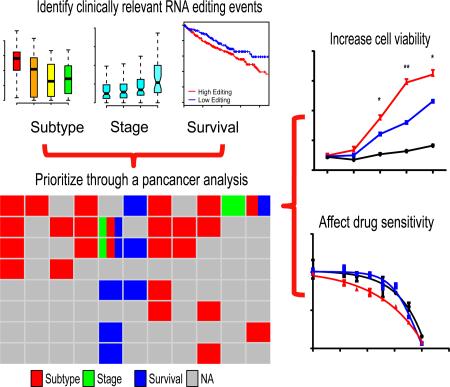
Summary
Adenosine-to-inosine (A-to-I) RNA editing is a widespread post-transcriptional mechanism, but its genomic landscape and clinical relevance in cancer have not been investigated systematically. We characterized the global A-to-I RNA editing profiles of 6236 patient samples of 17 cancer types from The Cancer Genome Atlas and revealed a striking diversity of altered RNA-editing patterns in tumors relative to normal tissues. We identified an appreciable number of clinically relevant editing events, many of which are in noncoding regions. We experimentally demonstrated the effects of several cross-tumor nonsynonymous RNA editing events on cell viability and provide the evidence that RNA editing could selectively affect drug sensitivity. These results highlight RNA editing as an exciting theme for investigating cancer mechanisms, biomarkers and treatments.
Graphical Abstract
Introduction
RNA editing is a widespread post-transcriptional mechanism that confers specific and reproducible nucleotide changes in selected RNA transcripts (Bass, 2002; Keegan et al., 2001). As for functional consequences, RNA editing events can result in missense codon changes (Maas and Rich, 2000), modulation of alternative splicing (Rueter et al., 1999), or modification of regulatory RNAs (Kawahara et al., 2007; Tomaselli et al., 2015) and their binding sites (Liang and Landweber, 2007). In humans, the most common type of RNA editing is adenosine to inosine (A to I)(Piskol et al., 2013), which is catalyzed by ADAR enzymes (Bass et al., 1997). Despite some issues in earlier attempts, recently several groups have developed computational methods for accurately detecting A-to-I RNA editing from next-generation sequencing data on a large scale (Bahn et al., 2012; Peng et al., 2012; Ramaswami et al., 2012; Ramaswami et al., 2013). As a result, more than a million A-to-I RNA editing sites have been confidently detected in the human genome (Bazak et al., 2014; Ramaswami et al., 2013). However, the vast majority of these sites are in noncoding and repetitive element regions of the genome and have unknown functional relevance. Therefore, the research focus on RNA editing has moved from identification of novel sites to characterization of the mechanisms by which they mediate their functions and their consequences on cellular function.
To date, a critical role of A-to-I RNA editing in human cancer has been reported for only individual examples. In prostate cancer, A-to-I RNA editing in the androgen receptor impairs the protein's ability to interact with androgenic or anti-androgenic ligands (Martinez et al., 2008); in liver cancer, the edited form of AZIN1 has a stronger affinity for antizyme and induces cytoplasmic-to-nuclear translocation of AZIN1, and a low editing level is sufficient to confer more aggressive tumor behavior (Chen et al., 2013); in colorectal cancer, A-to-I RNA editing in RHOQ promotes the invasion potential (Han et al., 2014); and in gliboblastoma, ADAR2-mediated RNA editing in CDC14B modulates the Skp2/p21/p27 pathway and plays a critical role in the pathogenesis of this disease (Galeano et al., 2013). Despite these intriguing findings, the global pattern of A-to-I RNA editing in human cancer genomes have not been systematically characterized, and the functional importance and clinical relevance of RNA editing in cancer remain largely unknown. Here, we aimed to address these questions through a systematic analysis of A-to-I RNA editing events using RNA-sequencing data from The Cancer Genome Atlas (TCGA) project (The Cancer Genome Atlas Research Network et al., 2013).
Results
Overview of A-to-I RNA editing patterns across major cancer types
To perform a comprehensive, high-quality analysis of A-to-I RNA editing in cancer genomes, we developed a computational pipeline based on ~1.4 million high-confidence RNA editing sites annotated in the Rigorously Annotated Database of A-to-I RNA Editing (RADAR) (Ramaswami and Li, 2014) (Figure S1A). The RNA editing sites in RADAR were collected from recent transcriptome-wide RNA-editing identification studies and underwent extensive manual curation. We further applied a series of filters to remove the potential contamination of SNPs or somatic mutations (Experimental Procedures). Thus, this RNA editing dataset represent a reliable and global candidate set to start with. From TCGA RNA-seq data, we assessed the RNA-editing signals at these candidate sites in 6236 samples of 17 cancer types or related normal tissues (Table 1 and Figure 1A). For each cancer type, we detected a large number of RNA editing candidate sites with editing signals but many of them were only sufficiently covered in a limited sample set. Therefore, we defined “informative” RNA editing sites as those sites with detected signals and coverage ≥10× in ≥30 tumor samples (and related normal samples) for a cancer type (Experimental Procedures) and focused on these sites in subsequent analyses to ensure adequate statistical power. The number of informative RNA editing sites per cancer type ranged from 8493 in CRC to 76555 in BRCA (Figure 1B). This large variation among cancer types is mainly because (i) the number of tumor samples per cancer type varied markedly (from 66 in KICH to 837 in BRCA, Table 1) and (ii) the number of mappable reads per sample varied greatly among cancer types due to different sequencing strategies (from 22 million in CRC to 174 million in KICH, Table 1). Indeed, across the 17 cancer types, the number of informative editing sites showed a strong linear correlation with the total number of mappable bases or the total number of mappable reads (Figure 1B, Pearson Correlation R = 0.84, p = 2.0×10−5, Spearman Correlation Rs = 0.75, p = 7.4×10−4; Figure S1B, R = 0.89, p = 2.0×10−6, Rs = 0.82, p = 7.0×10−5). These results also indicate that the informative editing sites we identified show no significant bias towards one or a few well-studied cancer types.
Table 1.
