Abstract
The amygdala, a crucial hub of the emotional processing neural system, has been implicated in late-life depression (LLD) pathophysiology. However, the overlapping and diverging amygdala network function abnormalities underlying two clinical LLD phenotypes (i.e., LLD alone and LLD with mild cognitive impairment [LLD-MCI]) are unknown. The aim of this study is to investigate the amygdala functional connectivity (FC) differences between LLD alone, LLD-MCI and healthy controls, and to examine the relationships between amygdala network dysfunction and symptom dimensions. A resting-state functional connectivity magnetic resonance imaging study was conducted to probe amygdala FC in a total of 63 elderly participants (LLD [n=22], LLD-MCI [n=15], and age- and gender-equated healthy older adults [n=26]) using a seed-based voxelwise R-fcMRI approach. LLD-only adults showed increased FC in the posterior default mode and vermis, and diminished connections in the fronto-parietal, salience and temporal areas, relative to controls. The LLD-MCI participants showed diminished FC in the default mode, cognitive control, salience and visual regions, whereas increased FC was limited to lateral parietal cortex compared with healthy controls. The LLD-MCI group also showed diminished FC in the occipital and posterior default mode areas, relative to the LLD-only group. Distinct amygdala FC abnormalities that explain depressive and anxiety symptom severity, and executive functioning were identified. The amygdala FC impairments may distinguish LLD phenotypes. These functional network abnormalities may also explain the heterogeneity seen in the LLD clinical presentations.
Keywords: Depression, mild cognitive impairment, amygdala, functional connectivity, MRI, depressive symptoms, elderly, late-life, symptom dimensions
INTRODUCTION
Late-life major depression (LLD) presents with considerable cognitive symptom heterogeneity; while some have intact cognitive functioning, others with greater illness severity demonstrate impairments in memory, information processing speed and executive function performances (Butters et al., 2004). In addition, depression often coexists with mild cognitive impairment (MCI) in the elderly (Bhalla et al., 2009). The complex bidirectional and reciprocal relationships between depressive and cognitive symptomatology in late life suggests that distinct, as well as overlapping, neurophysiologic features might underlie two common phenotypic presentations of LLD (i.e., LLD with and without comorbid MCI).
Despite age-related decline in many cognitive domains, emotional processing function is well preserved and sometimes enhanced in healthy older adults (Charles, 2010). The improvements in emotional processing with age are supported by the ‘cognitive control’ hypothesis of healthy aging. This theoretical construct postulates that the positivity effect seen in older adults is a result of executive control brain regions exerting greater regulation on limbic areas that process negative emotional stimuli (Mather, 2012; Mather and Knight, 2005). The amygdala, a medial temporal lobe (MTL) brain region and a crucial hub of the emotional processing neural system, plays a vital role in processing threats and triggering various responses to emotionally valenced stimuli. The amygdala is closely interconnected with important brain regions that subserve multidomain cognitive functions, and is central to diverse cognitive-emotional interactions (Pessoa, 2008). In line with the cognitive control hypothesis, extensive observations of dampened amygdala and posterior cortical regional reactivity, and enhanced frontal activation in response to emotionally laden stimuli, have been reported in older adults compared with their younger counterparts (Gunning-Dixon et al., 2003; St Jacques et al., 2009; Tessitore et al., 2005). Emotional processing dysregulation is considered a core feature of major depression, including LLD. Although accumulating studies implicate the amygdala in LLD pathophysiology (Burke et al., 2011), the amygdala network function abnormalities in LLD were not previously investigated.
Task-based functional magnetic resonance imaging (T-fMRI) studies have provided unique insights into the role of fronto-limbic circuitry underlying emotional and cognitive changes associated with LLD (Aizenstein et al., 2005; Aizenstein et al., 2009; Wang et al., 2008; Wang et al., 2012). Using various emotional and cognitive paradigms, prior studies have reported frontal hypoactivity (Aizenstein et al., 2005; Aizenstein et al., 2009) and increased limbic activation in some (Aizenstein et al., 2005) but not all (Naismith et al., 2010) LLD studies. Although T-fMRI studies provide unique information as to how specific brain regions respond during a particular task, they offer little information into how functionally related structures serve as interconnected nodes of a dynamic brain network. Moreover, this imaging modality is prone to task-related motion artifacts and false-negative findings because of compromised performance during demanding stimuli in older depressed adults with varying cognitive function levels.
Resting-state functional connectivity MRI (R-fcMRI) is a task-free imaging method increasingly utilized to probe brain network dysfunction in neuropsychiatric disorders, including LLD (Li et al., 2014; Tadayonnejad and Ajilore, 2014). The R-fcMRI technique measures temporal interregional correlations of spontaneous low-frequency blood oxygenation level-dependent fluctuations between functionally connected but spatially separated brain regions at rest (Biswal et al., 1995). Abnormal functional connectivity (FC) in the default mode, executive control and reward processing brain networks has been previously reported in LLD compared with normal older individuals (Alexopoulos et al., 2012; Alexopoulos et al., 2013; Wu et al., 2011). Recently, more pronounced FC vulnerabilities in the hippocampal memory networks were demonstrated in patients with LLD and MCI comorbidity compared with older adults with either disorders occurring alone (Xie et al., 2013). A lone study focusing on the amygdala also examined main and interactive relationships between depressive symptoms and memory performance in elderly subjects (Xie et al., 2012a); however, amygdala FC abnormalities in different phenotypic presentations of LLD have not yet been examined.
This study’s primary objective was to investigate the amygdala FC in individuals with LLD alone, LLD comorbid with MCI and age- and gender-equated healthy elderly. Based on the evidence from previously published functional activation and R-fcMRI aging studies using healthy and LLD participants, we hypothesized that the LLD-only group would show diminished amygdala FC with executive control nodes and increased FC with posterior default mode network regions. We also hypothesized that the comorbid group would show globally diminished amygdala FC in similar brain regions that were associated with poorer cognitive performance in older adults, as evidenced in a previous study. Secondarily, we examined the amygdala FC differences that explained the variance in depressive symptom severity and multidomain cognitive performance within each group.
