Abstract
Interleukin (IL)-6 is a multi-functional cytokine that can either promote or suppress tissue inflammation depending on the specific disease context. IL-6 is elevated in the exocrine glands and serum of patients with Sjögren’s syndrome (SS), but the specific role of IL-6 in the pathogenesis of this disease has not been defined. In this study, we showed that IL-6 expression levels were increased with age in C56BL/6.NOD-Aec1Aec2 mice, a primary SS model, and higher than the control C57BL/6 mice. To assess the role of IL-6 during the immunological phase of SS development, a neutralizing anti-IL-6 antibody was administered into 16 week-old female C56BL/6.NOD-Aec1Aec2 mice, 3 times weekly for a consecutive 8 weeks. Neutralization of endogenous IL-6 throughout the immunological phase of SS development led to increased apoptosis, caspase-3 activation, leukocytic infiltration, and IFN-γ- and TNF-α production in the salivary gland. To further determine the effect of IL-6 on the apoptosis of exocrine gland cells, recombinant human IL-6 or the neutralizing anti-IL-6 antibody was injected into female C57BL/6 mice that received concurrent injection of anti-CD3 antibody to induce the apoptosis of exocrine glands. Neutralization of IL-6 enhanced, whereas administration of IL-6 inhibited apoptosis and caspase-3 activation in salivary and lacrimal glands in this model. The apoptosis-suppressing effect of IL-6 was associated with up-regulation of Bcl-xL and Mcl-1 in both glands. Moreover, IL-6 treatment induced activation of STAT3 and up-regulated Bcl-xL and Mcl-1 gene expression in a human salivary gland epithelial cell line. In conclusion, IL-6 inhibits the apoptosis of exocrine gland tissues and exerts a tissue-protective effect under inflammatory conditions including SS. These findings suggest the possibility of using this property of IL-6 to preserve exocrine gland tissue integrity and function under autoimmune and inflammatory conditions.
Keywords: Interleukin-6, apoptosis, inflammation, Sjögren’s syndrome, exocrine gland
1. Introduction
Interleukin (IL)-6 is a pleiotropic cytokine with both pro- and anti-inflammatory properties [1–3]. IL-6 promotes the pathogenesis of a variety of autoimmune or inflammatory disorders such as experimental autoimmune encephalomyelitis, rheumatoid arthritis and psoriasis [1, 4–6]. However, a growing number of reports have shown that IL-6 can also exert anti-inflammatory effects in a variety of autoimmune or inflammatory diseases [7–12]. IL-6 increases serum IL-1Rα, TNF receptor p55 and IL-10 levels in humans receiving IL-6 therapy and induces IL-1Rα production by human macrophages [13, 14]. IL-6 deficiency leads to exacerbated skin inflammation, accompanied by increased inflammatory mediators including TNF-α, IL-1β, in a mouse model of irritant dermatitis [7]. Furthermore, exogenous and endogenous IL-6 increases IL-1Rα in skin and serum in a mouse model of organ-specific autoimmune bullous dermatoses and plays a protective and anti-inflammatory role in this disease [12]. Moreover, our recent study has shown that IL-6 can induce the generation of type 1 regulatory T (Tr1) cells and suppress multi-organ inflammation in mice [15].
In addition to affecting immune cells, IL-6 can also directly act on intestinal epithelial cells to promote their survival and maintain intestinal tissue integrity during ischaemia reperfusion, bacterial infection, and dextran sulfate sodium (DSS)-induced colitis [10, 16, 17]. IL-6 protects against lung epithelial and endothelial cell death induced by reactive oxygen species and hyperoxic injury [18, 19]. Moreover, IL-6 inhibits the apoptosis of pancreatic acinar cells in a LPS-induced pancreatitis model [20]. The anti-apoptotic effect of IL-6 is often associated with activation of STAT3 and up-regulation of anti-apoptotic molecules that include Bcl-xL, Mcl-1 and Bcl-2 [10, 16, 17, 19].
Sj gren’s syndrome (SS) is a chronic autoimmune disease with the lacrimal gland (LAC) and salivary gland as the primary target organs, causing dryness of mouth and eyes [21–24]. The disease often affects multiple other organs and causes systemic symptoms [21–24]. Apoptosis of exocrine gland cells is one of the early pathologic events that promote the innate immune activation and lymphocyte-mediated autoimmune responses, which propel the development and onset of SS [25–29]. IFN-γ and TNF-α, two pro-inflammatory cytokines that are dramatically elevated in the salivary gland and serum of SS patients [30–32], have been shown to induce caspase-3 activation and apoptosis in salivary gland epithelial cells and disrupt the function of tight junctions [33–37]. Exocrine gland-specific transgenic expression of retinoblastoma-associated protein 48 (RbAP48) in mice results in apoptosis of the LAC and salivary gland tissues [38, 39]. Moreover, epithelial-specific deletion of STAT3 in mice causes apoptosis of LAC epithelial cells and subsequent development of SS-like dacryoadenitis [40]. Therefore, one crucial need in SS research is to better define the causes and regulatory mechanisms of exocrine gland tissue apoptosis and identify the factors that can protect tissue integrity. IL-6 levels are significantly elevated in the salivary gland and serum of SS patients and are correlated with the disease manifestation [41, 42]. However, the in vivo function of IL-6 in the development and onset of SS has not been investigated. Since IL-6 possesses both pro-inflammatory and tissue-protective properties, it is important to determine whether the excess IL-6 acts as a disease promoting factor or as a defense mechanism to protect exocrine gland tissue integrity in SS and other inflammatory conditions.
The current study was undertaken to determine the in vivo role of IL-6 in exocrine gland inflammatory disorders, including SS. Our results demonstrate that IL-6 reduces the apoptosis and inflammation of salivary and lacrimal gland tissues, thereby serving as a tissue protective factor in exocrine gland inflammations.
2. Material and methods
2.1. Mice
C57BL/6 mice were purchased from the Jackson Laboratory. C57BL/6.NOD-Aec1Aec2 mice were kindly provided by Dr. Cha at University of Florida. Mice were kept under specific pathogen-free conditions. All experiments were carried out under the guidelines of the Institutional Animal Care and Use Committee at the Forsyth Institute. The human salivary gland epithelial cell line, HSG cells, was kindly provided by Dr. Cha at University of Florida.
2.2. Antibodies and cytokines
Purified monoclonal hamster anti-mouse CD3 (145–2C11), rat-anti-mouse IL-6 (NJ5-20F3) and its isotype control rat IgG1 (HRPN) were from BioXCell. Recombinant human IL-6 was obtained from Peprotech. Purified polyclonal rabbit anti-mouse Mcl-1 (Poly6163) and rabbit anti-Bcl-2 (Poly6119) antibodies were purchased from Biolegend. Monoclonal rabbit anti-mouse Bcl-xL (54H6) antibody was from Cell Signaling Technology.
2.3. Histology
Tissue samples were fixed in 4 % paraformaldehyde, embedded in paraffin and sectioned to 5 μm thickness. Sections were then stained with hematoxylin and eosin (H&E) and examined for leukocytic infiltration. The numbers of leukocytic foci in each of the two non-consecutive sections from each sample were counted, and the higher number between the two was used for further calculation and statistical analysis.