Summary of TCGA RNA-seq data used in this study
| Cancer Type Name (TCGA code) | Num of normal samples | Num of tumor Samples | Sequence strategy | Read length | Average mappable reads | Num of informative editing sites |
|---|---|---|---|---|---|---|
| Colorectal cancer (CRC) | 0 | 228 | Single-end | 76 | 21793066 | 8493 |
| Uterine corpus endometrioid carcinoma (UCEC) | 4 | 316 | Single-end | 76 | 25324332 | 14217 |
| Glioblastoma multiforme (GBM) | 0 | 154 | Paired-end | 76 | 106403279 | 37934 |
| Lung Adenocarcinoma (LUAD) | 58 | 488 | Paired-end | 48 | 133297582 | 54362 |
| Liver hepatocellular carcinoma (LIHC) | 50 | 200 | Paired-end | 48 | 139117210 | 23540 |
| Bladder Urothelial Carcinoma (BLCA) | 19 | 252 | Paired-end | 48 | 144059158 | 39270 |
| Kidney renal papillary cell carcinoma (KIRP) | 30 | 198 | Paired-end | 48 | 146793890 | 36686 |
| Prostate adenocarcinoma (PRAD) | 52 | 374 | Paired-end | 48 | 147246105 | 43078 |
| Brain Lower Grade Glioma (LGG) | 0 | 486 | Paired-end | 48 | 149851835 | 51806 |
| Head and Neck Squamous Cell Carcinoma (HNSC) | 42 | 426 | Paired-end | 48 | 157436457 | 35510 |
| Cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) | 3 | 196 | Paired-end | 48 | 161207521 | 32797 |
| Breast invasive carcinoma (BRCA) | 105 | 837 | Paired-end | 50 | 161673379 | 76555 |
| Kidney renal clear cell carcinoma (KIRC) | 67 | 448 | Paired-end | 50 | 166049114 | 63717 |
| Stomach adenocarcinoma (STAD) | 33 | 285 | Paired-end | 75 | 169720033 | 26389 |
| Lung squamous cell carcinoma (LUSC) | 17 | 220 | Paired-end | 50 | 171002267 | 36822 |
| Thyroid carcinoma (THCA) | 59 | 498 | Paired-end | 48 | 171399819 | 52701 |
| Kidney Chromophobe (KICH) | 25 | 66 | Paired-end | 48 | 174113816 | 22317 |
Figure 1. Overview of A-to-I RNA editing patterns in human cancer.
(A) Numbers of TCGA tumor and normal samples analyzed in this study. (B) A correlation between the number of total mappable RNA-seq bases and the number of informative RNA editing sites across different cancer types. (C) The editing-level distributions at informative editing sites in different cancer types. Dashed and solid lines denote average and median for each cancer type, respectively. (D) The distributions of informative RNA editing sites in different types of RNA regions. See also Figure S1.
We first quantified the editing levels at the informative RNA editing sites (defined as the proportion of edited reads among the total mapped reads at a given site in a TCGA BAM file). As a quality control, we randomly selected a few samples, remapped the raw RNA-seq reads using the previously established mapping pipeline that can accurately detect both Alu and non-Alu RNA editing events (Ramaswami et al., 2012; Ramaswami et al., 2013), and obtained very consistent RNA-editing levels (Spearman correlation Rs > 0.93). Thus, for the informative edited sites surveyed, the employed RNA-seq mapping procedures had little effect on the quantification of RNA-editing levels. Figure 1C shows the overall editing-level distributions at informative RNA editing sites in different cancer types (Figure S1C shows the distributions in normal tissues). We next examined the distribution of these editing sites in different types of transcribed regions (Figure 1D). Across different cancer types, most of the informative RNA editing sites were in 3′ UTRs and intronic regions, as observed previously in mouse tissues (Gu et al., 2012), but the editing sites in coding regions were relatively limited (Figure S1D shows the numbers of nonsynonymous and synonymous RNA editing sites in different cancer types). Furthermore, we did not detect any correlation between RNA-editing level and the local GC content (Figure S1E). Since our analysis was based on RNA-seq data, the observed genomic distribution of informative RNA editing sites could be affected by the coverage bias of the mRNA-seq platform. However, due to the large number of candidate editing sites examined, we still obtained sufficient sampling power to survey RNA editing activities in different transcribed regions.
Diversity of RNA editing patterns in tumors relative to normal samples
The global RNA-editing differences between cancer samples and related normal tissues remain largely uncharacterized, and previous studies have suggested this is a complex topic (Jiang et al., 2013; Maas et al., 2001; Nemlich et al., 2013; Qin et al., 2014; Tomaselli et al., 2015). For example, an earlier study found reduced editing in brain tumors (Paz et al., 2007); while a recent study suggests a mixture of gene-specific hyper- and hypo-editing activities in liver cancer (Chan et al., 2014). However, these studies are either based on a small set of RNA editing sites or limited to a single cancer type. To obtain a comprehensive view of RNA editing patterns in tumor samples, we focused on 12 tumor types with available RNA-seq data for matched normal tissues from the same patients (Figure 2A). For each cancer type, we identified RNA editing sites with significantly differential editing activity between matched tumor and normal samples (paired Wilcoxon test, false discovery rate [FDR] < 0.05 and the mean editing-level difference among comparison groups, Diff ≥ 5%). Although with this criterion the editing levels at the most sites remain similar, we observed a great diversity of “altered” RNA editing patterns across these cancer types: significant numbers of RNA editing sites showed over-editing patterns in HNSC, BRCA, THCA and LUAD tumors, while significant numbers of editing sites showed under-editing patterns in KICH and KIRP tumors.
Figure 2. Comparison of the overall A-to-I RNA editing patterns between paired tumor and normal samples.
(A) Numbers of over-editing sites and under-editing sites across different cancer types. (B) The correlation between the “net” proportion of over-editing sites (defined as the percentage of over-editing sites minus the percentage of under-editing sites) and the relative mRNA expression of ADAR1 (left), ADAR2 (middle), and ADAR3 (right) (fold change relative to normal tissues). To robustly detect a meaningful relation, the rank-based Spearman correlations were used and plotted. (C) Distribution of editing-level difference in BRCA relative to matched normal breast tissue samples (left panel) and the mRNA expression level of ADAR1 (right panel) (red in tumor and blue in normal). (D) Distribution of editing level difference in KICH samples relative to matched normal kidney samples. (A) and (B) over-editing sites are in red; under-editing sites are in blue. (C) and (D) The paired Wilcoxon test was used to assess the difference between paired tumor and normal samples. The boxes show the median±1 quartile, with whiskers extending to the most extreme data point within 1.5 interquartile range from the box boundaries. See also Table S1.