METHODS
Participants
A total of 63 participants aged 60 or older participated in this cross-sectional study. The participant groups included cognitive normal healthy controls (CN: n = 26), late-life depression (LLD: n = 22), and LLD with mild cognitive impairment (LLD-MCI: n = 15). All patients diagnosed as having LLD and/or MCI were recruited from the Medical College of Wisconsin (MCW) Geriatric Psychiatry and Memory Disorders Clinics. Control subjects were recruited from the community through local advertisements. All participants provided written informed consent according to MCW Institutional Review Board-approved protocols.
Study participants received detailed clinical and neuropsychiatric assessments, as described previously. The core neuropsychological battery administered to all participants included the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), Mattis Dementia Rating Scale-2 (MDRS-2) (age- and education-corrected MOANS-scaled score of ≥ 5) (Lucas et al., 1998), education-adjusted Logical Memory II Delayed paragraph recall (LMII-DR) subscale from the Wechsler Memory Scale-Revised (Wechsler, 1987), Physical Self Maintenance Scale/Instrumental Activities of Daily Living (PSMS/IADL) (Lawton and Brody, 1969), 30-item Yesavage Geriatric Depression Scale (GDS) (Yesavage et al., 1982), Diagnostic assessment for Axis 1 disorders, including the depression module from the Structured Clinical Interview for DSM IV (SCID) (First et al., 2002), and Hamilton Anxiety Scale (HAM-A) (Hamilton, 1959). All participants scored ≤ 4 on the modified Hachinski Ischemic Scale (HIS). Clinical assessment findings were reviewed during the weekly consensus conferences attended by neurologists, neuropsychologists and a geriatric psychiatrist. Age of onset of first episode of major depression was obtained from all depressed participants.
Inclusion criteria
LLD included a GDS score of 10 or above, MMSE ≥ 24, PSMS ≤ 6 and IADL ≤ 9, score above the education-adjusted cutoff on the LMII-DR (Delayed recall score > 8 for 16 or more years of education or score > 4 for 8–15 years of education), and SCID depression module positive for major depression. Because clinically significant anxiety often coexists with LLD, we did not exclude patients with HAM-A scores ≥ 17, if the study psychiatrist determined that the primary diagnosis was a depressive disorder.
-
LLD-MCI: All subjects who met the MCI criteria (amnestic MCI: n = 13; nonamnestic MCI: n = 2), scored 10 or above on the GDS and were SCID depression module positive for major depression were included in this group. To be included in this group, participants had to meet the MCI criteria prior to being diagnosed with the current depressive episode. One amnestic MCI subject who scored 9 on the GDS and met the SCID criteria for dysthymic disorder was included in this group. One subject in this group also had an HAM-A score ≥ 17.
MCI was operationally defined according to the established criteria (Winblad et al., 2004): subjective report of cognitive decline, objective cognitive impairment that includes scoring 1.5 SD below on memory and/or nonmemory measures (see below), intact activities of daily living (ADLs) and relatively preserved instrumental ADLs (IADLs) and no dementia. Participants met the clinical diagnosis of amnestic MCI or nonamnestic MCI, MMSE score ≥ 24, age- and education-corrected MOANS-scaled score of ≥ 5, and score in the normal range on the PSMS and IADL scales. (a) Amnestic MCI: In meeting the criteria for objective cognitive impairment, participants had to score below the education-adjusted cutoff on the LMII-DR (i.e., ≤ 8 for 16 or more years of education, and ≤ 4 for 8–15 years of education), and score 1.5 SD below the mean on one or more subscales (one of the impairments had to be memory) of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Immediate Memory, Visuospatial Ability, Language, Attention, Delayed Memory) (Randolph, 1998). One amnestic MCI subject did not complete the RBANS. (b) Nonamnestic MCI: For meeting the criteria for objective cognitive impairment, participants had to score within normal limits on the LMII-DR and 1.5 SD below the mean on one or more nonmemory subscales of the RBANS.
CN: The eligibility criteria were similar to those used for the LLD cohort, except these subjects could not meet criteria for any Axis 1 disorders or be on psychoactive medications. Exclusion criteria included past or current history of concurrent Axis 1 psychiatric disorders, such as psychotic or bipolar disorders; alcohol or substance abuse/dependence during the past five years; active suicidality; MMSE scores < 24; history of neurological disease, including Parkinson’s disease, dementia, multiple sclerosis, seizures, or stroke; head injury with loss of consciousness; MRI contraindications and unstable chronic medical conditions.
MRI Data Acquisition
All MRI scans were acquired using a GE 3T whole-body scanner (GE, Waukesha, Wisconsin, USA) with a standard transmit-receive quadrature head coil. These included an 8-min task free R-fcMRI scan using a single-shot gradient echo-echo planar (EPI) imaging and a 6-min high-resolution three-dimensional spoiled gradient-recalled echo (SPGR) pulse sequences. All participants were instructed to close their eyes and relax during the scans. The R-fcMRI imaging parameters were: TE = 25 ms, TR = 2000 ms, flip angle (FA) = 90°, number of slices = 36, slice thickness = 4 mm, matrix size = 64 × 64, and field of view (FOV) = 240 × 240 mm. The SPGR axial images were acquired for anatomical reference with parameters: TE = 3.2 ms, TR = 8.2 ms, TI = 450 ms, FA = 12°, number of slices = 150, slice thickness = 1 mm, matrix size = 256 × 192, and FOV = 240 × 240 mm2.
MRI Data Processing
R-fcMRI data preprocessing was performed using AFNI software (http://afni.nimh.nih.gov/afni), FSL software (http://fsl.fmrib.ox.ac.uk/) and MATLAB programs (The MathWorks Inc., Natick, Mass.), as described previously (Li et al., 2014; Xie et al., 2012a; Xie et al., 2013). Briefly, outlier points in the time series were removed followed by volume registration, estimation of the motion parameters, and detrending using linear least squares. Participants who had images with translational motion > 2mm or rotational 2° were re scanned to minimize motion artifacts. Next, nuisance signals, such as from physiological noise (cardiac and respiratory), white matter (WM), cerebrospinal fluid (CSF), and six rigid-body motion vectors were regressed out from each time series. The only difference was that global signal regression was not performed because of concerns that this procedure may artificially introduce anti-correlated FC results (Murphy et al., 2009). Finally, a band-pass filter (0.015Hz to 0.1Hz) was applied to obtain only the low-frequency fluctuations portion of the data.