2.4. Immunohistochemistry
Paraffin sections were de-paraffinized and stained with anti-mouse Bcl-xL or anti-mouse Mcl-1 antibodies at 4oC overnight using VECTASTAIN Elite ABC Kit (Vector Labs) following the manufacturers’ instructions. Active Caspase-3 was detected by SignalStain® Apoptosis (Cleaved Caspase-3) IHC Detection Kit (Cell Signaling Technology), according to the manual.
2.5. Preparation of single cell suspension
Submandibular glands, extra-orbital lacrimal glands and submandibular lymph nodes were cut into small fragments and ground into cell suspensions between frosted glass slides. Cell suspensions were then filtered through a 200 μm nylon mesh, washed, and resuspended in culture medium.
2.6. Real-time RT-PCR
Total RNA was reverse-transcribed into cDNA using Oligo (dT) and M-MLV reverse transcriptase (Promega). The cDNA was subjected to SYBR Green-based realtime PCR amplification (Qiagen) for 40 cycles with annealing and extension temperature at 60°C, on a LightCycler 480 Real-Time PCR System (Roche). Primer sequences are as follows: mouse IFN-γ 5’-GGATGCATTCATGAGTATTGC-3’(forward) and 5’-CTTTTCCGCTTCCTGAGG-3’(reverse); mouse TNF-α, 5’-CCTTTCACTCACTGGCCCAA-3’(forward) and 5’-AGTGCCTCTTCTGCCAGTTC-3’(reverse); mouse Bcl-xL, 5’-CACCTAGAGC CTTGGATCCA-3’(forward) and 5’-TTGAAGCGCTCCTGGCCTTT-3’(reverse); mouse Mcl-1, 5’-TTGTAAGGACGAAACGGGACT-3’(forward) and 5’-ACATTTCTGATGCCGCCTTCT-3’(reverse). Other sequences will be provided upon request.
2.7. In vivo administration of antibodies and cytokines
Female B6.NOD-Aec mice were intraperitoneally (i.p).-injected with 100 μg of control rat IgG1 or anti-IL-6 antibody 3 times weekly for 8 weeks, starting from 16 weeks of age. Mice were then sacrificed and organs harvested for analysis. Anti-CD3-induced tissue injury model was established by i.p.-injecting 20 ug of anti-CD3 antibody or control rat IgG1 into 7 week-old C57BL/6 (B6) mice on day 0 and day 2. In order to examine the function of IL-6, we also i.p.-administered 1 ug of hu IL-6 on day 0, 1 and 2 or 100 ug of anti-mouse IL-6 antibody on day 0 and 2 to the mice treated with anti-CD3. Tissues harvested at 3 h or 24 h after the last injection were used for RNA or protein level detection, respectively.
2.8. Detection of serum antinuclear antibodies (ANA)
ANA in mouse sera were detected using HEp-2 human epithelial cell substrate slides (INOVA Diagnostics) following the manufacturer’s instructions. The stained samples were examined with inverted wide-field fluorescence microscope (Zeiss) at a magnification of 400X. Images presented here were processed using Zeiss software (ZEN blue edition).
2.9. Measurement of salivary flow rate
Non-anesthetized mice were weighed and given an i.p injection of 100 μl PBS-based secretagogue solution containing isoproterenol (0.02 mg/ml) and pilocarpine (0.05 mg/ml). One minute after secretagogue injection, saliva was collected continuously for 5 minutes from the oral cavity of mice with a micropipette. The volume of saliva was measured and normalized to the body weight.
2.10. In vitro culture and treatment of HSG cells
The HSG cells were seeded at a density of 0.2 × 106 cells per well in 24-well culture plates. The cells were cultured in freshly replenished medium for 4 hours before rh IL-6 (10 ng/ml) was added into the culture. Viable cell numbers were counted after 5 days of culture.
2.11. In situ apoptosis detection
Paraffin embedded tissue sections were de-paraffinized, hydrated and then subjected to in situ apoptosis assay using TACS.XL In Situ Apoptosis Detection kit (Trevigen) according to the manufacturer’s instruction. Briefly, tissue sections were partially digested with proteinase-K for 20 min, and then incubated in 3% H2O2 to inactivate endogenous peroxidases. DNA fragmentation was then detected following the manufacturer’s protocol.
2.12. Statistical analysis
Statistical significance was determined by Student’s t-test (two-tailed, two-sample equal variance). P values equal to less than 0.05 were considered as statistically significant.
3. Results
3.1. Levels of IL-6 and its receptor are elevated in salivary glands in B6. NOD-Aec mice
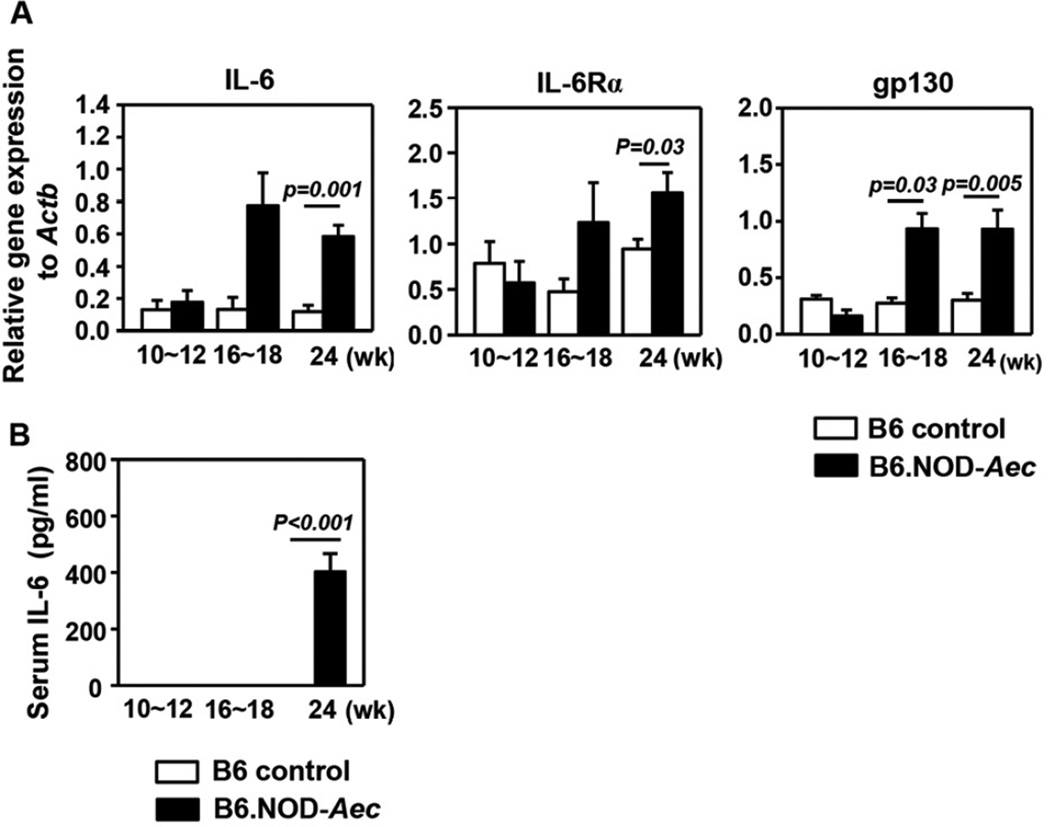
We first assessed whether IL-6 levels are elevated in C56BL/6.NOD-Aec1Aec2 (B6.NOD-Aec) mice, which has been observed in human SS patients. Real-time PCR analysis showed that the mRNA levels of IL-6 and IL-6 receptor, which includes IL-6Rα and gp130 subunits, in submandibular glands (SMG) were comparable between B6.NOD-Aec mice and control B6 mice at 10–12 weeks of age (pre-immunological phase). However, IL-6 and IL-6 receptor levels were markedly increased with aging in B6.NOD-Aec mice but not B6 mice (Fig. 1A). As a result, IL-6, IL-6Rα and gp130 levels in B6.NOD-Aec mice were significantly higher than those in B6 mice at 16–18 weeks (immunological phase) and 24 weeks of age (clinical phase). Moreover, serum IL-6 concentrations in B6.NOD-Aec mice, but not B6 mice, were also increased between age 16–18 weeks and 24 weeks (Fig. 1B). Hence, B6.NOD-Aec mice, which spontaneously develop SS-like exocrinopathy, have elevated expression of IL-6 and IL-6 receptor in the salivary gland.