To identify the molecular determinants underlying these patterns, we performed two complementary analyses: one focusing on the general pattern across cancer types and the other focusing the editing abundance within each cancer type. We first analyzed the correlations of ADAR expression with the “net” proportion of over-editing RNA sites (defined as the percentage of over-editing sites minus the percentage of under-editing sites) and found that the proportion was highly correlated with the relative ADAR1 mRNA expression level (defined as the fold change relative to normal samples) (Spearman correlation Rs = 0.70, p = 0.014, Figure 2B), but not with that of ADAR2 (Rs = 0.38, p = 0.22, Figure 2B) nor ADAR3 (Rs = −0.007, p = 0.99, Figure 2B). Figure 2C and 2D show the detailed RNA-editing change patterns in two representative cancer types. In BRCA, 12770 (16.7%) informative RNA editing sites showed significant over-editing in the tumor samples than matched normal samples; whereas only 553 (1.2%) showed significant under-editing in tumor samples (paired Wilcoxon test, FDR < 0.05, Figure 2C, left panel). In contrast, in KICH, only 110 (0.5%) informative RNA editing sites showed over-editing in the tumor samples; whereas 4318 (19.3%) showed under-editing in tumor samples (paired Wilcoxon test, FDR < 0.05, Figure 2D, left panel). Indeed, ADAR1 mRNA expression was much higher in BRCA than in matched normal samples (fold change = 1.81, paired Wilcoxon test, p < 2.2×10−16, Figure 2C, right panel); while ADAR1 was significantly under-expressed in KICH (fold change = 0.76, paired Wilcoxon test, p = 1.6×10−4, Figure 2D, right panel). We further performed sample-based analysis within each cancer type and found that the number of informative sites with editing signals shows the strongest correlation with the ADAR1 mRNA expression among the three ADAR enzymes (Table S1). These results suggest that the global altered RNA-editing patterns in tumors are more likely to be affected by ADAR1 than the other two editing enzymes. However, since the mRNA expression of ADAR may not directly reflect enzyme editing activity (Wahlstedt et al., 2009) and the dimer formation and the interactions among the ADAR enzymes could be important for editing activity (Chen et al., 2000; Chilibeck et al., 2006; Cho et al., 2003), further efforts are required to elucidate the relative contributions of the three ADAR enzymes to the observed RNA editing patterns.
An appreciable level of clinically relevant RNA editing sites in various cancer types
Given the large number of A-to-I RNA editing events observed across tumor types and distinct editing patterns at some sites between tumor and normal tissues, a fundamental question is what fraction of RNA editing events in tumors are functionally tumorigenic or clinically valuable. To address this question, we focused on the RNA editing sites showing correlations with tumor subtype, clinical stage and patient survival. Clinical stage and patient survival are well-established clinical variables, while tumor subtype often facilitates clinical decisions. In a sense, they all characterize intertumoral heterogeneity among the same disease. Thus, we referred to RNA editing sites showing non-random editing patterns in regard to these biologically and clinically meaningful parameters as “clinically relevant editing sites”. Specifically, we identified such sites within each cancer type using three complementary computational analyses (Figure 3A): (i) differential analysis of RNA editing level among established tumor subtypes (FDR < 0.01, Diff ≥ 5%), which identified 2660 RNA editing sites in total; (ii) differential analysis of RNA editing level among tumor stages (FDR < 0.05, Diff ≥ 5%), which identified 684 RNA editing sites in total; and (iii) correlation analysis of RNA editing level with patient overall survival (FDR < 0.05, Diff ≥ 5%), which identified 1,130 RNA editing sites in total. Among the 17 cancer types, 12 cancers contained such clinically relevant sites, ranging from 4 in PRAD to 2059 in BRCA (Table S2). To rule out the potential confounding effect of tumor purity, we repeated the analysis using ABSOLUTE-based (Carter et al., 2012) tumor purity as a covariate. For the nine cancer types with available tumor purity data, we found that 97.9% of the clinically relevant sites originally identified still remain significant (Table S2). We also calculated the correlation of ADAR expression levels with tumor purity and found no strong correlation (Table S2). Therefore, tumor purity appeared to have little effect on our results.
Figure 3. Identification and patterns of clinically relevant RNA editing sites.
(A) The overview of clinically relevant RNA editing sites identified by three complementary computational analyses: differential analysis among tumor subtypes, differential analysis among tumor stages, and correlation analysis with patient overall survivals. An explicative cartoon is shown for illustration purposes. (B-D) Statistical significance for the enrichment or depletion patterns of clinically relevant RNA editing sites through coverage-dependent permutation tests across 12 tumor types for different types of RNA regions: gene annotation (B), non-repetitive (C), non-Alu repetitive and Alu elements, and evolutionary conservation (D). See also Table S2.
In order to investigate the distributions of clinically relevant editing sites in different types of RNA regions, we classified the RNA editing sites from three parallel perspectives: gene annotation, sequence repetitive elements and evolutionary conservation. Since the power to detect clinically relevant editing sites in our analysis was affected by sample size and quality of clinical data (e.g., the follow-up time) in a given cancer type, we examined the distribution patterns for each cancer type separately. Further, given the potential effects of coverage bias in different RNA regions due to gene expression or the mRNA-seq platform, instead of directly comparing the proportions of clinically relevant RNA editing sites among different RNA regions, we performed a coverage-dependent permutation test to assess the enrichment/depletion patterns (Experimental Procedures). In terms of gene annotation, we found that clinically relevant RNA editing sites tend to be in noncoding RNAs as well as in nonsynomous and intronic regions in some cancer types (Figure 3B). In terms of sequence repetitive elements, clinically relevant sites show consistent depletion patterns in Alu elements (Figure 3C). In terms of evolutionary conservation, clinically relevant sites tend to be conserved among humans, chimpanzees and macaques (Figure 3D). Together, these analyses based on different types of RNA-region classification help to understand which factors affect the overall distributions of clinically relevant RNA editing sites.
“Driver” functional effects of clinically relevant nonsynonymous RNA editing events
Since clinically relevant RNA editing events at nonsynonymous sites could directly result in amino acid changes, we focused on these RNA editing sites and assessed their functional effects experimentally. To boost the discovery power, we performed the above analyses for nonsynonymous RNA editing sites with a relaxed FDR cutoff and identified 35 RNA editing sites with potential clinical relevance (FDR < 0.2, Diff > 5%, Table S3). Interestingly, 8 out of these RNA editing events (22.9%) showed clinically relevant patterns in more than one cancer type (Figure 4A, Figure S2). This pan-cancer analysis suggests that some A-to-I nonsynonymous RNA editing may be a “master” driver event and play a critical functional role in different tumor contexts. We focused on four top editing candidate sites (S367G in AZIN1, I164V in COPA, I635V in COG3 and R764G in GRIA2) for further investigation (Figure 4A and Figure S2B). The functional effects of RNA editing at the residue S367G in AZIN1 (identified in eight cancer types by our analysis, Figure 4B and Figure S2C) have been characterized in liver cancer (Chen et al., 2013). Differential editing activity at I164V in COPA (identified in seven cancer types, Figure 4C and Figure S2D) between tumor and normal samples has been reported in liver cancer (Chan et al., 2014) but has not been functionally characterized. I635V at COG3 (identified in six cancer types, Figure 4D and Figure S2E) was only reported in a recent RNA-editing methodology study (Ramaswami et al., 2012). GRIA2 (also known as GluR-B) contains two known RNA editing sites: the Q607R editing in the second transmembrane domain is well studied (Herb et al., 1996; Higuchi et al., 1993) but has insufficient coverage in our dataset; the role of R764G (identified in two cancer types, Figure 4E) has not been functionally characterized in cancer. We confirmed the occurrence of these RNA editing events in an independent set of breast tumor samples using an orthogonal Sequenom approach (Figure 5A and Figure S3A).