Structural MRI data was segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using VBM8 toolbox in Statistical Parametric Mapping (SPM) software (http://www.fil.ion.ucl.ac.uk/spm).
Amygdala Functional Connectivity
The seed-based R-fcMRI method was used to examine the whole-brain voxelwise resting-state FC of the amygdala. The bilateral amygdala seed region of interest (ROI) was manually traced on the axial slices with reference to the sagittal and coronal slices of the high-resolution SPGR images, as described previously (see supplement for details) (Honeycutt et al., 1998; Xie et al., 2012a).
The high-resolution amygdala tracing (0.98 × 0.98 × 1 mm3) was then downsampled to match the resolution of the R-fcMRI data (3.75 × 3.75 × 4 mm3). Only voxels in the R-fcMRI that were at least 70% occupied by the traced amygdala voxels in the structural images were included in the amygdala seed ROI in the further analyses to accommodate the resolution difference. Pearson product-moment correlation was used to obtain a whole-brain amygdala functional connectivity (AFC) map for each participant by cross-correlating between the mean time course of the amygdala seed and the time courses of all voxels within the entire brain. Next, the correlation coefficient (cc) maps were subjected to Fisher’s z-transformation to obtain an approximately normally distributed m = 0.5*ln{(1+cc)/(1-cc)}. Finally, each individual AFC map was spatially transformed to the Montreal Neurological Institute (MNI) space, followed by smoothing of the image, using a 6-mm Gaussian kernel.
Statistical Analysis
Demographic information (age and education), except gender (using χ2-tested), and neuropsychological measurements were compared among the three participant groups, using analysis of variance (SPSS 18.0; SPSS Inc., Chicago, Ill.). Post-hoc Fisher’s Least Significant Difference Test examined the sources of the differences between the means of the three groups.
One-sample t-tests were used to obtain the AFC patterns in each participant group separately. Analysis of covariance (ANCOVA) was used to examine the differences in voxelwise AFC among the three participant groups, while controlling for age, gender, education and voxelwise whole-brain GM volume (GMV). Post-hoc t-tests revealed sources of between-group differences within the ANCOVA results. We adopted a built-in AFNI program (3dClustSim), a type of Monte Carlo simulation method, to correct for the multiple comparisons (voxelwise p < 0.05, α < 0.05, and cluster size > 3960 mm3).
Stepwise linear regression was used to investigate the relationships between neuropsychological measures (GDS, LMII-DR, HAM-A and MDRS-2 Initiation/Perseveration [Init/Pers] scores, respectively) as dependent variables and the set of regressors, including age, gender, education, GMV, and mean AFC of brain regions (10 ROIs determined by ANCOVA), as independent variables. The regression analyses were conducted separately in each participant group. The forward selection approach was used to determine the subset of regressors that significantly contributed to the linear model by individually adding the most statistically significant regressors until no further regressor met the p-value entrance criterion. The statistical threshold for a regressor to be added to the model was set at p < 0.05. We used this approach to reduce the number of regressors included in the linear model, because the smaller sample sizes, particularly for the LLD-MCI group (N = 15), limited the degrees of freedom available to determine the best model fit.
RESULTS
Demographic and Neuropsychiatric Characteristics
No significant (p > 0.05) differences were found in age, gender, and education among the three groups. Age of depression onset did not significantly differ between the two LLD groups (p= 0.225). The neuropsychiatric characteristics are summarized in Table 1.
Table 1.
Demographics and Neuropsychological Characteristics
| CN (n = 26) Mean ± SD |
LLD (n = 22) Mean ± SD |
LLD-MCI (n = 15) Mean ± SD |
p-value | |
|---|---|---|---|---|
| Age | 71.19 ± 6.95 | 68.14 ± 5.79 | 71.73 ± 7.00 | 0.177 |
| Gender (F/M) | 15/11 | 18/4 | 8/7 | 0.123 |
| Education | 15.54 ± 2.49 | 14.91 ± 2.74 | 13.93 ± 2.96 | 0.193 |
| Age of depression onset | N/A | 32.95 ± 14.93 | 41.33 ± 22.86 | 0.225 |
| Neuropsychiatric measurements | ||||
| GDS | 1.96 ± 2.05 | 16.36 ± 3.67 | 17.53 ± 7.23 | <0.001a,b |
| MMSE | 28.77 ± 1.39 | 28.27 ± 1.20 | 26.80 ± 1.82 | <0.001b,c |
| HAM-A | 1.17 ± 1.17 | 10.09 ± 4.93 | 9.33 ± 4.03 | <0.001a,b† |
| Recall Scores | ||||
| Immediate | 14.85 ± 3.59 | 14.18 ± 3.80 | 7.60 ± 4.03 | <0.001b,c |
| Delayed | 12.96 ± 3.17 | 12.50 ± 3.95 | 4.47 ± 4.49 | <0.001b,c |
| DRS-2 raw scores | ||||
| Attention | 36.46 ± 0.51 | 36.41 ± 0.59 | 36.07 ± 1.10 | 0.217 |
| Init/Pers | 36.42 ± 1.17 | 36.36 ± 1.92 | 32.07 ± 5.12 | <0.001b,c |
| Construct | 5.96 ± 0.20 | 5.95 ± 0.21 | 5.87 ± 0.52 | 0.601 |
| Conceptual | 37.65 ± 1.35 | 36.73 ± 3.51 | 35.47 ± 3.02 | 0.049b |
| Memory | 23.81 ± 1.10 | 24.00 ± 1.15 | 20.33 ± 3.68 | <0.001b,c |
| Total | 140.35 ± 2.43 | 139.95 ± 3.11 | 130.13 ± 6.47 | <0.001b,c |
| Current antidepressants (%) | ||||
| No antidepressant | N/A | 2 (9.1) | 2 (13.3) | |
| SSRI monotherapy | N/A | 3 (13.6) | 3 (20.0) | |
| SNRI monotherapy | N/A | 5 (22.7) | 3 (20.0) | |
| Other | N/A | 3 (13.6) | 1 (6.7) | |
| Combination treatment | N/A | 9 (40.9) | 6 (40.0) | |
| Current cognitive enhancers (%) | ||||
| No cognitive enhancer | N/A | N/A | 11 (73.3) | |
| ChEI monotherapy | N/A | N/A | 2 (13.3) | |
| Memantine monotherapy | N/A | N/A | 2 (13.3) | |
| Combination treatment | N/A | N/A | N/A | |
Notes - ANOVA showed significant differences in GDS, MMSE, HAM-A, Recall scores (immediate and delayed), and DRS-2 raw scores (except for Attention, Construct, and Conceptual).
