Whereas myocardial depolarization is a fast event mediated almost exclusively by rapid Na currents, myocardial repolarization is a much slower and more complex affair for the heart. It is regulated by a carefully orchestrated interplay of many different inward and outward currents that allows for a precise regulation of action potential (AP) shape and duration (Figure 1). Intact repolarization is essential for excitation-contraction coupling and helps protect the myocardium against spontaneous ectopic activity and reentrant arrhythmias. During hypokalemia, the fine balance of ion currents across the cell membrane is disrupted, resulting in action potential prolongation (Figure 2). This phenomenon, known as loss of repolarization reserve, has been also implicated in the cell pathophysiology of heart failure even during normokalemia.1 Recent reports indicate that a small-conductance Ca- activated outward K (SK) current is upregulated in failing ventricular myocytes, which partially restores repolarization reserve and AP duration.2 Since the Ca-activated K+ current is blocked by neurotoxin apamin, it is also known as apamin sensitive K current (IKAS). The molecular correlate is likely the type 2 SK channel. However, previous studies had indicated that in healthy hearts, IKAS plays a role only in atrial, but not ventricular electrophysiology.2
Figure 1.
Major ionic currents that shape the ventricular action potential (AP). Inward currents (black ↓) depolarize the cell membrane and prolong the action potential; outward currents (grey ↑) repolarize the cell membrane and shorten the action potential. INa, Na+ current, ICa, L-type Ca2+ current, Ito, transient outward K+ current, IKs, slowly-activating delayed rectifier K+ current, IKr rapidly activating delayed rectifier K+ current, IK1, inward rectifier K+ current, IKAS, apamin-sensitive Ca-activated K+ current, INaK, Na/K-ATPase pump current
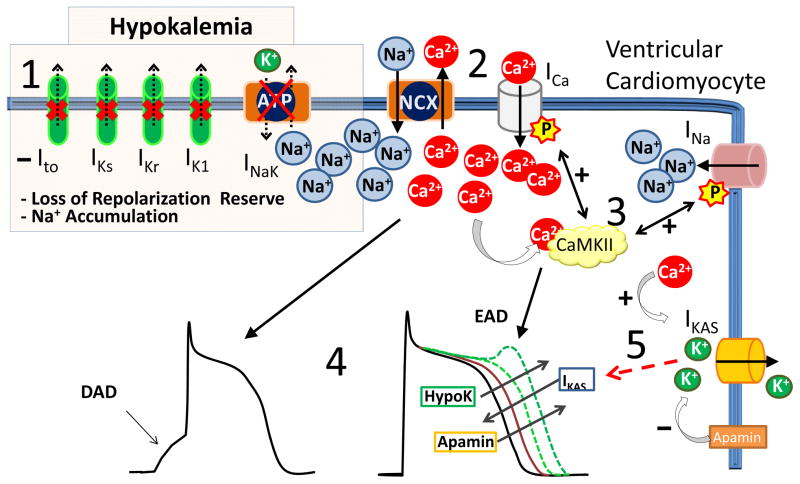
Figure 2.
Cardiomyocyte electrophysiology during hypokalemia. 1) Hypokalemia inhibits outward potassium currents (Ito, IKr, IKs and IK1) and the Na/K ATPase pump current (INaK) with consequent loss of repolarization reserve and increased intracellular Na. 2) High intracellular [Na+] inhibits Ca2+ removal via the Na/Ca exchanger (NCX) and Ca2+ accumulates in the cytosol. 3) High [Ca2+]cytosol activates CaMKII which increases ICa and late INa inward currents. 4) Together with the hypokalemia-induced block of K+ currents, these changes in ionic currents cause AP prolongation and promote EAD and DAD triggered arrhythmias. 5) During hypokalemia, the increased [Ca2+]cytosol activates apamin-sensitive Ikas currents, which shorten the AP. When Ikas is inhibited by apamin, AP is not restored and ventricular arrhythmogenesis ensues.