Figure 4. Clinical relevance of nonsynonymous A-to-I RNA editing sites.
(A) The clinical relevance of 8 nonsynonymous RNA editing sites identified in multiple cancer types. For each cancer type, the grey box indicates not significant, the red box indicates the significant differential editing among tumor subtypes (FDR < 0.2, Diff ≥ 5%), the green box indicates the significant differential editing among stages (FDR < 0.2, Diff ≥ 5%), the blue box indicates the association with the overall survival (FDR < 0.2, Diff ≥ 5%). (B-E) The representative plots showing clinical relevance of nonsynonymous RNA editing events in AZIN1S367D (CRC subtype: CIN, chromosomal instability; MSI, microsatellite instability) (B), COPAI164V (STAD subtype: CIN, chromosomal instability; EBV, Epstein–Barr virus (EBV)-positive; GS, genomically stable; MSI, microsatellite instability) (C), COG3I635V (D) and GRIA2R764G (E). The boxes show the median±1 quartile, with whiskers extending to the most extreme data point within 1.5 interquartile range from the box boundaries. See also Figure S2, Table S3.
Figure 5. Sequenom validation and functional effects of nonsynonymous RNA editing sites on cell viability.
(A) Sequenom validation of AZIN1S367D. The upper panels show the results of a group of samples at cDNA and gDNA, respectively, where each blue symbol represents the AG genotype of a sample; while the bottom panels show the results of an individual sample in cDNA and gDNA, respectively, where there are one “A” peak and one “G” peak in cDNA but only one “A” peak in gDNA. (B) The effects of AZIN1S367D, GRIA2R764G and COG3I635V in MCF10A cell viability assays. (C) The effects of AZIN1S367D, GRIA2R764G and COG3I635V in BaF3 cell viability assays. Two-sided t-test was used to assess the difference. Error bars denote +/− SEM, * denotes p < 0.05, ** denotes p <0.001, and *** denotes p < 0.0001. See also Figure S3.
Given the availability of high-quality antibodies, we assessed the functional effects of the editing events in AZIN1, GRIA2 and COG3 using various functional assays. To examine the effects on cell proliferation or survival, we performed cell viability assays (upon overexpression) in MCF10A cells, a normal human breast epithelial cell line. Given similar levels of wild-type and edited proteins (Figure S3B), the edited AZIN1 (AZIN1S367G), GRIA2 (GRIA2R764G) and COG3(COG3I635V) significantly increased cell survival relative to the wild-type gene (t-test, p < 0.05, Figure 5B; see Experimental Procedures). We obtained similar results of cell viability assays based on cell counting (Figure S3C). Since these RNA editing events show cross-tumor clinical relevance, we further examined their effects in a different lineage. We performed similar viability assays in Ba/F3 cells, which is a murine leukemia cell line and an established drug screening platform for subsequent investigation (Cheung et al., 2014; Liang et al., 2012), and observed the same patterns (t-test, p < 0.05, Figure 5C). To examine the effects on cell survival, we assessed levels of active caspase 3 in MCF10A and found no significant changes (Figure S3D). These results were confirmed by a cell death detection ELISA kit (data not shown). To examine the effects on cell migration, we performed wound healing assays in MFC10A and observed no substantial effects (Figure S3E).
Therapeutic liability of clinically relevant nonsynonymous RNA editing sites
A critical question about RNA editing is whether some RNA editing could affect the response of cancer therapies. This question has significant clinical implications but has never been investigated. Given their confirmed “driver” behaviors in Ba/F3 (Figure 5C), we focused on the RNA editing in AZIN1, GRIA2 and COG3 and examined whether these events alter drug sensitivity using a high-throughput Ba/F3 differential cytotoxicity screen (Cheung et al., 2014; Quayle et al., 2012). Ba/F3 cells depend on interleukin-3 (IL-3) for proliferation, but readily become IL3-independent in the presence of an oncogene or oncogenic event (Liang et al., 2012). We screened 145 compounds targeting major signaling pathways in Ba/F3 addicted to these RNA editing events (in the absence of IL-3) and performed a “counterscreen” with the same Ba/F3 cells cultured with exogenous IL-3 to control for the cytotoxic activity of the compounds. In addition, we used a spontaneously transformed Ba/F3 cell line (originally transfected with PIK3R1 but not expressing significant levels of PIK3R1) as negative controls. Strikingly, compared to the wild-type genes, the edited genes selectively affected the sensitivity of Ba/F3 cells to several targeted therapeutics, including AZIN1S367G for the IGF-1R inhibitor BMS536924, GRIA2R764G for MEK inhibitors CI1040 and PD0325901, COG3I635V for MEK inhibitors CI1040, PD0325901 and trametinib (Figure 6A shows representative examples).
Figure 6. Effects of nonsynonymous RNA editing sites on drug sensitivity.
(A) Spontaneously transformed Ba/F3 cells (negative control), Ba/F3 cells stably expressing AZIN1 and AZIN1S367D, GRIA2 and GRIA2R764G , COG3 and COG3I635V were screened against the drug library with or without IL-3 for 72 hr. Dose-response curves for the IGF-1R inhibitor BMS536924, the MEK inhibitors CI1040 and trametinib. The drugs were dissolved in DMSO, and only DMSO was added at the drug concentration of 0 as a control. At each drug dosage, the relative cell viability (measured based on three independent replicates) was obtained by normalizing the absolute cell viability to the DMSO control to remove the baseline difference between Ba/F3 cells with and without IL-3. Error bars denote +/− SD. (B) A heatmap showing the correlations of the RNA editing levels of 35 clinically relevant nonsynonymous sites with the IC50 values of 24 clinical drugs across CCLE cell line. The highlighted boxes indicate significant correlations at FDR < 0.1.
Furthermore, we examined the editing levels of the 35 clinically relevant nonsynonymous RNA editing sites (Table S3) in cell lines from the Cancer Cell Line Encyclopedia (CCLE) (Barretina et al., 2012) and examined their correlations with the sensitivity data (IC50) of 24 drugs available at the CCLE portal. Interestingly, we found that the editing levels of 16 RNA editing sites were significantly correlated with drug sensitivity (FDR < 0.1, Figure 6B). Furthermore, across RNA editing sites, the drug clustering analysis showed meaningful patterns: three chemotherapy agents, paclitaxel, irinotecan and topotecan, were clustered together and their sensitivities were associated with the editing in AZIN1 and other sites; erlotinib was in the same cluster as the HER2 agent lapatinib; two RAF inhibitors, PLX4720 and RAF265, were adjacent to one another; and two MEK inhibitors, AZD6244 and PD0325901, were tightly correlated. These results suggest that the effects of RNA editing on drug response are not limited to the cases we examined.