Post-hoc analyses revealed the source of ANOVA (a: LLD vs CN, b: LLD-MCI vs CN, c: LLD-MCI vs LLD).
Two CN subjects did not have HAM-A scores, the Mean and SD are based on the remaining 24 subjects.
Abbreviations: CN, cognitively normal healthy controls; LLD, late-life depression; LLD-MCI, late-life depression with mild cognitive impairment; SD, standard deviation; F/M, female/male; GDS, geriatric depression scale; MMSE, mini-mental state examination; HAM-A, Hamilton anxiety; DRS-2, dementia rating scale-2; Init/Pers, Initiation/Preservation
Voxelwise Whole-Brain Amygdala Functional Connectivity
(1) Within group AFC pattern
The within group AFC network patterns for CN, LLD, and LLD-MCI groups are shown in Figure S1. The positive AFC was found in the middle temporal gyrus (MTG), middle and inferior occipital gyrus (MOG and IOG), fusiform gyrus (FFG), superior parietal lobe (SPL), inferior frontal gyrus (IFG), insula, caudate, anterior and posterior cingulate gyrus (ACC and PCC), parahippocampal gyrus (PHG), and hippocampus. The negative AFC was found in the dorsolateral prefrontal gyrus (DLPFC), lateral inferior parietal lobe (Lat. IPL), thalamus, and retrosplenial cortex.
(2) ANCOVA and post-hoc differences
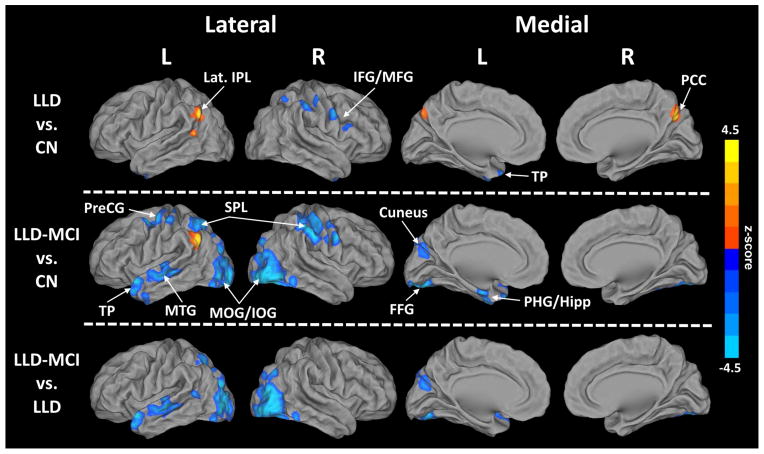
The ANCOVA showed brain regions with altered AFC in the depressed groups compared with the CN group (p < 0.05, cluster size > 3960 mm3) (Figure 1 and Table 2). Post-hoc t-tests revealed sources of between-group differences (Figure 2).
Figure 1.
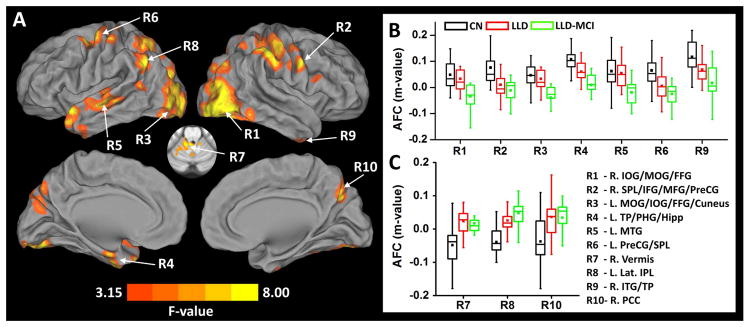
Analysis of covariance (A) illustrates the brain regions with significantly altered Amygdala Functional Connectivity (AFC) in LLD and LLD-MCI patients, when compared between the patient groups and CN group (p < 0.05 and cluster size > 3960 mm3). Boxplots (B) and (C) indicate the data distribution in individual groups with decreased AFC and increased AFC in the patient groups compared with CN group, respectively. In the boxplots, the band inside the box indicates the median point, the bottom and top box indicate 25% and 75% respectively, and the whiskers indicate 1.5 standard deviation of the data. The mean is displayed as colored squares within each boxplots.
Abbreviations – IOG: inferior occipital gyrus, MOG: middle occipital gyrus, FFG: fusiform gyrus, SPL: superior parietal lobule, IFG: inferior frontal gyrus, MFG, middle frontal gyrus, PreCG: precentral gyrus, TP: temporal pole, PHG: parahippocampal gyrus, Hipp: hippocampus, MTG: middle temporal gyrus, Lat. IPL: lateral inferior parietal lobule, ITG: inferior temporal gyrus, PCC: posterior cingulate gyrus, CN: cognitively normal healthy controls, LLD: late-life depression, LLD-MCI: late-life depression with mild cognitive impairment.
Table 2.