In this issue of Circulation, the Chen laboratory reports a new role for IKAS during hypokalemia that is relevant even in the healthy ventricle:3 the authors discovered that, unlike in normokalemic conditions, during hypokalemia IKAS is increased and appears to have two main functions: 1) IKAS provides additional repolarization reserve and reduces the AP duration (APD) in particular at long pacing cycle lengths; and 2), IKAS is important for short-term cardiac memory. Using isolated rabbit hearts as a model system, the authors found that in normokalemia IKAS does not appear to be relevant because selective blockade of IKAS with apamin does not prolong ADP significantly in intact hearts. In contrast, in hypokalemia, IKAS is upregulated due to the increase in cytosolic Ca (Figure 2), and IKAS block with apamin causes significant APD prolongation, activation wavebreak and spatially discordant APD alternans. This results in increased susceptibility to pacing induced ventricular fibrillation in hypokalemic hearts after apamin treatment. The authors suggest that IKAS is an “emergency” K current that acts as a defense mechanism against ventricular arrhythmias in a setting of reduced repolarization reserve and myocyte Ca overload due to hypokalemia.
Effects of Hypokalemia on cardiac electrophysiology
Hypokalemia is widely recognized as being associated with increased risk for ventricular arrhythmias, in particular in the setting of preexisting heart disease such as cardiac ischemia, bundle branch block, ventricular pacing or heart failure. The main effect of low extracellular [K] appears to be the reduction of repolarization reserve due to inhibition of outward K currents (Figure 2). Particularly affected are the rapid component of the delayed rectifier (IKr) and the inward rectifier (IK1) that together determine the fast downslope of the repolarization phase of the AP (Fig. 1).4 In addition, chronic hypokalemia causes an internalization of channels that leads to reduced IKr density. Conductance of the delayed rectifier K current (IKs) and the transient outward K current (Ito) are also regulated by extracellular K concentrations. In addition, hypokalemia inhibits the cell membrane Na/K ATPase pump, which further decreases the repolarization reserve due to the reduced outward Na/K pump current. As a result of the altered repolarization process, AP duration is prolonged which can trigger early afterdepolarizations (EAD). However, Weiss et al5 recently reported in an elegant combination of experimental studies and computer simulation that reduction of repolarization reserve by hypokalemia was not, by itself, sufficient to induce EADs, unless Na and Ca homeostasis were also altered. The underlying mechanism is illustrated in Figure 2: The hypokalemia-induced Na/K pump inhibition not only reduces repolarization reserve but also leads to accumulation of intracellular Na (Figure 2), which in turn reduces Ca extrusion via the Na/Ca exchanger thereby causing intracellular Ca overload and delayed afterdepolarizations (DADs).6 The increased intracellular [Ca] activates the Ca/calmodulin-dependent protein kinase II (CaMKII), which seems to be another key component in Ca-induced arrhythmia risk. Activated CaMKII phosphorylates L-type Ca channels and Na channels thus generating a positive feedback that further raises intracellular Ca and late Na current, both involved in afterdepolarization and arrhythmia triggering.5 Inhibition of CaMKII prevents EAD and arrhythmias in rabbit hearts.7 In addition, the inhomogeneous distribution of APD changes and conduction slowing promote an arrhythmogenic substrate for reentrant circuits that sustain ventricular arrhythmias.8 As a result, hypokalemia produces significant arrhythmia risk even in structurally normal hearts, and Ca overload appears necessary to increase the risk of ventricular arrhythmias in the setting of hypokalemia.
The article by the Chen group3 clearly documents the hypokalemia-induced increase in intracellular Ca. The authors suggest that high cytosolic Ca is responsible for the activation of SK channels that likely generate the potentially antiarrhythmic outward IKAS. Consistent with this mechanism, apamin had a greater effect on the AP duration in sites remote to the pacer where [Ca], and supposedly repolarizing IKAS activity, are higher. The authors also report that IKAS activation flattens the AP restitution curve and prevents discordant alternans, both of which are considered to reduce the likelihood of ventricular fibrillation,9 as confirmed experimentally by Chan et al. on intact perfused rabbit hearts.3 Taken together, the article by Chan et al. provides compelling evidence that IKAS activation protects the heart against ventricular fibrillation in the setting of hypokalemia3.
IKAS as a pro arrhythmic current in diseased hearts?