Figure 1. Expression of IL-6 and its receptor in the SMG of B6.NOD-Aec mice.
(A) IL-6, IL-6Rα and gp130 mRNA levels in the SMG of B6 or B6.NOD-Aec mice aged 10–12, 16–18 and 24 weeks. Expression levels were presented relative to that of β-actin. (B) Serum IL-6 concentrations in the same mice described above. Data are the average of analyses of at least 6 mice for each group from 3 independent experiments. The histograms and error bars represent the average and SEM, respectively.
3.2. Neutralization of endogenous IL-6 exacerbates leukocytic infiltration and apoptosis of salivary gland tissues
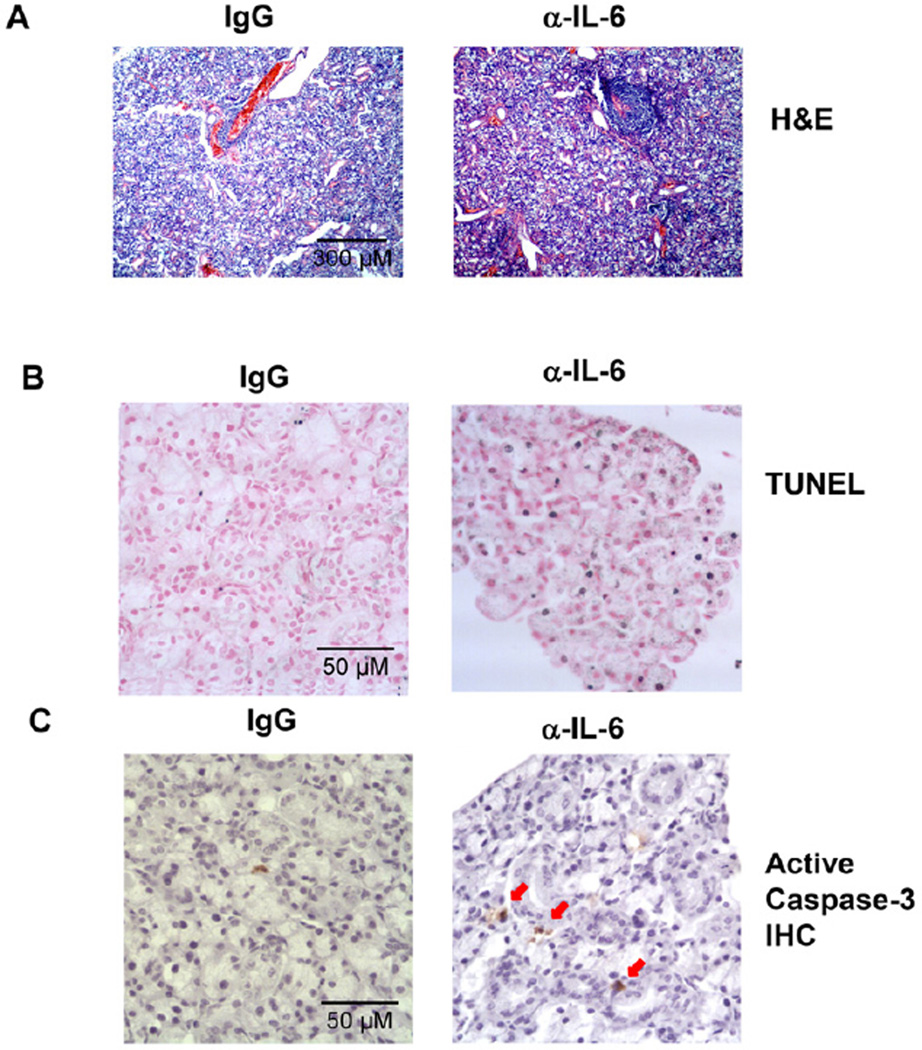
To determine the role of endogenous IL-6, which is up-regulated in B6.NOD-Aec mice during the immunological phase of SS development, in the pathogenesis of this disease, we administered a neutralizing anti-IL-6 antibody to B6.NOD-Aec mice to inhibit IL-6 activity throughout the immunological phase. We i.p.- injected 100 ug anti-IL-6 or its isotype control IgG, rat IgG1, into B6.NOD-Aec mice 3 times weekly starting from 16 weeks of age, and analyzed the mice after 8 weeks of consecutive injection. H&E staining showed that neutralization of IL-6 increased the percentage of mice that have SMG inflammation, as indicated by the presence of at least one leukocytic focus in the SMG section (Fig. 2A, and Table 1). Moreover, neutralization of IL-6 increased the percentage of mice that have multiple leukocytic foci in the SMG section (Fig. 2A, and Table 1). To assess the apoptosis of salivary gland tissues, we performed in situ terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay on SMG sections, which showed that neutralization of IL-6 increased the amount of apoptotic cells in the SMG (Fig. 2B). Consistent with this observation, the level of cleaved, active caspase-3 in the SMG was also increased by anti-IL-6 treatment as assessed by immunohistochemical staining (Fig. 2C).
Figure 2. Neutralization of IL-6 in B6.NOD-Aec mice leads to increased inflammation and apoptosis in SMG.
Anti-IL-6 or control IgG was injected to 16 week-old B6.NOD-Aec mice, 3 times weekly for 8 weeks. (A) H&E staining of SMG sections showing leukocytic foci. Original magnification: ×10. (B) In situ TUNEL staining of apoptotic cells in SMG sections. Original magnification: ×40. (C) Immunohistochemical staining of active caspase-3 in SMG sections. Data are representative of the analyses of 9 mice for each group from 5 independent experiments. Original magnification: ×40.
Table 1.
Leukocytic infiltration of the SMG
| SMG | LF > 0 | LF > 1 | Mean number of LF |
|---|---|---|---|
| IgG | 54% | 15% | 0.84 |
| α-IL-6 | 80% | 47% | 1.47 |
Percentage of mice with any leukocytic foci (LF>0) or more than one LF (LF>1) in the SMG section from mice treated with IgG or anti-IL-6 antibody. Mean number of LF from each treatment group are also shown. Data are from 13 IgG-treated controls and 15 anti-IL-6-treated mice.
IL-6 neutralization did not alter the percentage of mice that were positive for serum antinuclear antibody (ANA) or the levels of serum ANA and did not affect the salivary flow rate (data not shown). Hence, inhibition of endogenous IL-6 activity throughout the immunological phase of SS development leads to exacerbated apoptosis and leukocytic infiltration of the SMG during the development of SS in B6.NOD-Aec mice, without affecting the secretory function of the SMG.