Discussion
The advent of next-generation sequencing data has drawn widespread attention to the analysis of RNA editing (Li et al., 2011; Peng et al., 2012; Piskol et al., 2013; Ramaswami et al., 2012; Ramaswami et al., 2013); however, these studies have mainly focused on RNA editing events in normal tissues. More recently, a functional role for RNA editing in tumorigenesis has begun to emerge, but related studies have been limited to individual examples. The present study represents a systematic investigation of the global pattern and clinical relevance of A-to-I RNA editing across a broad range of cancer types and normal tissues.
The number of A-to-I RNA editing sites in humans is huge, but most sites exhibit editing at very low levels (Bazak et al., 2014), leading to a great challenge in detecting editing sites in a comprehensive manner. To ensure the high-quality analysis, we started with the high-confidence RNA editing sites reported in previous studies rather than calling novel editing sites without prior knowledge. We focused on RNA editing events with detected editing signals in multiple TCGA samples and further filtered those with potential mutational signals at the DNA level. Although “false” RNA-editing sites due to SNPs or mutations might not be completely removed, such noise in our data should be very rare. Because of the large number of RNA editing candidates identified in normal tissues in the RADAR database, we obtained sufficient numbers of RNA editing sites to assess the global patterns of A-to-I RNA editing. Further, the strong linear correlation between informative editing sites per cancer type and the total number of mapped reads (or bases) across cancer types indicates that our RNA editing sets are not biased towards well-studied cancer types.
The rich TCGA dataset allowed us to address some important questions about RNA editing on a large scale. We revealed a diversity of altered RNA editing events in tumor samples relative to normal tissues, which correlates best with the ADAR1 expression level globally. Note that this observation does not rule out the important role of ADAR2-mediated editing events in specific cancer types, as demonstrated in previous studies (Cenci et al., 2008; Galeano et al., 2013; Maas et al., 2001; Tomaselli et al., 2015). Based on the correlations of RNA editing levels with tumor subtype, stage or survival, we detected an appreciable number of RNA editing sites with potential clinical relevance (~3.5% of the total informative RNA editing sites examined). These editing sites show marked editing difference for distinct patient groups within a cancer type, and they may represent promising biomarker candidates for further assessment. An alternative way to infer clinically relevance could be based on levels of edited transcripts. However, unlike the editing level, which is a parameter independent from the expression level of the edited gene, the levels of edited transcripts are linked to the gene expression level itself. Indeed, we observed that for a large proportion of RNA editing sites with clinical correlations based on the level of edited transcripts, their gene expression levels also showed corresponding correlations, suggesting the potential confounding effects of gene expression on detecting clinical relevance. Therefore, we focused on the editing-level-based clinically relevant sites in this study. Our datasets (both raw data and clinically relevant sites) have been made publically available through Synapse (Omberg et al., 2013), and thus provide a valuable resource for systematically dissecting the clinical utility of RNA editing.
Importantly, we experimentally investigated the functional effects of several nonsynonymous RNA editing events with potential clinical relevance across multiple tumor types, including the well-studied editing site in AZIN1 and the other two previously functionally uncharacterized RNA editing sites in COG3 and GRIA2. Moreover, our study provides the evidence that a specific RNA editing event could selectively affect therapeutic responses. We demonstrated that the RNA editing event in COG3 and GRIA2 increased sensitivity to some targeted agents whereas the editing in AZIN1 engendered decreased sensitivity. Mutations in cancer genes can increase or decrease sensitivity to the same therapeutic agent based on where they are located in the targeted pathway. For example, mutations in the EGFR receptor increase sensitivity to drugs targeting the EGFR. However, mutations in KRAS which is clearly a driver can result in resistance to EGFR inhibitors. Furthermore, if the editing is a neomorph, it could either increase or decrease to the sensitivity to a specific drug. Thus, some RNA editing events may be functionally equivalent to “driver” mutations, making a notable contribution to tumor initiation and growth as well as playing a critical role in response to cancer therapy. Together, our findings highlight RNA editing as an exciting theme for investigating cancer mechanisms, identifying biomarkers, and developing therapeutic targets. Further efforts should be made to characterize the function of other clinically relevant RNA editing events (especially those in noncoding regions), to elucidate the interactions of these editing events with other types of molecular aberrations, and to investigate their utility in clinical practice.
Experimental Procedures
Characterization of A-to-I RNA editing profiles
We downloaded RNA-seq BAM files of 5672 patient tumor samples across 17 TCGA cancer types and their related 564 non-tumor tissue samples (if available) from the UCSC Cancer Genomics Hub (CGHub, https://cghub.ucsc.edu/). We also downloaded 740 BAM files of CCLE cell lines from CGHub. The detailed read mapping procedure (BAM generation) was previously described in TCGA marker papers (Brennan et al., 2013; The Cancer Genome Atlas Research Network, 2008; The Cancer Genome Atlas Research Network, 2012a; The Cancer Genome Atlas Research Network, 2012b; The Cancer Genome Atlas Research Network, 2012c; The Cancer Genome Atlas Research Network, 2013a; The Cancer Genome Atlas Research Network, 2013b).
We obtained a comprehensive collection of ~1.4 million A-to-I RNA editing sites from the Rigorously Annotated Databases of A-to-I RNA Editing (RADAR, http://rnaedit.com/) (Ramaswami and Li, 2014). Note that these RNA editing sites were directly called from RNA-seq data from normal tissues and tumor samples, not from the comparison of editing profiles upon ADAR perturbation. We re-annotated them by ANNOVAR (Wang et al., 2010), and then filtered ~4,000 sites annotated in dbSNP (version 137), COSMIC and TCGA somatic mutations. Based on the RNA-seq reads mapped to the human reference genome (hg19), the editing level at a specific site in a given sample was calculated as the number of edited reads divided by the total number of reads (Ramaswami et al., 2013), and only the nucleotides with a base quality ≥ 20 were used. Those editing sites with at least 3 edited reads in at least 3 samples per tissue type were considered to be detected RNA editing sites. To ensure adequate statistical power, we further identified the informative RNA editing sites among the detected RNA editing sites by requiring at least 30 samples (including normal samples if available) with a coverage ≥ 10 in a tissue/tumor type. Thus, given a cancer type, the tumor samples and their related normal samples had the same set of informative RNA editing sites in our analysis. To further rule out the possibility of potential contamination due to undetected SNPs or somatic mutations, we obtained whole-genome sequencing data from International Cancer Genome Consortium (ICGC) and whole-exome sequencing data from TCGA for the cancer types we surveyed and assessed if there were some potential mutational signals at informative RNA editing sites. We only found potential mutational signals at 310 sites out of 112572 across the 17 cancer types (0.28%) and excluded them from our analysis. RNA editing profiles were uploaded to Synapse (syn2374375), and are publically available.