IKAS has been studied not only in failing ventricular myocytes but also in other conditions such as tachycardia/ventricular pacing and myocardial infarction.10 IKAS is upregulated after an infarction, although potential regional differences in current expression between border zone and remote zone have not been thoroughly investigated.11 Besides its seemingly antiarrhythmic role in reducing APD, a proarrhythmic effect of IKAS has been hypothesized related to its heterogeneous expression in the human heart. Patch clamp studies show a higher IKAS density in the endocardial and epicardial layers in failing hearts. This transmural dispersion might generate different APD across the ventricular tissue and cause conduction blocks. In addition, excessive IKAS activation was associated with transient shortening of APD following defibrillation shocks in failing rabbit hearts. After a DC shock, cells displayed persistent cytosolic Ca accumulation that might activate IKAS and excessively reduce AP, thereby increasing the likelihood of post-shock reentry. Consistent with this mechanism, failing rabbit hearts developed spontaneous after-shock ventricular tachycardia attributed to ectopic ventricular activity during phase 3 of the AP. Interestingly, apamin administration prevented transient AP shortening and recurrent ventricular fibrillation after DC shock.12 Hence, IKAS activation and therefore IKAS blockers might be both proarrhythmic and antiarrhythmic depending on the underlying heart disease.13
IKAS and short-term cardiac memory
The other main observation of the Chen article is that IKAS seems to be important for short-term cardiac memory.3 Cardiac memory refers to the phenomenon that a change in the direction of cardiac activation and/or the pacing rate can transiently modify the AP duration and the T-wave morphology on the EKG. It is called “memory” because the T wave maintains the same vector even after the altered activation has ceased. Cardiac memory has also been implicated as risk factor for ventricular fibrillation.14 The mechanisms underlying short-term cardiac memory are poorly understood and multifactorial. Suppression of Ito current seems to be one of the changes responsible for the phenomenon of cardiac memory.15 Angiotensin, through its AT1 receptor, might promote Kv4.3 internalization with consequent loss of Ito. Interestingly, pacing frequency appears to be not as important in determining short-term memory as the pacing site and dyssynchrony. This suggests a role of mechanical stretch in mediating the electrical effects observed during ventricular pacing. However, the link between mechanical strain and altered electrical activity is not clear.16 In their article,3 the authors hypothesize a role of IKAS in cardiac memory based on the observation that apamin prolonged the AP more at late activated sites compared to sites close to the pacer. The time needed for an AP to reach a stable and constant morphology after an altered activation sequence, as during cardiac pacing, is an expression of cardiac memory.17 Since IKAS reduces AP duration and IKAS block with apamin affects the AP restitution curve,13 the prolonged AP shows a positive correlation with the AT, suggesting a role of IKAS in cardiac memory modulation. However, all studies on cardiac memory were done in hypokalemic hearts, where IKAS plays a prominent role in regulating AP duration, and IKAS may be upregulated because of increased intracellular Ca concentration regardless of the pacing condition (Figure 2). In normokalemic paced hearts, IKAS essentially has no effect on AP duration. Therefore, a role of IKAS on cardiac memory in normokalemic setting cannot be definitively inferred from the data presented. Clearly, multiple factors play a role in electrical memory such as strain induced intracellular signal activation, altered Ca handling and abnormal connexin distribution. Further experiments are required to interpret the complex changes associated with electrical remodeling and cardiac memory.
IKAS has generated a lot of interest in recent years because it is upregulated in various condition of altered electrical activity. However, two or more of these conditions often coexist as hypokalemia and heart failure or dyssynchrony and heart failure and it is difficult to discern what determines IKAS activation and its relative contribution to arrhythmia risk. Moreover, amiodarone, possibly the most effective anti-arrhythmic drug on the market, blocks IKAS. Finally, we still do not know if other antiarrhythmic drugs affect IKAS as part of their therapeutic effect. Nevertheless, the article by Chan et al has provided us with a new understanding of the physiological role of IKAS in the hypokalemic heart.
Footnotes
Disclosures: None.
