Interleukin-10 (IL-10) is a key anti-inflammatory cytokine1, 2 and loss of function mutations in IL-10 or the IL-10 receptor (IL-10R) have been implicated as a common cause of infantile IBD3, 4, 5, 6. These patients typically present in the first months of life with severe colitis, perianal disease, folliculitis, and, on occasion, arthritis, and are classically refractory to various immunosuppressive agents. Hematopoietic stem cell transplantation (HSCT) has been shown to be curative, but is not available for all patients4, 5, 6. The long-term risks of IL-10R-deficiency are unclear; however B-cell lymphomas have recently been reported in untransplanted young children with a known diagnosis of IL-10R deficiency7. Here we report the discovery of IL-10R deficiency in a patient who presented with severe IBD as an infant and developed a mature B cell lymphoma in adolescence.
Through our interNational Early Onset Pediatric IBD Cohort Study (NEOPICS)/Care-for-Rare IBD Alliance we were referred a 15 year-old female patient with history of infantile IBD. She developed bloody diarrhea and anal fissures in the first weeks of life, failure to thrive and anemia requiring several blood transfusions by 6 months, and a rectovaginal fistula at 8 months of age. Endoscopic evaluation revealed severe pan-colitis, a distal colonic stricture, and pseudopolyps; biopsies demonstrated patchy areas of cryptitis, ulcerations, and lymphocytic infiltration. Her disease was resistant to various medications including steroids and aminosalicylates for the first 5 years, Imuran for the next 3 years, and combination of anti-TNF antibodies and methotrexate for 3 more years. Persistent symptoms led to a partial colectomy and colostomy at 8 months of age and at 5 years of age a subtotal colectomy, Hartmann's pouch construction, and permanent ileostomy. Despite these interventions, the patient continued to suffer from severe perianal fistulizing disease.
At 12 years of age the patient presented with two months history of right-sided abdominal pain and hepatosplenomegaly. Blood tests demonstrated mild thrombocytopenia, hyperuricemia and abnormal liver tests (Table 1). An abdominal CT showed hepatosplenomegaly with multiple focal liver lesions accompanied by enlarged mesenteric lymph nodes, all confirmed to be hypermetabolic on PET scan (Figure 1). Liver biopsy revealed CD20 positive, EBV-encoded RNA (EBER)-negative small round blue cells leading to a diagnosis of mature large B cell lymphoma. Despite successful initial treatment with cyclophosphamide, vincristine, prednisone, ritixumab, cytarabine, doxorubicin as well as intrathecal methotrexate, cytarabine, and hydrocortisone, remission was maintained for only two years. At time of relapse at age 15, salvage chemotherapy of rituximab, ifosfamide, carboplatin, and etoposide was initiated and consolidation with autologous HSCT was being considered at the time of referral.
Table 1. Laboratory results at presentation with diffuse large B cell lymphoma.