3.3. Neutralization of IL-6 results in increased IFN-γ and TNF-α expression in the salivary gland
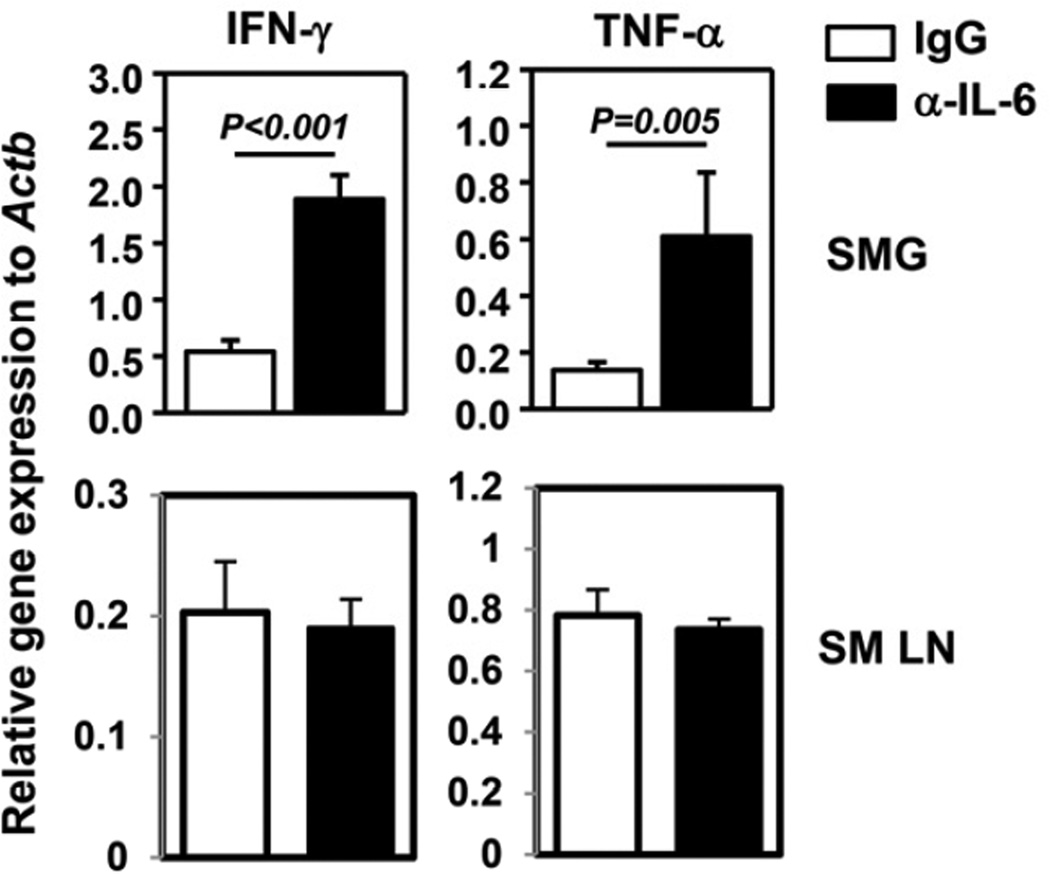
We next assessed the effect of IL-6 neutralization on the production of cytokines, particularly the ones that are mainly derived from effector T cells and shown to be required for SS pathogenesis. Real-time PCR analysis showed a significant increase in T helper cell (Th) 1 cytokines, IFN-γ and TNF-α, in the SMG of anti-IL-6-treated mice compared to IgG-treated controls (Fig. 3). The levels of IL-4 and IL-17 mRNA were extremely low in the SMG of both IgG- and anti-IL-6-treated groups (data not shown). Moreover, the amounts of IFN-γ, TNF-α, IL-4 and IL-17 transcripts in the submandibular draining lymph nodes were not affected by anti-IL-6 treatment (Fig. 3). Thus, neutralization of IL-6 results in increased Th1 cytokines in the salivary gland but not in the draining lymph nodes.
Figure 3. Neutralization of IL-6 in B6.NOD-Aec mice leads to increased IFN-γ and TNF-α expression in the SMG.
IFN-γ and TNF-α mRNA levels in the submandibular gland (SMG) and submandibular draining lymph nodes (SM LN) of B6.NOD-Aec mice injected with anti-IL-6 or control IgG as described in Figure 2. Expression levels were presented relative to that of β-actin. Data are the average of the analyses of 7 mice for each group from 4 independent experiments. The histograms and error bars represent the average and SEM, respectively.
3.4. IL-6 activates STAT3 and up-regulates Mcl-1 and Bcl-xL expression in a human salivary gland cell line
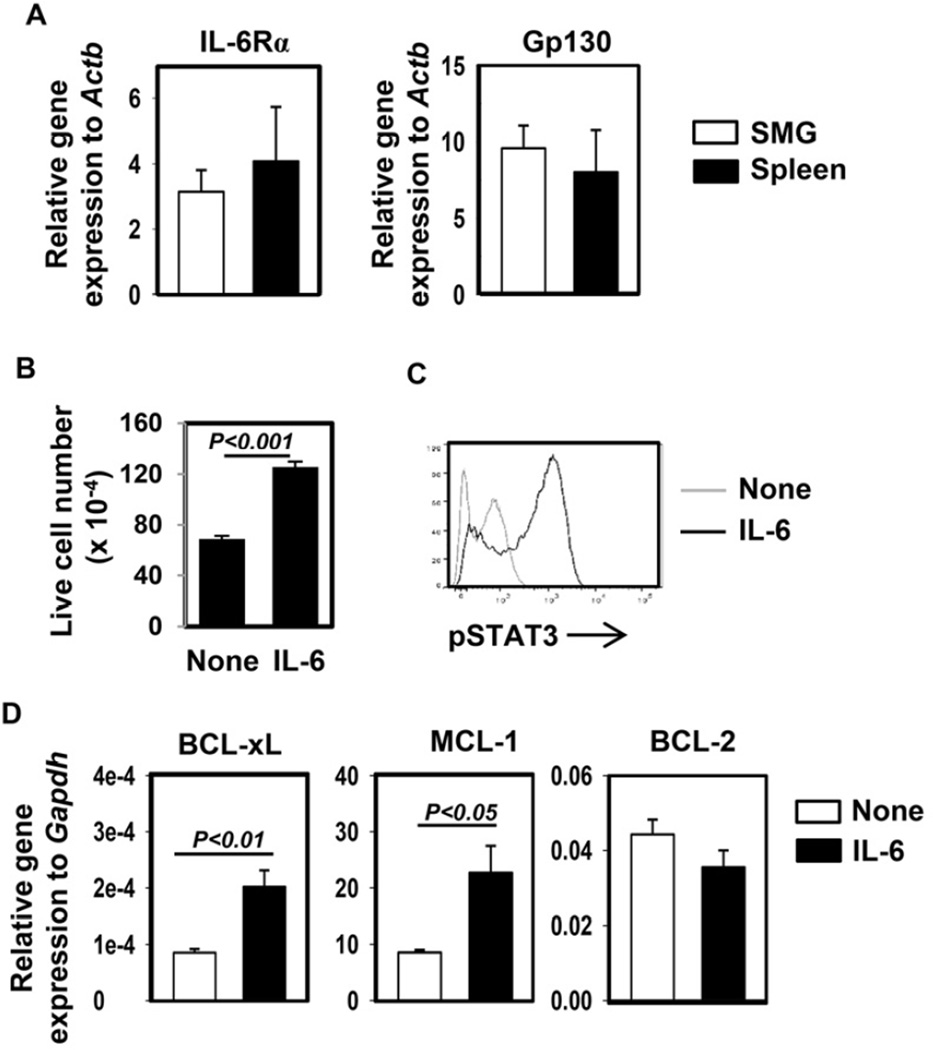
Since IL-6 can inhibit apoptosis and promote survival of intestinal and lung epithelial cells, we next determined whether IL-6 can also exert similar effect on salivary gland epithelial cells. Many types of tissue cells do not express IL-6Rα and receive IL-6 signaling via a trans-signaling mechanism. We therefore first examined the mRNA expression levels of IL-6Rα in SMG cells and compared them with splenocytes, which are mostly lymphocytes that express abundant IL-6Rα. Real-time PCR assay showed that SMG cells expressed comparable levels of both IL-6Rα and gp130 as splenocytes, suggesting that SMG cells express their own IL-6 receptors and can directly receive IL-6 signal through the classic signaling pathway (Fig. 4A). Next, we cultured HSG cells, a human salivary gland epithelial cell line, in vitro in the presence or absence of exogenous recombinant human (rh) IL-6 for 5 days and found that IL-6 treatment significantly increased the number of viable HSG cells (Fig. 4B). Moreover, brief IL-6 stimulation induced substantial STAT3 phosphorylation in HSG cells (Fig. 4C), further confirming that IL-6 can directly affect these cells. IL-6 treatment markedly up-regulated Mcl-1 and Bcl-xL gene expression, without affecting Bcl-2 (Fig. 4D). In addition, IL-6 treatment did not affect TNF-α expression in HSG cells (data not shown), indicating that the pro-survival effect of IL-6 on HSG cells is not a result of decreased TNF-α production from these cells. Hence, IL-6 can directly act on HSG cells and up-regulate Mcl-1 and Bcl-xL expression.