Amygdala Functional Connectivity (AFC) ANCOVA Results
| Regions | Side | Cluster size (mm3) | Coordinates (LPI) | Post-hoc z-score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | F-value | LLD vs CN | LLD-MCI vs CN | LLD-MCI vs LLD | ||||
| R1 | IOG | R | 26856 | −34 | 85 | −7 | 10.97 | −4.06 | −3.34 | |
| MOG | −32 | 85 | 10 | 8.06 | −2.97 | −3.48 | ||||
| FFG | −36 | 69 | −13 | 10.75 | −4.06 | −2.99 | ||||
| R2 | SPL | R | 26712 | −56 | 25 | 48 | 12.33 | −2.66 | −4.27 | |
| IFG | −54 | −14 | 22 | 5.81 | −3.19 | −2.59 | ||||
| MFG | −54 | −28 | 24 | 7.67 | −3.64 | −2.58 | ||||
| PreCG | −54 | 0 | 32 | 8.12 | −2.52 | −3.33 | ||||
| R3 | MOG | L | 21792 | 27 | 85 | 14 | 9.51 | −3.18 | −3.86 | |
| IOG | 43 | 83 | −3 | 8.36 | −3.57 | −2.84 | ||||
| FFG | 31 | 73 | −15 | 7.57 | −3.63 | −2.77 | ||||
| Cuneus | 15 | 73 | 20 | 5.81 | −3.06 | −2.66 | ||||
| R4 | TP | L | 7208 | 51 | −10 | −13 | 11.05 | −3.92 | −3.87 | |
| PHG/Hipp | 17 | 9 | −25 | 6.44 | −2.96 | |||||
| R5 | MTG | L | 6024 | 55 | 19 | −5 | 6.89 | −3.23 | −3.24 | |
| R6 | PreCG | L | 5208 | 39 | 7 | 56 | 6.89 | −2.83 | ||
| SPL | 45 | 21 | 52 | 4.65 | −2.54 | |||||
| R7 | Vermis | R | 4728 | −14 | 45 | −25 | 7.28 | 3.39 | ||
| R8 | Lat. IPL | L | 4329 | 53 | 49 | 42 | 8.16 | 2.41 | 3.39 | |
| R9 | ITG/TP | R | 4200 | −34 | −2 | 43 | 8.17 | −2.57 | ||
| R10 | PCC | R | 4016 | −10 | 63 | 30 | 5.89 | 2.7 | ||
Abbreviations – IOG: inferior occipital gyrus, MOG: middle occipital gyrus, FFG: fusiform gyrus, SPL: superior parietal lobule, IFG: inferior frontal gyrus, MFG, middle frontal gyrus, PreCG: precentral gyrus, TP: temporal pole, PHG: parahippocampal gyrus, Hipp: hippocampus, MTG: middle temporal gyrus, Lat. IPL: lateral inferior parietal lobule, ITG: inferior temporal gyrus, PCC: posterior cingulate gyrus, CN: cognitively normal healthy controls, LLD: late-life depression, LLD-MCI: late-life depression with mild cognitive impairment, L/R: left/right.
Figure 2.
Post-hoc t-tests depict the sources of analysis of covariance results (p < 0.05 and cluster size > 3960 mm3). Warm colors indicate an increased Amygdala Functional Connectivity (AFC) and cool colors indicate a decreased AFC.
Abbreviations – IOG: inferior occipital gyrus, MOG: middle occipital gyrus, FFG: fusiform gyrus, SPL: superior parietal lobule, IFG: inferior frontal gyrus, MFG, middle frontal gyrus, PreCG: precentral gyrus, TP: temporal pole, PHG: parahippocampal gyrus, Hipp: hippocampus, MTG: middle temporal gyrus, Lat. IPL: lateral inferior parietal lobule, ITG: inferior temporal gyrus, PCC: posterior cingulate gyrus, CN: cognitively normal healthy controls, LLD: late-life depression, LLD-MCI: late-life depression with mild cognitive impairment, L/R: left/right.
a) LLD versus CN
Relative to CN subjects, LLD subjects showed significantly diminished AFC in the right SPL, IFG, middle frontal (MFG), precentral (PreCG) and inferior temporal gyri (ITG), and temporal pole (TP). The left Lat. IPL and right PCC and the cerebellar vermis showed increased AFC in the LLD group (Figure 2, top).
b) LLD-MCI versus CN
The largest AFC difference was observed between LLD-MCI and CN groups. Compared with the CN, the LLD-MCI group showed decreased AFC in the bilateral IOG and MOG, FFG, SPL, PreCG; right IFG and MFG; and left TP, PHG, hippocampus, MTG, and cuneus. Only the left Lat. IPL showed increased AFC in the LLD-MCI compared with the CN group (Figure 2, middle).
c) LLD-MCI versus LLD
Decreased AFC in IOG, MOG and FFG bilaterally; and cuneus, TP, and MTG on the left were seen in LLD-MCI compared with LLD group (Figure 2, bottom).
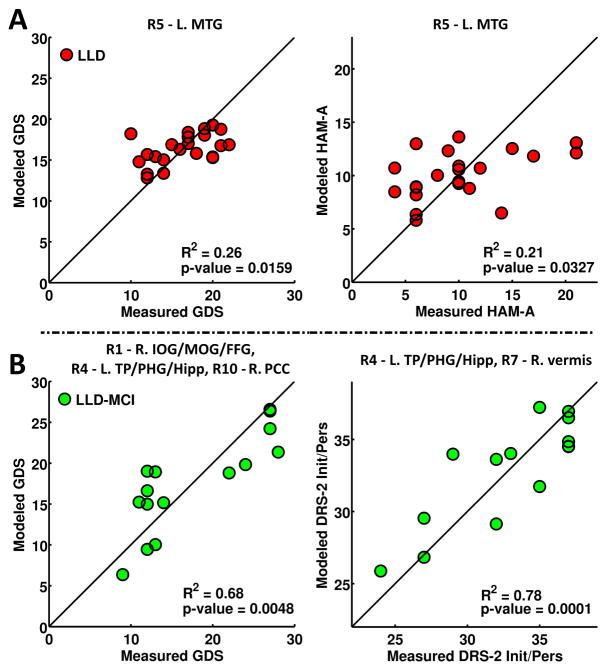
Stepwise Linear Regression
In the LLD group (Figure 3A), GDS (R2 = 0.26, p = 0.0159) and HAM-A (R2 = 0.21, p = 0.0327) correlated with the mean AFC in the left MTG. In the LLD-MCI group (Figure 3B), GDS (R2 = 0.68, p = 0.0048) correlated with the mean AFC in right IOG/MOG/FFG, PCC, and left TP/PHG/hippocampus clusters. The DRS-2 Init/Pers (R2 = 0.78, p = 0.0001) correlated with the mean AFC in the left TP/PHG/hippocampus, and right vermis clusters. LMII-DR (R2 = 0.16, p = 0.0397) and DRS-2 Init/Pers (R2 = 0.15, p = 0.0479) correlated with education and GMV in the CN, respectively (Figure S2).