| Laboratory: | Patient values: | Normal range: |
|---|---|---|
| Complete blood count: | ||
| White blood cell count | 12,000 | 4,500-13,5000 cells/mm3 |
| Absolute neutrophil count | 4,716 | 1,800-10,100 cells/mm3 |
| Absolute lymphocyte count | 6,240 | 1,100-6,100 cells/mm3 |
| Hemoglobin | 14.0 | 11.5-16.0 g/dL |
| Platelet count | 93,000 | 150-400,000 cells/mm3 |
|
| ||
| Renal panel: | ||
| Sodium | 133 | 136-146 mEq/L |
| Potassium | 4.4 | 3.5-5.0 mmol/L |
| Chloride | 97 | 98-108 mmol/L |
| Bicarbonate | 19 | 22-34 mmol/L |
| Blood urea nitrogen | 13 | 8-20 mg/dL |
| Creatinine | 0.5 | 0.5-1.0 mg/dL |
| Glucose | 56 | 73-110 mg/dL |
| Corrected calcium | 9.1 | 8.6-10.3 mg/dL |
| Magnesium | 2.1 | 1.5-2.4 mg/Dl |
| Phosphorus | 3.8 | 2.7-4.6 mg/dL |
|
| ||
| Liver function test: | ||
| AST | 237 | 8-30 IU/L |
| ALT | 121 | 7-35 IU/L |
| Alkaline phosphatase | 368 | 90-420 IU/L |
| Total bilirubin | 1.2 | 0.2-1.2 mg/dL |
| GGT | 308 | 8-35 IU/L |
| Albumin | 3.1 | 3.8-4.9 g/dL |
|
| ||
| Coagulation: | ||
| PT | 13.5 | 9.5-12.0 sec |
| INR | 1.3 | |
| PTT | 26.3 | 22.0-30.0 sec |
|
| ||
| Uric acid | 6.8 | 2.2-6.0 mg/dL |
| LDH | 2771 | 120-240 IU/L |
|
| ||
| Inflammatory markers: | ||
| ESR | 26 | 0-20 mm/hr |
| CRP | 8.9 | 0-0.6 mg/dL |
Figure 1. Imaging and histology results at time of diagnosis with diffuse large B cell lymphoma.
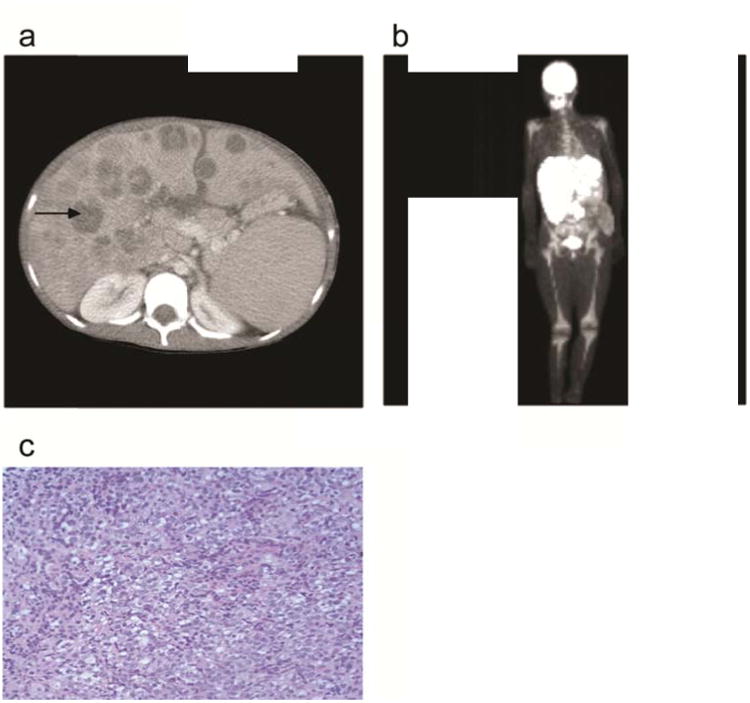
(a) Abdominal CT scan showing multiple focal liver lesions (arrow pointing to representative lesion) confirmed to be hypermetabolic on PET scan (b). (c) Hematoxylin & Eosin staining of liver biopsy (40×) showing numerous small round blue tumor cells.
We performed IL-10R functional testing on freshly isolated peripheral blood mononuclear cells obtained from the patient and her father who served as a healthy control. Our flow cytometry-based assay measures IL-10-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3), which is a key transcription factor down-stream of the IL-10 receptor; IL-6-induced STAT3 phosphorylation serves as an internal positive control. While the patient's IL-6-dependent STAT3 phosphorylation was intact, IL-10-dependent phosphorylation of STAT3 was completely abrogated (Figure 2A), suggesting abnormalities in the IL-10 receptor or downstream signaling components. Targeted sequencing of IL10R genes revealed novel compound heterozygous mutations in IL10RB (NM000628.4) comprised of a variant on exon 2 (c. 172 A>G; p. S58R) leading to a serine to arginine substitution, predicted by PolyPhen analysis as highly deleterious, and a variant on exon 5 (c. 611 G>A; p. W204X) leading to a stop codon (Figure 2B). Additional functional assays with monocytes isolated from this patient, as well as other IL-10R deficient patients, demonstrating an increase in proinflammatory macrophage function and a defect in anti-inflammatory macrophage generation and function have been recently published 2. Since autologous HSCT would be predicted to be ineffective in patients with IL-10R deficiency given the broad requirement of IL-10-dependent signalling in the hematopoietic compartment, the patient was referred for allogeneic HSCT. Four months after matched unrelated allogeneic transplantation, the patient is fully engrafted without any signs of active colitis or lymphoma recurrence, and the rectovaginal fistula is resolving.
