Intramural VSDs were associated with significant morbidity and mortality in the initial case series reported by Preminger et al1, in which all eight patients described required multiple reoperations and three died. Four subjects with an intramural VSD in another series2 required surgical reoperation to close the VSD. However, it was unclear if these cases represented all subjects with intramural VSDs or if there was a reporting bias of the most severe defects. Indeed, Belli et al. were the first to note that not all intramural VSDs were clinically significant3. Three of the five subjects they reported with intramural VSD required reoperation while the other two had trivial, insignificant intramural VSDs seen incidentally during angiography performed for outflow tract obstruction.
By systematically reviewing all patients at our institution who underwent conotruncal repair, we observed an association between intramural VSDs and post-operative morbidity and mortality. Subjects with intramural VSDs were more likely to have postoperative mortality, require ECMO support, and require re-intervention compared to subjects with non-intramural residual VSDs or no residual VSD. This difference persisted after adjusting for known risk factors for post-operative morbidity and mortality. Moreover, while the PLOS for our total cohort were similar to other reports for patients after repair of conotruncal defects 6, 13, we found that those with intramural VSDs had a significantly longer PLOS compared to those with a non-intramural VSD or no VSD after surgery. This difference persisted after correcting for potential confounders. Overall, the hospital course of subjects with intramural VSDs was more complicated, with more cardiac arrests, arrhythmia, and days of mechanical ventilation and vasopressive support. While the majority of children with intramural VSDs still did not require re-intervention, these children may benefit from VSD re-intervention if the defect is large by TTE, there is a large shunt by cardiac catheterization, or they are unable to be weaned from respiratory or circulatory support.
The mechanism of how intramural VSDs cause increased morbidity and mortality is not fully understood. It is likely that patients with intramural VSDs have higher morbidity because these VSDs become larger and more hemodynamically significant early in the postoperative period. It is also possible that these VSDs are more difficult to detect accurately on intraoperative transesophageal echocardiography and thus may be underappreciated until the postoperative TTE. Patients with conotruncal defects may poorly tolerate a significant residual VSD in the postoperative period because of preferential shunting across the VSD resulting in low cardiac output syndrome. In addition, the hearts of children with tetralogy of Fallot or double outlet right ventricle with pulmonary stenosis have not previously been exposed to a volume load. This sudden volume load may lead to ventricular dysfunction and need for ECMO support as well as other intensive care morbidities such as longer ventilation time and the development of pleural and pericardial effusions.
Despite the association of intramural VSDs with poor postoperative outcome, it is important to note that the majority of patients with these defects do not require re-intervention. In our study, 71% of patients with an intramural VSD did not have an outcome event. We hypothesize that there is an early post-operative period of vulnerability during which these intramural VSDs may become hemodynamically significant. However, if the intramural VSD does not substantially enlarge during this time period, then the outcomes can be similar to the favorable results seen with non-intramural residual VSDs. This is supported by our finding that only 30% of patients with intramural VSD on the postoperative TTE who did not require re-intervention had a residual VSD seen on most recent follow-up TTE, which was a rate similar to the rate of closure seen in patients with non-intramural VSD (34%). Similarly, approximately 30% of tetralogy of Fallot patients with VSD on early post-operative TTE are known to have residual VSD in follow up4.
Limitations
There are several limitations to this study. This was a single center study that reflects the population at one large referral hospital. It was a retrospective review of acquired TTE images, so it is possible that residual VSDs were not noted due to lack of adequate image acquisition. This would cause a bias in underreporting the prevalence of residual VSDs. Our standard hospital TTE protocol includes sweeps to examine the ventricular septum in multiple views, which should have adequately identified the majority of these VSDs. We also excluded subjects who did not have adequate post-operative TTE; some of these subjects may not have undergone post-operative imaging because they were unwell. However, the number of subjects excluded was small (n=19, 4%), and any bias would be unlikely to change our results significantly. The diagnosis of intramural and non-intramural VSD was dependent on the TTE read by the study authors; we believe the criteria for diagnosing intramural VSDs were standardized in our echocardiography laboratory and this was reflected in our high inter-rater agreement. Furthermore, because of the relatively small number of composite outcome events, there is a possibility of over-fitting the final multivariable models for primary outcome event or length of stay. However, the magnitude of our odds ratios and width of the 95% confidence intervals did not change substantially for any of the covariates going from bivariable to multivariable testing, suggesting that these models are valid. Additionally, logistic regression has been shown in simulation studies to be valid at event:variable ratios comparable to what we have employed14. Finally, while attempts were made to adjust for confounding variables in evaluating the association of residual VSDs to the outcome events, it is possible there are additional confounding events that were not taken into account.