Comparisons of RNA editing patterns between cancer and normal samples
For the comparison between tumor and normal samples, we required the informative RNA editing sites with at least 5 pairs of tumor and normal samples with a coverage ≥ 10. If sufficient matched normal samples in which a site had adequate coverage were not available, the site was excluded from our analysis. We used the Wilcoxon test to detect RNA editing sites with differential editing between tumor and normal samples, and defined significantly differential editing sites as FDR < 0.05 and a mean editing level difference ≥ 5%. TCGA mRNA expression data were obtained from TCGA Synapse portal (syn300013) (Omberg et al., 2013). We used the paired student t-test to detect differentially expressed ADAR enzymes between normal and tumor samples.
Identification of clinically relevant RNA editing sites
We obtained clinical information, including tumor subtypes, disease stage, and patient overall survival time from TCGA marker papers, or TCGA data portal (https://tcga-data.nci.nih.gov/tcga/). We used the Wilcoxon test or Kruskal-Wallis nonparametric ANOVA to detect RNA editing sites with differential editing among different tumor subtypes, and considered FDR < 0.01 to be statistically significant. We used the Wilcoxon test or Kruskal-Wallis nonparametric ANOVA to detect RNA editing sites with differential editing among different tumor stages, and considered FDR < 0.05 to be statistically significant. We used the univariate Cox test to examine whether the RNA editing level was significantly correlated with patient survival, and considered FDR < 0.05 to be statistically significant. We chose different FDR cutoffs based on the signal abundance in each analysis. Groups with fewer than 5 samples were excluded from the analysis. We required a mean RNA editing level difference, Diff ≥ 5% for at least two groups, thereby ensuring a sufficient biological difference. The gene-annotation-based RNA type of an RNA editing site was annotated by ANNOVAR, and the sequence repetitive status and evolutionary conservation status (i.e., the conservation among humans, chimpanzees, and macaques) were annotated as in RADAR (Ramaswami and Li, 2014). To test the effect of tumor purity, we obtained the tumor purity data based on ABSOLUTE from synapse (syn1710466, https://www.synapse.org/#!Synapse:syn1710466)(Carter et al., 2012), and repeated the analysis with the tumor purity as a covariate in the ANOVA. We repeated the above analysis for the nonsynonymous RNA editing sites only, and considered FDR < 0.2 to indicate statistical significance. We then ranked the nonsynonymous RNA editing sites based on the number of cancer types with detected significance.
To assess if clinically relevant RNA editing sites are enriched in some RNA regions, we performed a coverage-dependent permutation test. First, for each cancer type, we classified all the informative RNA editing sites into 10 coverage groups (each with the same number of editing sites) based on the median coverage of a given RNA editing site across all sufficiently covered samples. Second, given the numbers of clinically relevant sites observed in each group, we randomly selected the same number of RNA editing sites as “pseudo clinically relevant sites”, so the whole pseudo set would have the same coverage distribution as the true clinically relevant sites. We then counted the frequencies of pseudo clinically relevant sites for each type of RNA region. We repeated this process for 1000 times, and based on the obtained distributions of these permutations, we assessed the statistical significance of the enrichment of the clinically relevant sites relative to the random expectation (defined as the frequency of permutations with the number of pseudo clinically relevant sites no fewer than the observed true clinically relevant sites). We did this analysis for each cancer type separately.
Sequenom validation
Four selected RNA editing sites, AZIN1S367D, COPAI164V, COG3I635V, and GRIA2R764G, were validated on in-house breast cancer samples by Sequenom MassARRAY at MD Anderson Sequenome Core Facility, as previously described (Liang et al., 2012).
Generation of stable BaF3 and MCF10A cell lines
The mutant open reading frames (ORFs) corresponding to the RNA editing sites in AZIN1, GRIA2 (the mutation was introduced at the R764G site only and the codon at Q607R remained as wild-type CAG) and COG3 were made by site-directed mutagenesis and confirmed by Sanger sequencing. Virus were produced by transfecting HEK293PA cells with the GFP control vectors or pHAGE-V5-puromycin expression vectors (carrying AZIN1-WT, AZIN1-S367D, GRIA2-WT, GRIA2-R764G, COG3-WT or COG3-I635V), and the Lentiviral Packaging Mix (psPAX2 and pMD2.G). BaF3 cells were transduced by the virus and were added RPMI 1640 medium/5% FBS in the low IL3 (0.0001ng/ml) and put back into the incubator for 4 weeks, followed by selection with puromycin (0.6 g/ml) and IL-3 withdrawal. Stable Ba/F3 cells were maintained in medium without IL-3. MCF10A cells were transduced by the virus followed by selection with puromycin (0.6 μg/ml). Stable MCF10A cells were maintained in completed DMEM/F12 (Invitrogen) full medium with 5% Horse serum (Invitrogen), 20 ng/ml EGF (Peprotech), 10 ug/ml insulin (Sigma), 100ng/ml Cholera Toxin (Sigma), 0.5mg/ml Hydrocortisone. After 7 days of antibiotic selection, expression of the constructs was verified by Western blots.
Cell extract preparation and western blotting
Whole-cell lysates for western blotting were extracted with RIPA (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, protease, and phosphatase inhibitor cocktail). Cell lysates (20 ug) were loaded onto 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane and protein expression was depicted with an enhanced chemiluminescence western blot detection kit (Amersham Biosciences). Antibodies used were AZIN1, Antizyme inhibitor 1Polyclonal antibody (Proteintech), GRIA2, AMPA Receptor (GluR2) (E1L8U) Rabbit mAb (Cell Signaling Technology), COG3 polyclonal antibody (Proteintech), V5 Tag Mouse Monoclonal Antibody (Life technologies), and ERK2 (Santa Cruz biotechnology).
BaF3 and MCF10A cell viability assay
BaF3 cells were transduced by the virus, and resuspended in BaF3 low IL-3 medium (0.0001 ng/ml). Then the cells were transferred to a 96-well plate and the assays were performed at week 1.5, 2, 3 and 4 time points. Stable MCF10A cell lines were seeded into 96-well plates, and the assays were performed at day 0, 4, 8, 10 and 12 time points. CellTiter-Glo (Promega, Madison, WI, USA) was added to access cell viability according to manufacturer's instructions. The cell viability measurement was also performed based on cell counting after trypsin digestion. The significance of differences was analyzed with Student's t test, and p < 0.05 was considered statistically significant.
Apoptosis assay
Cells (1.5×104) were seeded into 6-well plates for 24 hr before incubation with MCF10A full medium, MEBM added BPE (Lonza, Allendale, NJ), or DMEM medium without glucose and L-glutamine for another 24 hr. Apoptosis-induced DNA fragmentation was measured using the Cell Death Detection ELISA Kit (Roche Applied Science, Indianapolis, IN) according to manufacturer's instructions. Apoptosis-induced the active form of caspase-3 was tested using the PE Rabbit Anti-Active Caspase-3 (BD Biosciences, San Jose, CA) according to manufacturer's instructions. BD Canto II analyzer is used to read active caspase-3 in the PE channel.