Figure 4. IL-6 activates STAT3 and up-regulates expression of anti-apoptotic molecules in HSG cells.
(A) Numbers of viable HSG cells after being cultured in vitro in the presence or absence of rh IL-6 for 5 days. Data are the average of 6 independent samples from 2 experiments. The histograms and error bars represent the average and SEM, respectively. (B) Flow cytometry of intracellular p-STAT3 in HSG cells transiently stimulated with rh IL-6. Data are representative of 4 independent analyses from 4 experiments. (C) Bcl-xL, Bcl-2 and Mcl-1 mRNA levels in HSG cells cultured with or without rh IL-6 for 1 day, presented relative to that of human Gapdh gene. Data are the average the analyses of 4 independent samples for each group. The histograms and error bars represent the average and SEM, respectively.
3.5. Endogenous and exogenous IL-6 inhibits anti-CD3 antibody-induced exocrine gland tissue apoptosis in B6 mice
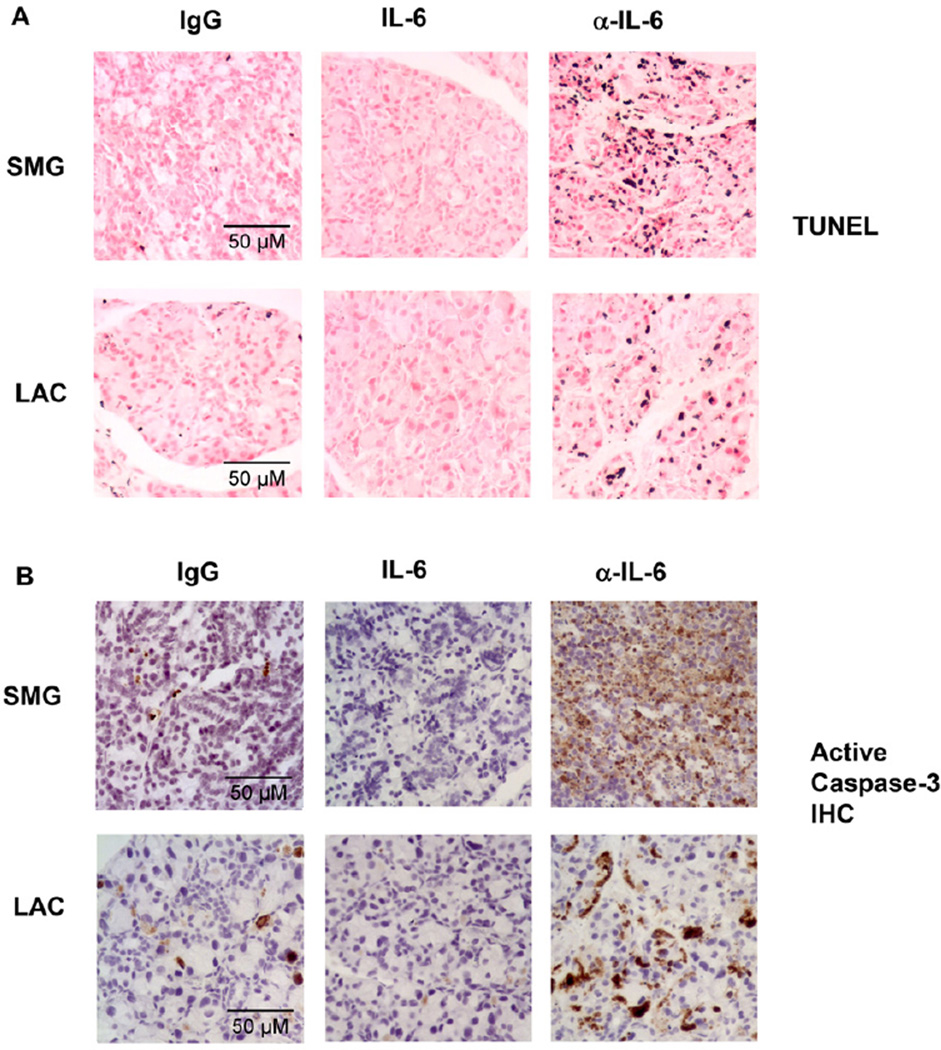
We next further assessed the effect of IL-6 in another model of exocrine gland injury and inflammation. Systemic administration of anti-CD3 antibody in mice induces production of a variety of cytokines by T cells, including TNF-α, IFN-γ, IL-6 and IL-10, and causes acute inflammation in multiple organs [15, 43, 44]. Since TNF-α and IFN-γ are reported to induce apoptosis in salivary gland epithelial cells, we reasoned that anti-CD3 administration may induce exocrine gland tissue apoptosis as a result of increased TNF-α and IFN-γ production. Indeed, we found that i.p.-injection of anti-CD3 antibody induced cell apoptosis in both SMG and LAC in B6 mice, as assessed by TUNEL-based in situ apoptosis detection assay (data not shown). We hence employed this model to assess the effect of exogenous and endogenous IL-6 on the apoptosis of exocrine gland tissues. We i.p.-administered anti-CD3 antibody to B6 mice in conjunction with control IgG for anti-IL-6 antibody, recombinant human IL-6 or the neutralizing anti-IL-6 antibody. After two consecutive injections, we measured apoptosis and active caspase-3 levels in the exocrine glands. Exogenous IL-6 inhibited, whereas anti-IL-6 significantly exacerbated the degree of apoptosis in both SMG and LAC as assessed by TUNEL assay (Fig. 5A). Moreover, the level of active caspase-3 in these glands exhibited similar changes upon treatment with IL-6 and anti-IL-6 (Fig. 5B). Therefore, exogenous and endogenous IL-6 inhibits apoptosis of exocrine gland tissues induced by anti-CD3 treatment.