References
- 1.Nabauer M, Kaab S. Potassium channel down-regulation in heart failure. Cardiovasc Res. 1998;37:324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- 2.Chang PC, Chen PS. Sk channels and ventricular arrhythmias in heart failure. Trends Cardiovasc Med. 2015;25:508–514. doi: 10.1016/j.tcm.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan YHTW, Ko JS, Yin D, Chang PC, Rubart M, Weiss JN, Everett T, Lin SF, Chen PS. Small conductance calcium-activated potassium current is activated during hypokalemia and masks short term cardiac memory induced by ventricular pacing. Circulation. 2015;132:XX–XXX. doi: 10.1161/CIRCULATIONAHA.114.015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osadchii OE. Mechanisms of hypokalemia-induced ventricular arrhythmogenicity. Fundam Clin Pharmacol. 2010;24:547–559. doi: 10.1111/j.1472-8206.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 5.Pezhouman A, Singh N, Song Z, Nivala M, Eskandari A, Cao H, Bapat A, Ko CY, Nguyen T, Qu Z, Karagueuzian HS, Weiss JN. Molecular basis of hypokalemia-induced ventricular fibrillation. Circulation. 2015 Aug 12; doi: 10.1161/CIRCULATIONAHA.115.016217. pii: CIRCULATIONAHA.115.016217. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronsen JM, Skogestad J, Lewalle A, Louch WE, Hougen K, Stokke MK, Swift F, Niederer S, Smith NP, Sejersted OM, Sjaastad I. Hypokalaemia induces ca(2+) overload and ca(2+) waves in ventricular myocytes by reducing na(+),k(+)-atpase alpha2 activity. J Physiol. 2015;593:1509–1521. doi: 10.1113/jphysiol.2014.279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ME, Braun AP, Wu Y, Lu T, Schulman H, Sung RJ. Kn-93, an inhibitor of multifunctional ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 8.Yu CC, Corr C, Shen C, Shelton R, Yadava M, Rhea IB, Straka S, Fishbein MC, Chen Z, Lin SF, Lopshire JC, Chen PS. Small conductance calcium-activated potassium current is important in transmural repolarization of failing human ventricles. Circ Arrhythm Electrophysiol. 2015;8:667–676. doi: 10.1161/CIRCEP.114.002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, Garfinkel A, Karma A. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–1240. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 10.Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF, Chen PS. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm. 2013;10:1516–1524. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YS, Chang PC, Hsueh CH, Maruyama M, Park HW, Rhee KS, Hsieh YC, Shen C, Weiss JN, Chen Z, Lin SF, Chen PS. Apamin-sensitive calcium-activated potassium currents in rabbit ventricles with chronic myocardial infarction. J Cardiovasc Electrophysiol. 2013;24:1144–1153. doi: 10.1111/jce.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh YC, Chang PC, Hsueh CH, Lee YS, Shen C, Weiss JN, Chen Z, Ai T, Lin SF, Chen PS. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol. 2013;6:410–418. doi: 10.1161/CIRCEP.111.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baher A, Qu Z, Hayatdavoudi A, Lamp ST, Yang MJ, Xie F, Turner S, Garfinkel A, Weiss JN. Short-term cardiac memory and mother rotor fibrillation. Am J Physiol Heart Circ Physiol. 2007;292:H180–189. doi: 10.1152/ajpheart.00944.2005. [DOI] [PubMed] [Google Scholar]
- 15.Jeyaraj D, Wan X, Ficker E, Stelzer JE, Deschenes I, Liu H, Wilson LD, Decker KF, Said TH, Jain MK, Rudy Y, Rosenbaum DS. Ionic bases for electrical remodeling of the canine cardiac ventricle. Am J Physiol Heart Circ Physiol. 2013;305:H410–419. doi: 10.1152/ajpheart.00213.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeyaraj D, Wilson LD, Zhong J, Flask C, Saffitz JE, Deschenes I, Yu X, Rosenbaum DS. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115:3145–3155. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 17.Jordan PN, Christini DJ. Determining the effects of memory and action potential duration alternans on cardiac restitution using a constant-memory restitution protocol. Physiol Meas. 2004;25:1013–1024. doi: 10.1088/0967-3334/25/4/018. [DOI] [PubMed] [Google Scholar]