Wound healing assay
MCF10A cells (3.5×104) were seeded into 96-well ImageLock plates for 24 hr in DMEM/F12 medium included with 1% Horse serum, 4 ng/ml EGF, 2 ug/ml insulin, 20 ng/ml Cholera Toxin, 0.1 mg/ml Hydrocortisone. Automated 96-well cell migration (scratch wound) on IncuCyte was analyzed by IncuCyte™ Cell Migration Kit (Essen BioScience, Ann Arbor, Michigan, USA), which comprises 96-pin woundmaking tool (WoundMaker™), Cell Migration Analysis software module and starter batch of 96-well ImageLock Plates.
Ba/F3 drug screening assay
The IL-3–dependent Ba/F3 parental cell line was maintained in RPMI 1640 medium containing 5% FBS and 5 ng/ml of IL-3. The spontaneously transformed Ba/F3 cell line was maintained in RPMI 1640 medium containing 5% FBS without IL-3. Stable Ba/F3 cell lines expressing the wild-type and edited genes were obtained and maintained by selection of puromycin (0.6 ug/ml) and IL-3 withdrawal. The 145-compound library was purchased from the John S. Dunn Gulf Coast Consortium for Chemical Genomics (Houston, TX). These compounds were dissolved in DMSO as 10 mM stock solutions. The day before treatment, cells (1×104) were seeded in 96-well plates in medium with or without IL-3. Eight serial dilutions of each compound were prepared in media, and final drug concentrations ranged from 0 to 10 μM. Cells were treated with DMSO or drug compounds in the presence or absence of IL-3 for 72 hr. Cell viability was determined using PrestoBlue (Promega, Madison, WI) for mitochondrial dehydrogenase activity. Drug screening was repeated independently to ensure the reproducibility of the results.
To comprehensively assess the effects of RNA editing sites on drug sensitivity, we downloaded the drug screening data from CCLE (http://www.broadinstitute.org/ccle/home), and calculated the correlations between the RNA editing level and IC50.
Supplementary Material
Significance.
ADAR-mediated A-to-I RNA editing represents a widespread, phylogentically conserved, post-transcriptional mechanism to engender genomic diversity by reproducibly changing RNA sequences without a concomitant change in DNA sequences. The role of RNA editing in human cancer is only beginning to emerge from early studies of individual candidates in a few cancer types. Our systematic analysis of RNA editing across 17 cancer types demonstrates an appreciable number of RNA editing events associated with clinical characteristics of tumors and patient outcomes, some of which show functional effects on cell viability and drug sensitivity. Thus, aberrant RNA editing provides an underexplored mechanism to reproducibly alter protein or regulatory RNA sequences that could act as drivers and represent potential biomarkers or therapeutic targets in cancer.
Acknowledgements
We gratefully acknowledge contributions from TCGA Research Network and TCGA Pan-Cancer Analysis Working Group. This study was supported by the National Institutes of Health (CA168394, CA098258 and U24CA143883 to G.B.M., R01CA175486 to H.L., R01GM102484 to J.B.L. and CCSG grant CA016672); NIH/NCI Uterine SPORE Career Development award; the R. Lee Clark Fellow Award from The Jeanne F. Shelby Scholarship Fund; a grant from the Cancer Prevention and Research Institute of Texas (RP140462 to H.L.); the Lorraine Dell Program in Bioinformatics for Personalization of Cancer Medicine (to H.L.); the Adelson Medical Research Foundation (to G.B.M.); and the Ellison Medical Foundation (to J.B.L.). We thank the MD Anderson high-performance computing core facility for computing, and LeeAnn Chastain for editorial assistance. G.B.M. has sponsored research support from AstraZeneca and is on the Scientific Advisory Board for AstraZeneca, ImmunoMet, Nuevolution and Precision Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
H.L. conceived of and supervised the project. L.H., G.B.M. and H.L. designed and performed the research. L.D., R.Z., Y. Yang, Y. Yuan, Jun L., J.R., Z.J., S.W. and J.B.L. contributed to the data analysis. S.Y., X.X., Jie L., H.M.W., A.K.E., N.N., R.M., Y.H. L., L.W.C, K.J.J., Y.L., and S.L.K. performed the experiments. L.H., X.X., G.B.M., and H.L. wrote the manuscript, with input from all other authors.
References
- Bahn JH, Lee JH, Li G, Greer C, Peng G, Xiao X. Accurate identification of A-to-I RNA editing in human by transcriptome sequencing. Genome Research. 2012;22:142–150. doi: 10.1101/gr.124107.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity (vol 483, pg 603, 2012). Nature. 2012;492:290–290. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annual Review of Biochemistry. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Nishikura K, Keller W, Seeburg PH, Emeson RB, OConnell MA, Samuel CE, Herbert A. A standardized nomenclature for adenosine deaminases that act on RNA. RNA. 1997;3:947–949. [PMC free article] [PubMed] [Google Scholar]
- Bazak L, Haviv A, Barak M, Jacob-Hirsch J, Deng P, Zhang R, Isaacs FJ, Rechavi G, Li JB, Eisenberg E, Levanon EY. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Research. 2014;24:365–376. doi: 10.1101/gr.164749.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng SY, Chakravarty D, Sanborn JZ, Berman SH, et al. The Somatic Genomic Landscape of Glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA, et al. Absolute quantification of somatic DNA alterations in human cancer. Nature Biotechnology. 2012;30:413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci C, Barzotti R, Galeano F, Corbelli S, Rota R, Massimi L, Di Rocco C, O'Connell MA, Gallo A. Down-regulation of RNA editing in pediatric astrocytomas: ADAR2 editing activity inhibits cell migration and proliferation. The Journal of Biological Chemistry. 2008;283:7251–7260. doi: 10.1074/jbc.M708316200. [DOI] [PubMed] [Google Scholar]
- Chan TH, Lin CH, Qi L, Fei J, Li Y, Yong KJ, Liu M, Song Y, Chow RK, Ng VH, et al. A disrupted RNA editing balance mediated by ADARs (Adenosine DeAminases that act on RNA) in human hepatocellular carcinoma. Gut. 2014;63:832–843. doi: 10.1136/gutjnl-2012-304037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Cho DSC, Wang QD, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single-and double-stranded RNA binding domains. RNA. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Li Y, Lin CH, Chan THM, Chow RKK, Song YY, Liu M, Yuan YF, Fu L, Kong KL, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nature Medicine. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LW, Yu S, Zhang D, Li J, Ng PK, Panupinthu N, Mitra S, Ju Z, Yu Q, Liang H, et al. Naturally occurring neomorphic PIK3R1 mutations activate the MAPK pathway, dictating therapeutic response to MAPK pathway inhibitors. Cancer Cell. 2014;26:479–494. doi: 10.1016/j.ccell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilibeck KA, Wu T, Liang C, Schellenberg MJ, Gesner EM, Lynch JM, MacMillan AM. FRET analysis of in vivo dimerization by RNA-editing enzymes. The Journal of Biological Chemistry. 2006;281:16530–16535. doi: 10.1074/jbc.M511831200. [DOI] [PubMed] [Google Scholar]