Figure 5. IL-6 inhibits exocrine gland cell death induced by in vivo anti-CD3 administration.
B6 mice were injected with anti-CD3 in the presence or absence of rh IL-6 or anti-IL-6. (A) In situ TUNEL staining of apoptotic cells in SMG and LAC sections. (B) Immunohistochemical staining of active caspase-3 in SMG and LAC sections. Data are representative of the analyses of 8 mice for each group from 3 independent experiments. Original magnification: × 40.
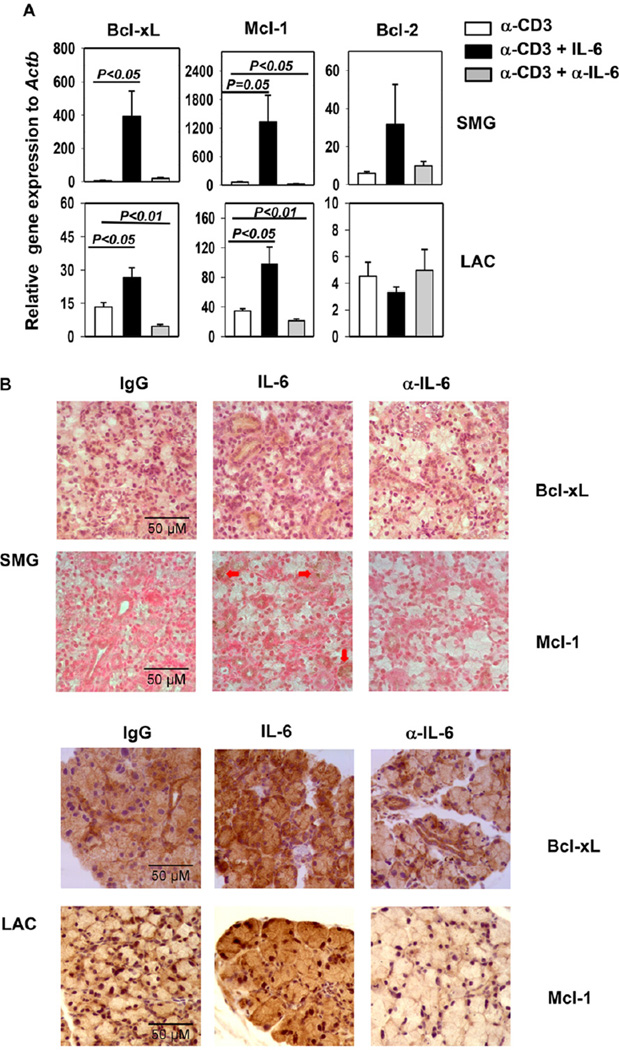
3.6. IL-6 up-regulates Bcl-xL and Mcl-1 expression in exocrine glands of anti-CD3-treated B6 mice
To understand the molecular mechanisms by which IL-6 inhibits apoptosis of exocrine glands, we examined the effect of IL-6 on the expression of a number of anti-apoptotic proteins and pro-inflammatory cytokines. Consistent with the findings in HSG cells, in anti-CD3-treated B6 mice, IL-6 treatment substantially up-regulated, whereas anti-IL-6 treatment significantly down-regulated Bcl-xL and Mcl-1 gene expression in SMG and LAC (Fig. 6A). In comparison, Bcl-2 gene expression was not altered by the treatment with IL-6 or anti-IL-6 in a statistically significant fashion (Fig. 6A). We next performed immunohistochemical staining on SMG and LAC tissue sections to assess the Bcl-xL and Mcl-1 protein expression. IL-6 treatment increased, whereas anti-IL-6 treatment decreased the protein levels of Bcl-xL and Mcl-1 in both SMG and LAC, based on the amount of cells stained positive for these proteins as well as the intensity of the staining (Fig. 6B).
Figure 6. IL-6 up-regulates Bcl-xL and Mcl-1 expression in exocrine glands in anti-CD3-treated mice.
(A) Bcl-xL, Mcl-1 and Bcl-2 mRNA levels in the SMG and LAC of B6 mice treated with anti-CD3 with or without rh IL-6 or anti-IL-6 as described in Figure 5. Expression levels were presented relative to that of β-actin. The histograms and error bars represent the average and SEM, respectively. (B) Immunohistochemical staining of Bcl-xL and Mcl-1 in SMG and LAC sections. Data are the average of or representative of the analyses of 7–8 mice each group from 3 independent experiments. Original magnification: × 40.
4. Discussion
In this study, we demonstrated that both endogenous and exogenous IL-6 can protect against the apoptosis of exocrine gland tissues and restrain tissue inflammation in mouse models of exocrine gland inflammation, including SS and anti-CD3-induced inflammation.
The anti-apoptosis and protective function of IL-6 is well-documented in intestinal epithelial cells during various tissue injuries, including ischaemia reperfusion, bacterial infection and DSS-induced colitis [10, 16, 17]. The apoptosis-suppressing effect of IL-6 on intestinal tissues is accompanied by the up-regulation of anti-apoptotic molecules such as Bcl-xL, Mcl-1 and c-Flip [10, 16, 17]. IL-6 also promotes Bcl-2 expression in lung epithelial cells during hyperoxic acute lung injury [19]. IL-6Rα is not expressed in many non-immune tissue cells, which nonetheless can receive IL-6 signal through a trans-signaling mechanism. We found that SMG cells expressed similar levels of IL-6Rα and gp130 mRNA as the splenocytes, which are mostly IL-6Rα-expressing lymphocytes, suggesting that the SMG cells express abundant endogenous IL-6Rα and gp130 to receive IL-6 signaling in a conventional fashion. Moreover, IL-6 treatment induced activation of STAT3 in HSG cells in vitro, further supporting that IL-6 can directly act on salivary gland epithelial cells. The anti-apoptotic effect of IL-6 was accompanied by the positive regulation of Bcl-xL and Mcl-1, which was demonstrated in HSG cells in vitro and in salivary and lacrimal gland tissues in vivo. In addition to Jak1/2-STAT3 pathway, IL-6 can also activate Ras-Raf-MEK-Erk and PI3 kinase-Akt signaling pathways, both of which have been shown to contribute to the anti-apoptotic function of IL-6 in different cells types [45–50]. Further investigations are needed to define the functional importance of these signaling pathways as well as Bcl-xL and Mcl-1 in IL-6-mediated protection of exocrine glands by loss-of-function approaches. Moreover, whether additional molecular players also contribute to the apoptosis-inhibiting effect of IL-6 should also be investigated.
IFN-γ and TNF-α, alone or working in concert, can induce the apoptosis of exocrine gland cells by both activating mitochondria/caspase-9-dependent pathway and by inducing Fas expression to enhance Fas/Fas ligand-caspase-8-dependent cell death [37, 51]. Both cytokine-induced and Fas/Fas ligand-mediated apoptosis are implicated in SS pathogenesis, and are likely also involved in anti-CD3-induced apoptosis of exocrine gland tissues, since anti-CD3 treatment can induce IFN-γ and TNF-α production and Fas ligand expression by T cells [35–37, 52–55]. In this study, we assessed the effect of endogenous and exogenous IL-6 on the activation of caspase-3, a substrate of both caspase-9 and-8 and the common executor of both mitochondria-dependent and Fas/FasL-mediated apoptosis. Further studies are needed to determine the involvement of upstream caspases, particularly caspase-9 and −8, in the apoptosis of exocrine gland tissues, and to understand the regulation of these caspases by endogenous and exogenous IL-6.