Figure 3.
Stepwise linear regression examining factors correlated with behavioral measures within the LLD and LLD-MCI groups. Modeled behaviors are based only on the significant regressor (listed on top of each plot) through forward selection. The regressors tested include age, gender, education, GMV, and the mean AFC values from the ten brain regions that showed differences.
Abbreviations – IOG: inferior occipital gyrus, MOG: middle occipital gyrus, FFG: fusiform gyrus, TP: temporal pole, PHG: parahippocampal gyrus, Hipp: hippocampus, MTG: middle temporal gyrus, PCC: posterior cingulate gyrus, GMV: gray matter volume, LLD: late-life depression, LLD-MCI: late-life depression with mild cognitive impairment, L/R: left/right.
DISCUSSION
The primary finding was that diminished amygdala functional connectivity with the fronto-parietal regions and increased connections in the posterior default mode areas and cerebellar vermis distinguished cognitively intact LLD patients from normal older individuals. The LLD-MCI subjects showed dampening in the enhanced amygdala-posterior DMN connectivity. Furthermore, globally decreased functional connections with other regions subserving emotional processing and cognitive functions were seen in individuals with comorbidity. In the secondary analysis, specific amygdala FC abnormalities that explain variations in the severity of depressive and anxiety symptoms, and executive function performance, were observed in the depressed groups. Our findings support the amygdala network function role and highlight the impairments in its functional interconnections with other emotional processing and intrinsic resting-state network hubs in differentiating common clinical presentations of LLD.
Amygdala Functional Connectivity Abnormalities in LLD
The amygdala FC pattern identified in healthy older adults includes brain regions that are commonly activated to emotional processing task paradigms in functional MRI studies of young and elderly subjects (Fusar-Poli et al., 2009; Gunning-Dixon et al., 2003; Tessitore et al., 2005). In using R-fcMRI, we were able to provide novel evidence supporting the role of the amygdala as a crucial emotional processing network hub in older adults.
Our findings demonstrated decreased FC between the amygdala and cognitive control regions in patients with LLD. DLPFC hypoactivity during cognitive control tasks was consistently observed in younger and older patients with depression (Aizenstein et al., 2005; Siegle et al., 2007). Diminished cognitive control network function was also reported in older depressed adults (Alexopoulos et al., 2012). The cognitive control neural system enables efficient information processing to flexibly adapt to changing environmental and personal demands, and is critical for carrying out attention-dependent executive function tasks. These brain areas also downregulate emotional responses to negatively valenced stimuli, thereby leading to positivity effect in the elderly (Mather, 2012). In this regard, dampened functional connections between the amygdala and cognitive control brain resources observed here may underlie executive dysfunction and/or impaired downregulation of emotional processing in LLD. Our LLD-only participants had relatively preserved executive function performance (i.e., normal DRS-2 Init/Pers scores). We postulate that abnormal amygdala-cognitive control connectivity is associated with executive dysfunction in LLD patients and should be examined in future studies. LLD patients showed increased amygdala connectivity primarily in the posterior DMN regions. Stimulus-induced heightened activity and a failure to downregulate activity within the DMN are reported in major depression (Sheline et al., 2009). Interestingly, a phenomenon of “double dissociation,” i.e., increased DMN and diminished cognitive control FC, has been previously reported in LLD compared with normal older adults (Alexopoulos et al., 2012; Smith et al., 2009). The DMN consists of multiple interacting subnetworks that are engaged when performing divergent cognitive and behavioral functions (Buckner et al., 2008). Abnormal amygdala FC in specific DMN nodes, therefore, may lead to different symptom dimensions in those with LLD. For instance, the increased amygdala FC with distinct DMN regions may be associated with abnormal self-referential thought processes and increased negative rumination, greater depressive and anxiety symptoms (e.g., MTG, as observed in this study), memory encoding and retrieval functions, and navigating social events (Buckner et al., 2008).
A substantial body of literature provides evidence of the involvement of the cerebellum in emotion and cognitive functions and is linked to major depression pathophysiology (Schmahmann, 2010)(Schmahmann et al., 2007). Aberrant cerebellar-cerebral connectivity was also associated with affective and cognitive dysfunction in geriatric depression in a prior investigation (Alalade et al., 2011). The increased amygdala-vermis connectivity observed here further highlights the region’s importance in the neurobiological underpinnings of LLD.
Amygdala Network Dysfunction in LLD-MCI
Consistent with the LLD-only group findings, those with coexisting LLD and MCI also exhibited diminished amygdala FC in the fronto-parietal areas compared with healthy elderly. This suggests that the disrupted downregulation of negative affect by the cognitive control neural system is also present in individuals with LLD-MCI comorbidity.
By contrast, amygdalar connections with the posterior default mode clusters showed somewhat divergent abnormalities compared to those observed in the LLD-only group. The amygdala-IPL connectivity remained enhanced, which was likely a result of diminished cognitive control associated with syndromal depression. Decreases in FC between the amygdala and posterior default mode nodes, as well as the visual cortices, were seen in those with coexisting LLD and MCI, relative to other groups. We recently demonstrated that decreased amygdala FC in certain default mode (i.e., PCC, MTG) and visual cortices (i.e., MOG) were associated with poorer memory scores in older adults. The interactive effects of depressive symptoms and memory performance on the amygdala network function also were seen in the PCC and temporal lobe regions in older nondepressed adults (Xie et al., 2012a). Longitudinal amygdalar FC declines in these regions also correlated with episodic memory changes over time in nondepressed MCI subjects (Yao et al., 2014). We did not find abnormal amygdala functional connections in DMN and visual cortices to be related to memory performance in the comorbid group; rather, amygdala FC abnormalities were associated with depressive symptoms (e.g., in the MTL and visual cortices) and executive function scores (e.g., in the MTL and vermis) in this study. These novel findings suggest that the amygdala FC abnormalities with specific DMN nodes, visual cortices, and the vermis may explain different behavioral dimensions commonly encountered in those with comorbidity.