Conclusion
Intramural VSDs occur after repair of conotruncal anomalies and are a distinct clinical entity from peripatch or muscular residual VSDs. In patients after conotruncal repair, intramural VSDs are associated with higher post-operative morbidity and mortality, more frequent re-intervention, and longer PLOS. Our findings suggest that early recognition of these lesions may identify a population at risk for post-operative events. Increased awareness of intramural VSDs and focus on surgical strategies to prevent their occurrence may improve overall outcome for patients with conotruncal anomalies who undergo biventricular repair. Further prospective work is needed to confirm these findings and characterize the intramural VSD prevalence and patient outcomes in a multicenter experience.
Supplementary Material
Acknowledgments
Funding Sources: J.K.P. is supported by a grant from the NIH (#5T32HL007915-15).
Footnotes
Disclosures: None.
References
- 1.Preminger TJ, Sanders SP, van der Velde ME, Castaneda AR, Lock JE. “Intramural” residual interventricular defects after repair of conotruncal malformations. Circulation. 1994;89:236–242. doi: 10.1161/01.cir.89.1.236. [DOI] [PubMed] [Google Scholar]
- 2.Yang SG, Novello R, Nicolson S, Steven J, Gaynor JW, Spray TL, Rychik J. Evaluation of ventricular septal defect repair using intraoperative transesophageal echocardiography: Frequency and significance of residual defects in infants and children. Echocardiography. 2000;17:681–684. doi: 10.1046/j.1540-8175.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 3.Belli E, Houyel L, Serraf A, Lacour-Gayet F, Petit J, Planche C. Transaortic closure of residual intramural ventricular septal defect. Ann Thorac Surg. 2000;69:1496–1498. doi: 10.1016/s0003-4975(00)01084-5. [DOI] [PubMed] [Google Scholar]
- 4.Dodge-Khatami A, Knirsch W, Tomaske M, Prêtre R, Bettex D, Rousson V, Bauersfeld U. Spontaneous closure of small residual ventricular septal defects after surgical repair. Ann Thorac Surg. 2007;83:902–905. doi: 10.1016/j.athoracsur.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 5.Kim JW, Gwak M, Shin WJ, Kim HJ, Yu JJ, Park PH. Preoperative factors as a predictor for early postoperative outcomes after repair of congenital transposition of the great arteries. Pediatr Cardiol. 2014;36:537–542. doi: 10.1007/s00246-014-1046-8. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch RE, Glatz AC, Gaynor JW, Nicolson SC, Spray TL, Wernovsky G, Bird GL. Results of elective repair at 6 months or younger in 277 patients with tetralogy of fallot: A 14-year experience at a single center. J Thorac Cardiovasc Surg. 2014;147:713–717. doi: 10.1016/j.jtcvs.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Egbe A, Mittnacht A, Nguyen K, Joashi U. Risk factors for morbidity in infants undergoing tetralogy of fallot repair. Ann Pediatr Cardiol. 2014;7:13. doi: 10.4103/0974-2069.126539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dongen EI, Glansdorp AG, Mildner RJ, McCrindle BW, Sakopoulos AG, VanArsdell G, Williams WG, Bohn D. The influence of perioperative factors on outcomes in children aged less than 18 months after repair of tetralogy of fallot. J Thorac Cardiovasc Surg. 2003;126:703–710. doi: 10.1016/s0022-5223(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 9.Dyamenahalli U, McCrindle BW, Barker GA, Williams WG, Freedom RM, Bohn DJ. Influence of perioperative factors on outcomes in children younger than 18 months after repair of tetralogy of fallot. Ann Thorac Surg. 2000;69:1236–1242. doi: 10.1016/s0003-4975(99)01441-1. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, Edwards FH. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 11.Hanna BM, El-Hewala AA, Gruber PJ, Gaynor JW, Spray TL, Seliem MA. Predictive value of intraoperative diagnosis of residual ventricular septal defects by transesophageal echocardiography. Ann Thorac Surg. 2010;89:1233–1237. doi: 10.1016/j.athoracsur.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 12.Rychik J, Norwood WI, Chin AJ. Doppler color flow mapping assessment of residual shunt after closure of large ventricular septal defects. Circulation. 1991;84:III153–161. [PubMed] [Google Scholar]
- 13.Oster ME, Dawson AL, Batenhorst CM, Strickland MJ, Kleinbaum DG, Mahle WT. Relationship between resource utilization and length of stay following tetralogy of fallot repair. Congenit Heart Dis. 2013;8:535–540. doi: 10.1111/chd.12023. [DOI] [PubMed] [Google Scholar]
- 14.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158:280–287. doi: 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