In B6.NOD-Aec mice, neutralization of IL-6 resulted in increased IFN-γ and TNF-α expression in the SMG, without affecting that in the lymphoid tissues. The cellular sources of IFN-γ and TNF-α and the precise mechanisms by which IL-6 affects the expression of these cytokines require further investigation. We also showed that IL-6 neutralization exacerbated leukocytic infiltration of the SMG. Tissue injuries that cause apoptosis can induce various degrees of local inflammatory responses by releasing intracellular DNA, RNA and proteins, which in turn can serve as inflammatory mediators and self-antigens. Growing amount of evidence indicates that the apoptosis of exocrine gland tissues is sufficient to initiate all the major SS-like pathological events, which include leukocytic infiltration of the exocrine glands [38, 40]. Hence, it is plausible that neutralization of IL-6 enhances the recruitment of immune cells into the exocrine gland tissues as a result of increased tissue apoptosis.
In this study, we administered anti-IL-6 to B6.NOD-Aec mice starting from 16 weeks of age, because we aimed at elucidating the role of IL-6 during the immunological phase of SS development, which precedes the clinical onset of the disease and starts around 16 weeks of age when the earliest leukocyte infiltration is detected [56]. IL-6 can promote Th17 differentiation in cooperation with TGF-β or IL-23 in vitro and in certain inflammatory and autoimmune disorders [57, 58]. However, in B6.NOD-Aec mice, neutralization of IL-6 did not have a measurable impact on IL-17 production either in the salivary glands or draining lymph nodes. Since several other Th17-promoting cytokines, including IL-23 and IL-21, are elevated in exocrine glands and serum of SS patients and mice [59–61], these cytokines may play a more dominant role in supporting the Th17 responses in SS. Moreover, whereas IL-6 is required for the optimal generation of Tr1 cells and production of IL-10 in several mouse inflammation models [15, 62], our data show that it is not required for these events in B6.NOD-Aec mice during the development of SS. We speculate that other Tr1-promoting and IL-10-inducing cytokines that are present in the SS settings, such as IL-21 and IL-27, may play a more critical role and thus make IL-6 dispensable in these events. Hence, whether IL-6 plays an indispensable role in a particular immunological process depends on specific disease settings, cytokine environments and tissue- and cell-specific factors. In SS-associated exocrine gland inflammations, IL-6 is indispensable for suppressing apoptosis and protecting tissue integrity, whereas largely dispensable for the immune responses. Therefore, the overall function of IL-6 during the immunological phase of SS development is tissue-protective and anti-inflammatory. It is important to note that the specific function of IL-6 in SS pathogenesis might be stage-dependent, and it will be important to further define its specific role at other stages of SS, including the early, non-immunological phase as well as the late, clinical phase of this disease.
Conclusions
In the present study, we demonstrated that the effect of IL-6 on exocrine epithelial tissues under autoimmune and inflammatory conditions is of a protective property. These results suggest that enhancing IL-6 activities specifically in exocrine gland tissues may be a new therapeutic approach to preserve exocrine gland integrity and function under autoimmune and inflammatory conditions.
Highlights.
Levels of IL-6 and its receptor are increased in salivary gland of a mouse model of SS
IL-6 reduces leukocytic infiltration of the salivary gland in a mouse model of SS
IL-6 inhibits apoptosis of exocrine gland tissues under two inflammatory conditions
IL-6 up-regulates Bcl-xL and Mcl-1 expression in exocrine gland cells
Acknowledgments
We thank Juan Du for technical assistance in several experiments. This study was supported by the National Institutes of Health (P30 DE020751, R01 DE023838).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jing Zhou, Email: jzhou@forsyth.org.
Jun-O Jin, Email: junojin1@gmail.com.
Ekta S Patel, Email: ektaspatel01@gmail.com.
Qing Yu, Email: qyu@forsyth.org.
References
- 1.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Danese S, Gao B. Interleukin-6: a therapeutic Jekyll and Hyde in gastrointestinal and hepatic diseases. Gut. 2010;59:149–151. doi: 10.1136/gut.2008.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabay C. Interleukin-6 and chronic inflammation. Arthritis research & therapy. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca JE, Santos MJ, Canhao H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Lindroos J, Svensson L, Norsgaard H, Lovato P, Moller K, Hagedorn PH, et al. IL-23-mediated epidermal hyperplasia is dependent on IL-6. The Journal of investigative dermatology. 131:1110–1118. doi: 10.1038/jid.2010.432. [DOI] [PubMed] [Google Scholar]
- 6.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EG, Mickle-Kawar BM, Gallucci RM. IL-6 deficiency exacerbates skin inflammation in a murine model of irritant dermatitis. Journal of immunotoxicology. 2013;10:192–200. doi: 10.3109/1547691X.2012.707700. [DOI] [PubMed] [Google Scholar]
- 8.Balto K, Sasaki H, Stashenko P. Interleukin-6 deficiency increases inflammatory bone destruction. Infect Immun. 2001;69:744–750. doi: 10.1128/IAI.69.2.744-750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiCosmo BF, Picarella D, Flavell RA. Local production of human IL-6 promotes insulitis but retards the onset of insulin-dependent diabetes mellitus in non-obese diabetic mice. International immunology. 1994;6:1829–1837. doi: 10.1093/intimm/6.12.1829. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun R, Tian Z, Kulkarni S, Gao B. IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners. Journal of immunology. 2004;172:5648–5655. doi: 10.4049/jimmunol.172.9.5648. [DOI] [PubMed] [Google Scholar]
- 12.Samavedam UK, Kalies K, Scheller J, Sadeghi H, Gupta Y, Jonkman MF, et al. Recombinant IL-6 treatment protects mice from organ specific autoimmune disease by IL-6 classical signalling-dependent IL-1ra induction. Journal of autoimmunity. 2013;40:74–85. doi: 10.1016/j.jaut.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 14.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. American journal of physiology Endocrinology and metabolism. 2003;285:433–437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 15.Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. Journal of autoimmunity. 2013;40:28–44. doi: 10.1016/j.jaut.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–196. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- 17.Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. Journal of immunology. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kida H, Yoshida M, Hoshino S, Inoue K, Yano Y, Yanagita M, et al. Protective effect of IL-6 on alveolar epithelial cell death induced by hydrogen peroxide. American journal of physiology Lung cellular and molecular physiology. 2005;288:342–349. doi: 10.1152/ajplung.00016.2004. [DOI] [PubMed] [Google Scholar]
- 19.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, et al. Interleukin-6-induced protection in hyperoxic acute lung injury. American journal of respiratory cell and molecular biology. 2000;22:535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 20.Chao KC, Chao KF, Chuang CC, Liu SH. Blockade of interleukin 6 accelerates acinar cell apoptosis and attenuates experimental acute pancreatitis in vivo. The British journal of surgery. 2006;93:332–338. doi: 10.1002/bjs.5251. [DOI] [PubMed] [Google Scholar]
- 21.Fox PC. Autoimmune diseases and Sjogren’s syndrome: an autoimmune exocrinopathy. Ann N Y Acad Sci. 2007;1098:15–21. doi: 10.1196/annals.1384.003. [DOI] [PubMed] [Google Scholar]
- 22.Mavragani CP, Moutsopoulos HM. Conventional therapy of Sjogren’s syndrome. Clinical reviews in allergy & immunology. 2007;32:284–291. doi: 10.1007/s12016-007-8008-3. [DOI] [PubMed] [Google Scholar]