- Cho DS, Yang W, Lee JT, Shiekhattar R, Murray JM, Nishikura K. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. The Journal of Biological Chemistry. 2003;278:17093–17102. doi: 10.1074/jbc.M213127200. [DOI] [PubMed] [Google Scholar]
- Galeano F, Rossetti C, Tomaselli S, Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco CM, Locatelli F, Gallo A. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene. 2013;32:998–1009. doi: 10.1038/onc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu TJ, Buaas FW, Simons AK, Ackert-Bicknell CL, Braun RE, Hibbs MA. Canonical A-to-I and C-to-U RNA Editing Is Enriched at 3′ UTRs and microRNA Target Sites in Multiple Mouse Tissues. PLoS One. 2012;7:e33720. doi: 10.1371/journal.pone.0033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Kim HP, Shin JY, Jeong EG, Lee WC, Kim KY, Park SY, Lee DW, Won JK, Jeong SY, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J Exp Med. 2014;211:613–621. doi: 10.1084/jem.20132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Higuchi M, Sprengel R, Seeburg PH. Q/R site editing in kainate receptor GluR5 and GluR6 pre-mRNAs requires distant intronic sequences. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1875–1880. doi: 10.1073/pnas.93.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Single FN, Kohler M, Sommer B, Sprengel R, Seeburg PH. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361–1370. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Crews LA, Barrett CL, Chun HJ, Court AC, Isquith JM, Zipeto MA, Goff DJ, Minden M, Sadarangani A, et al. ADAR1 promotes malignant progenitor reprogramming in chronic myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1041–1046. doi: 10.1073/pnas.1213021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan LP, Gallo A, O'Connell MA. The many roles of an RNA editor. Nat Rev Genet. 2001;2:869–878. doi: 10.1038/35098584. [DOI] [PubMed] [Google Scholar]
- Li MY, Wang IX, Li Y, Bruzel A, Richards AL, Toung JM, Cheung VG. Widespread RNA and DNA Sequence Differences in the Human Transcriptome. Science. 2011;333:53–58. doi: 10.1126/science.1207018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Cheung LWT, Li J, Ju ZL, Yu SX, Stemke-Hale K, Dogruluk T, Lu YL, Liu XP, Gu C, et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Research. 2012;22:2120–2129. doi: 10.1101/gr.137596.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Landweber LF. Hypothesis: RNA editing of microRNA target sites in humans? RNA. 2007;13:463–467. doi: 10.1261/rna.296407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Patt S, Schrey M, Rich A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14687–14692. doi: 10.1073/pnas.251531398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Rich A. Changing genetic information through RNA editing. Bioessays. 2000;22:790–802. doi: 10.1002/1521-1878(200009)22:9<790::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Martinez HD, Jasavala RJ, Hinkson I, Fitzgerald LD, Trimmer JS, Kung HJ, Wright ME. RNA Editing of Androgen Receptor Gene Transcripts in Prostate Cancer Cells. Journal of Biological Chemistry. 2008;283:29938–29949. doi: 10.1074/jbc.M800534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemlich Y, Greenberg E, Ortenberg R, Besser MJ, Barshack I, Jacob-Hirsch J, Jacoby E, Eyal E, Rivkin L, Prieto VG, et al. MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth. The Journal of Clinical Investigation. 2013;123:2703–2718. doi: 10.1172/JCI62980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omberg L, Ellrott K, Yuan Y, Kandoth C, Wong C, Kellen MR, Friend SH, Stuart J, Liang H, Margolin AA. Enabling transparent and collaborative computational analysis of 12 tumor types within The Cancer Genome Atlas. Nature Genetics. 2013;45:1121–1126. doi: 10.1038/ng.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz N, Levanon EY, Amariglio N, Heimberger AB, Ram Z, Constantini S, Barbash ZS, Adamsky K, Safran M, Hirschberg A, et al. Altered adenosine-to-inosine RNA, editing in human cancer. Genome Research. 2007;17:1586–1595. doi: 10.1101/gr.6493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZY, Cheng YB, Tan BCM, Kang L, Tian ZJ, Zhu YK, Zhang WW, Liang Y, Hu XD, Tan XM, et al. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nature Biotechnology. 2012;30:253–260. doi: 10.1038/nbt.2122. [DOI] [PubMed] [Google Scholar]
- Piskol R, Peng ZY, Wang J, Li JB. Lack of evidence for existence of noncanonical RNA editing. Nature Biotechnology. 2013;31:19–20. doi: 10.1038/nbt.2472. [DOI] [PubMed] [Google Scholar]
- Qin YR, Qiao JJ, Chan TH, Zhu YH, Li FF, Liu H, Fei J, Li Y, Guan XY, Chen L. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Research. 2014;74:840–851. doi: 10.1158/0008-5472.CAN-13-2545. [DOI] [PubMed] [Google Scholar]
- Quayle SN, Lee JY, Cheung LW, Ding L, Wiedemeyer R, Dewan RW, Huang-Hobbs E, Zhuang L, Wilson RK, Ligon KL, et al. Somatic mutations of PIK3R1 promote gliomagenesis. PloS One. 2012;7:e49466. doi: 10.1371/journal.pone.0049466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G, Li JB. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Research. 2014;42:D109–D113. doi: 10.1093/nar/gkt996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G, Lin W, Piskol R, Tan MH, Davis C, Li JB. Accurate identification of human Alu and non-Alu RNA editing sites. Nature Methods. 2012;9:579–581. doi: 10.1038/nmeth.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G, Zhang R, Piskol R, Keegan LP, Deng P, O'Connell MA, Li JB. Identifying RNA editing sites using RNA sequencing data alone. Nature Methods. 2013;10:128–132. doi: 10.1038/nmeth.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012a;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012b;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive molecular portraits of human breast tumours. Nature. 2012c;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013a;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinoma. Nature. 2013b;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network. Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM, Network, C. G. A. R. The Cancer Genome Atlas Pan-Cancer analysis project. Nature Genetics. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli S, Galeano F, Alon S, Raho S, Galardi S, Polito VA, Presutti C, Vincenti S, Eisenberg E, Locatelli F, Gallo A. Modulation of microRNA editing, expression and processing by ADAR2 deaminase in glioblastoma. Genome Biology. 2015;16:5. doi: 10.1186/s13059-014-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstedt H, Daniel C, Enstero M, Ohman M. Large-scale mRNA sequencing determines global regulation of RNA editing during brain development. Genome Research. 2009;19:978–986. doi: 10.1101/gr.089409.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Research. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.