Possible Pathophysiological Mechanisms
Our findings of diminished functional connections between the amygdala and fronto-parietal regions and enhanced connectivity with the posterior DMN and vermis lend support to the disrupted cognitive control model of LLD. The impaired abilities of the executive control brain areas in regulating emotions may be a result of ischemic damage to the fronto-limbic white matter tracts in older adults with depression. A few studies have examined the relationships between white matter compromise and brain network dysfunction in LLD, but the results are inconsistent (Aizenstein et al., 2011; Steffens et al., 2011). Thus, the role of WM tract compromise in explaining amygdala network dysfunction in LLD requires additional clarification. Our preliminary results in the comorbid group point to the possibility that multiple mechanisms may underlie network dysfunction when LLD emerges in individuals with MCI. While some findings in the comorbid group might still be explained by the disrupted cognitive control hypothesis of LLD, different mechanisms might be driving the diminished amygdala function with other default mode nodes and visual cortices. A possible additional mechanism in those with comorbidity could be neurodegeneration. While our results remain significant even after controlling for GMV, regional amygdala atrophy might still be driving our findings. Greater MTL atrophy is associated with the coexistence of LLD and MCI compared with either disease occurring alone (Xie et al., 2012b). Since age of depression onset has been associated with reduced MTL volume in LLD, we performed exploratory analysis, but did not find any associations between the age of onset and amygdala FC differences in either depression groups.
Limitations
The modest sample size in the comorbid group is an important limitation. The majority of our LLD participants (in both groups) were taking antidepressants. Therefore, we are unable to distinguish medication effects from those associated with the disease itself. Also, we have made interpretations based on these cross-sectional data. Therefore, we are unable to comment on causal relationships. Other variables related to depression that might influence functional connectivity and behavioral measures (such as duration of current depressive episode, past medication trials and antidepressant treatment response history) were not considered in this study. Spurious correlations in R-fcMRI data may continue to persist even after monitoring for head motion in real time and performing motion regression. An independent component analysis-denoising procedure may serve as an alternate method to improve overall FC measurements (Griffanti et al., 2014). Effective connectivity methods (Friston et al., 2013) may provide additional insight into the directionality of FC between the amygdala and other emotional processing and intrinsic network brain regions. Finally, multiple comparison corrections were not conducted for stepwise linear regression analyses. Regardless, the findings in the LLD-MCI group remain significant (Figure 3B) even if a Bonferroni correction procedure is applied (p=0.0125, i.e., correcting for separate regression analyses for four behaviors).
Conclusion
We demonstrate that distinct and overlapping abnormalities exist in the amygdala network function in LLD patients with and without MCI. The amygdala network function abnormalities might differentially contribute to the emergence and persistence of mood, anxiety, neurovegetative and cognitive symptoms in LLD phenotypes. Future research using comprehensive neuropsychological and behavioral evaluations coupled with multimodal neuroimaging approaches is essential to determine amygdala network function and structure in different LLD phenotypes, and to evaluate the underlying mechanisms that contribute to network dysfunction.
Supplementary Material
Patterns of the amygdala functional connectivity (AFC) for CN (top), LLD (middle), and LLD-MCI (bottom). Warm colors and cool colors indicate significant positive and negative AFC, respectively. The statistical threshold was set at p < 0.05 and cluster size > 3960 mm3.
Abbreviations – IOG: inferior occipital gyrus, MOG: middle occipital gyrus, FFG: fusiform gyrus, SPL: superior parietal lobule, IFG: inferior frontal gyrus, DLPFC: dorsolateral prefrontal cortex, PHG: parahippocampal gyrus, Hipp: hippocampus, MTG: middle temporal gyrus, Lat. IPL: lateral inferior parietal lobule, ITG: inferior temporal gyrus, AC: anterior cingulate gyrus, PCC: posterior cingulate gyrus, CN: cognitively normal healthy controls, LLD: late-life depression, LLD-MCI: late-life depression with mild cognitive impairment, L/R: left/right.
Highlights.
Diminished amygdala functional connectivity with the fronto-parietal regions and increased connections in the posterior default mode areas and cerebellar vermis distinguished cognitively intact late-life depression (LLD) patients from normal older individuals.
The LLD with mild cognitive impairment (LLD-MCI) subjects showed dampening in the enhanced amygdala-posterior default mode network connectivity.
Globally decreased functional connections with other regions subserving emotional processing function were seen in those with comorbidity, relative to the other groups.
Specific amygdala functional connectivity abnormalities that explain variations in the severity of depressive and anxiety symptoms, and executive function performance were observed in the depressed groups.
Our findings support the amygdala network function role and highlight the impairments in its functional interconnections with other emotional processing and intrinsic resting-state network hubs in differentiating common clinical presentations of LLD.
Acknowledgments
The authors thank Ms. Carrie M. O’Connor, M.A., for editorial assistance, Mr. Douglas Ward, M.S., for assistance with statistical analysis, and Ms. Qian Yin, Ms. Judi Zaferos-Pylant and Mr. Yu Liu, M.S., for MRI technical support.
FUNDING/SUPPORT: This work was supported by Alzheimer’s Association New Investigator Research Grant NIRG-11-204070 (JSG), Advancing Healthier Wisconsin Endowment at the Medical College of Wisconsin (JSG), the Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator program (JSG), Extendicare Foundation (JSG), R01 AD20279 (SJL) from the National Institute on Aging, and 8UL1TR000055 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: Li W, Antuono PG, Li SJ, Goveas JS.
Analysis and interpretation of data: Li W, Ward BD, Xie C, Jones JL, Antuono PG, Li SJ, Goveas JS.
Drafting the article: Li W, Ward BD, Goveas JS.
Revising the article critically for important intellectual content: Li W, Ward BD, Xie C, Jones JL, Antuono PG, Li SJ, Goveas JS.
Final approval: Li W, Ward BD, Xie C, Jones JL, Antuono PG, Li SJ, Goveas JS.