- 23.Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjogren’s Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. doi: 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thanou-Stavraki A, James JA. Primary Sjogren’s syndrome: current and prospective therapies. Seminars in arthritis and rheumatism. 2008;37:273–292. doi: 10.1016/j.semarthrit.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren’s syndrome: contributors to and regulators of pathophysiology. Clinical reviews in allergy & immunology. 2007;32:252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 26.Lee BH, Tudares MA, Nguyen CQ. Sjogren’s syndrome: an old tale with a new twist. Arch Immunol Ther Exp (Warsz) 2009;57:57–66. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolov NP, Illei GG. Pathogenesis of Sjogren’s syndrome. Curr Opin Rheumatol. 2009;21:465–470. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobon GJ, Pers JO, Youinou P, Saraux A. B cell-targeted therapies in Sjogren’s syndrome. Autoimmun Rev. 9:224–228. doi: 10.1016/j.autrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat Rev Rheumatol. 6:529–537. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 30.van Woerkom JM, Kruize AA, Wenting-van Wijk MJ, Knol E, Bihari IC, Jacobs JW, et al. Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjogren’s syndrome compared with non-Sjogren’s sicca syndrome. Annals of the rheumatic diseases. 2005;64:1474–1479. doi: 10.1136/ard.2004.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roescher N, Tak PP, Illei GG. Cytokines in Sjogren’s syndrome: potential therapeutic targets. Annals of the rheumatic diseases. 2010;69:945–948. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin JO, Yu Q. T Cell-Associated Cytokines in the Pathogenesis of Sjogren’s Syndrome. Journal of clinical & cellular immunology. 2013 doi: 10.4172/2155-9899.S1-009. S! [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. American journal of physiology Cell physiology. 2008;295:1191–1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. American journal of physiology Cell physiology. 2012;302:1331–1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauley KM, Gauna AE, Grichtchenko II, Chan EK, Cha S. A secretagogue-small interfering RNA conjugate confers resistance to cytotoxicity in a cell model of Sjogren’s syndrome. Arthritis and rheumatism. 2011;63:3116–3125. doi: 10.1002/art.30450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kajiya M, Ichimonji I, Min C, Zhu T, Jin JO, Yu Q, et al. Muscarinic type 3 receptor induces cytoprotective signaling in salivary gland cells through epidermal growth factor receptor transactivation. Molecular pharmacology. 2012;82:115–124. doi: 10.1124/mol.111.077354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamachi M, Kawakami A, Yamasaki S, Hida A, Nakashima T, Nakamura H, et al. Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor alpha and interferon gamma. The Journal of laboratory and clinical medicine. 2002;139:13–19. doi: 10.1067/mlc.2002.120648. [DOI] [PubMed] [Google Scholar]
- 38.Ishimaru N, Arakaki R, Yoshida S, Yamada A, Noji S, Hayashi Y. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjogren’s syndrome-like autoimmune exocrinopathy. The Journal of experimental medicine. 2008;205:2915–2927. doi: 10.1084/jem.20080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishimaru N, Arakaki R, Omotehara F, Yamada K, Mishima K, Saito I, et al. Novel role for RbAp48 in tissue-specific, estrogen deficiency-dependent apoptosis in the exocrine glands. Molecular and cellular biology. 2006;26:2924–2935. doi: 10.1128/MCB.26.8.2924-2935.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuma A, Hoshino K, Ohba T, Fukushi S, Aiba S, Akira S, et al. Enhanced apoptosis by disruption of the STAT3-IkappaB-zeta signaling pathway in epithelial cells induces Sjogren’s syndrome-like autoimmune disease. Immunity. 2013;38:450–460. doi: 10.1016/j.immuni.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Grisius MM, Bermudez DK, Fox PC. Salivary and serum interleukin 6 in primary Sjogren’s syndrome. J Rheumatol. 1997;24:1089–1091. [PubMed] [Google Scholar]
- 42.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren’s syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford) 2001;40:656–6561. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 43.Ferran C, Sheehan K, Dy M, Schreiber R, Merite S, Landais P, et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. European journal of immunology. 1990;20:509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- 44.Matthys P, Dillen C, Proost P, Heremans H, Van Damme J, Billiau A. Modification of the anti-CD3-induced cytokine release syndrome by anti-interferon-gamma or anti-interleukin-6 antibody treatment: protective effects and biphasic changes in blood cytokine levels. European journal of immunology. 1993;23:2209–2216. doi: 10.1002/eji.1830230924. [DOI] [PubMed] [Google Scholar]
- 45.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22:7809–7818. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- 46.Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cellular signalling. 2013;25:898–909. doi: 10.1016/j.cellsig.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 48.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 49.Jee SH, Chiu HC, Tsai TF, Tsai WL, Liao YH, Chu CY, et al. The phosphotidyl inositol 3-kinase/Akt signal pathway is involved in interleukin-6-mediated Mcl-1 upregulation and anti-apoptosis activity in basal cell carcinoma cells. The Journal of investigative dermatology. 2002;119:1121–1127. doi: 10.1046/j.1523-1747.2002.19503.x. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–2065. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Matsumura R, Umemiya K, Goto T, Nakazawa T, Ochiai K, Kagami M, et al. Interferon gamma and tumor necrosis factor alpha induce Fas expression and anti-Fas mediated apoptosis in a salivary ductal cell line. Clinical and experimental rheumatology. 2000;18:311–318. [PubMed] [Google Scholar]
- 52.Saito I, Haruta K, Shimuta M, Inoue H, Sakurai H, Yamada K, et al. Fas ligand-mediated exocrinopathy resembling Sjogren’s syndrome in mice transgenic for IL-10. Journal of immunology. 1999;162:2488–2494. [PubMed] [Google Scholar]
- 53.Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjogren’s syndrome through fas-mediated apoptosis. The American journal of pathology. 1999;155:173–181. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manganelli P, Fietta P. Apoptosis and Sjogren syndrome. Seminars in arthritis and rheumatism. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 55.Bolstad AI, Eiken HG, Rosenlund B, Alarcon-Riquelme ME, Jonsson R. Increased salivary gland tissue expression of Fas, Fas ligand, cytotoxic T lymphocyte-associated antigen 4, and programmed cell death 1 in primary Sjogren’s syndrome. Arthritis and rheumatism. 2003;48:174–185. doi: 10.1002/art.10734. [DOI] [PubMed] [Google Scholar]
- 56.Jin JO, Kawai T, Cha S, Yu Q. Interleukin-7 enhances the Th1 response to promote the development of Sjogren’s syndrome-like autoimmune exocrinopathy in mice. Arthritis and rheumatism. 2013;65:2132–2142. doi: 10.1002/art.38007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 58.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Seminars in immunology. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren’s syndrome: findings in humans and mice. Arthritis and rheumatism. 2008;58:734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren’s syndrome immunopathogenesis. The American journal of pathology. 2009;175:1167–1177. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang KY, Kim HO, Kwok SK, Ju JH, Park KS, Sun DI, et al. Impact of interleukin-21 in the pathogenesis of primary Sjogren’s syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis research & therapy. 2011;13:179. doi: 10.1186/ar3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]