Conflict of Interest Disclosures:
Dr. W. Li, Mr. Ward, Dr. Xie, and Ms. Jones report no biomedical financial interests or potential conflicts of interest. Within the past five years, Dr. Antuono has served on the speaker bureaus of Novartis and Pfizer. Dr. Antuono reports research support from Myriad, Glaxo Smith Kline, Pfizer, ICON, Premier Rach, Octa Pharma, Eisai, Bristol Myers Squibb, Janssen, Baxter and Elan; and DR. Shi-Jiang Li reports research grant funding from the National Institute on Aging and Pfizer; in addition, he has served as a consultant for Bristol-Meyers Squibb and BrainSymphonics, LLC. Dr. Goveas reports grant support from the Alzheimer’s Association International grant program, Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator program, Extendicare Foundation, and Advancing Healthier Wisconsin Endowment for Research to Medical College of Wisconsin.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wenjun Li, Email: wli@mcw.edu.
B. Douglas Ward, Email: ward@mcw.edu.
Chunming Xie, Email: cxie@mcw.edu.
Jennifer L. Jones, Email: jljones@mcw.edu.
Piero G. Antuono, Email: pantuono@mcw.edu.
Shi-Jiang Li, Email: sjli@mcw.edu.
References
- Aizenstein HJ, Andreescu C, Edelman KL, Cochran JL, Price J, Butters MA, Karp J, Patel M, Reynolds CF., 3rd fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168(10):1075–82. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58(4):290–6. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, Becker JT, Reynolds CF, 3rd, Carter CS. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alalade E, Denny K, Potter G, Steffens D, Wang L. Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS One. 2011;6(5):e20035. doi: 10.1371/journal.pone.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, Gunning FM. Functional connectivity in apathy of late-life depression: a preliminary study. J Affect Disord. 2013;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Becker JT, Houck PR, Snitz BE, Lopez OL, Aizenstein HJ, Raina KD, DeKosky ST, Reynolds CF., 3rd Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009;17(4):308–16. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burke J, McQuoid DR, Payne ME, Steffens DC, Krishnan RR, Taylor WD. Amygdala volume in late-life depression: relationship with age of onset. Am J Geriatr Psychiatry. 2011;19(9):771–6. doi: 10.1097/JGP.0b013e318211069a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Charles ST. Strength and vulnerability integration: a model of emotional well-being across adulthood. Psychol Bull. 2010;136(6):1068–91. doi: 10.1037/a0021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 2002 Biometrics Research. New York State Psychiatric Institute; New York: Structured Clinical Interview for DSM-1V TR Axis 1 disorders, research version, non-patient edition (SCID-I/NP) [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston K, Moran R, Seth AK. Analysing connectivity with Granger causality and dynamic causal modelling. Curr Opin Neurobiol. 2013;23(2):172–8. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–32. [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–47. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24(2):285–95. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, Pearlson GD. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res. 1998;83(2):85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- Li W, Douglas Ward B, Liu X, Chen G, Jones JL, Antuono PG, Li SJ, Goveas JS. Disrupted small world topology and modular organisation of functional networks in late-life depression with and without amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2014-309180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20(4):536–47. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol Aging. 2005;20(4):554–70. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith SL, Lagopoulos J, Ward PB, Davey CG, Little C, Hickie IB. Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. J Affect Disord. 2010;125(1–3):256–61. doi: 10.1016/j.jad.2010.02.114. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. The Psychological Corporation; San Antonio: 1998. [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6(3):254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D. The functional neuroanatomy of geriatric depression. International Journal of Geriatric Psychiatry. 2009;24(8):798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: fronto-amygdalar differences during emotional perception and episodic memory. J Int Neuropsychol Soc. 2009;15(6):819–25. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Taylor WD, Denny KL, Bergman SR, Wang L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One. 2011;6(7):e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol. 2014;27(1):5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139(1):9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am J Psychiatry. 2008;165(7):863–71. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- Wang L, Potter GG, Krishnan RK, Dolcos F, Smith GS, Steffens DC. Neural correlates associated with cognitive decline in late-life depression. Am J Geriatr Psychiatry. 2012;20(8):653–63. doi: 10.1097/JGP.0b013e31823e2cc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Goveas J, Wu Z, Li W, Chen G, Franczak M, Antuono PG, Jones JL, Zhang Z, Li SJ. Neural basis of the association between depressive symptoms and memory deficits in nondemented subjects: resting-state fMRI study. Hum Brain Mapp. 2012a;33(6):1352–63. doi: 10.1002/hbm.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Li W, Chen G, Douglas Ward B, Franczak MB, Jones JL, Antuono PG, Li SJ, Goveas JS. The co-existence of geriatric depression and amnestic mild cognitive impairment detrimentally affect gray matter volumes: voxel-based morphometry study. Behav Brain Res. 2012b;235(2):244–50. doi: 10.1016/j.bbr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Li W, Chen G, Ward BD, Franczak MB, Jones JL, Antuono PG, Li SJ, Goveas JS. Late-life depression, mild cognitive impairment and hippocampal functional network architecture. Neuroimage Clin. 2013;3:311–20. doi: 10.1016/j.nicl.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Zhou B, Zhang Z, Wang P, Guo Y, Shang Y, Wang L, Zhang X, An N, Liu Y Alzheimer’s Disease Neuroimaging I. Longitudinal alteration of amygdalar functional connectivity in mild cognitive impairment subjects revealed by resting-state FMRI. Brain Connect. 2014;4(5):361–70. doi: 10.1089/brain.2014.0223. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patterns of the amygdala functional connectivity (AFC) for CN (top), LLD (middle), and LLD-MCI (bottom). Warm colors and cool colors indicate significant positive and negative AFC, respectively. The statistical threshold was set at p < 0.05 and cluster size > 3960 mm3.
Abbreviations – IOG: inferior occipital gyrus, MOG: middle occipital gyrus, FFG: fusiform gyrus, SPL: superior parietal lobule, IFG: inferior frontal gyrus, DLPFC: dorsolateral prefrontal cortex, PHG: parahippocampal gyrus, Hipp: hippocampus, MTG: middle temporal gyrus, Lat. IPL: lateral inferior parietal lobule, ITG: inferior temporal gyrus, AC: anterior cingulate gyrus, PCC: posterior cingulate gyrus, CN: cognitively normal healthy controls, LLD: late-life depression, LLD-MCI: late-life depression with mild cognitive impairment, L/R: left/right.