Figure 2. Identification of loss of function mutations in IL10RB.
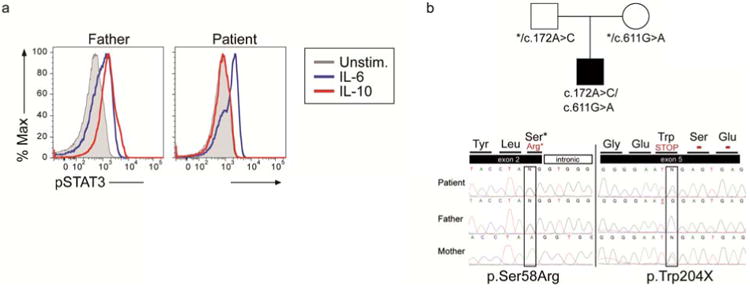
(a) Functional analysis of IL-10R was performed by assessing STAT3 phosphorylation using flow cytometry. Peripheral blood mononuclear cells were obtained from the patient and her father who served as a healthy control and cultured without stimuli (unstim.) or with IL-10 20 ng/mL for 30 minutes. Stimulation with IL-6 20ng/mL served as an internal positive control, since it also induces STAT3 phosphorylation. Cells were then fixed, permeabilized and stained for pSTAT3 (phosphorylated STAT3). (b) Targeted sequencing of IL10RB revealed compound heterozygote mutations.
Discussion
The long-term health risks of IL-10R deficiency in IBD patients who are not transplanted are unknown. While HSCT has been shown to be curative, the lack of suitable donors as well as cost, complexity and potential morbidity and mortality prevent its more widespread use. The patient reported here had a history of severe infantile IBD that was refractory to immunosuppressive medications and surgical interventions, and at 12 years of age developed B cell lymphoma. The diagnosis of loss of function mutations in the IL10RB gene was confirmed at 15 years of age and altered the treatment plan from autologous to allogeneic HSCT.
A recent study by Neven and colleagues showed an association between IL-10R deficiency and B cell lymphoma7. In their report, 5/14 (36%) patients with loss of function mutations in IL10RA or IL10RB that did not undergo HSCT developed lymphoma involving the spleen, liver, bone or lymph nodes, at the age of 5-6 years. Tumor cell analyses showed characteristics of EBV-negative diffuse large B cell lymphomas containing monoclonal germinal center B cells 7. Two additional cases of EBV-positive B cell lymphomas in IL-10R deficient patients have been reported elsewhere5.
The precise mechanism of how loss of IL-10R signaling leads to development of B cell lymphoma is unclear yet might be related to impaired immune surveillance. IL-10 has been shown to be a key factor for effective tumor immune surveillance by infiltrating CD8+ T cells. IL-10R is highly expressed on CD8+ T cells and, in response to IL-10, leads to increased IFN-and granzyme secretion and enhanced anti-tumor activity8. Granzyme B expression was shown to be reduced in lymphomas isolated from IL-10R-deficient patients7. Further support of a role for IL-10R signaling in B-cell lymphoma development is the finding that patients with dominant negative mutations in STAT3, a signaling molecule downstream of IL-10R, can develop large B cell lymphomas9. Finally, although it has been postulated that tumorigenesis in IBD patients may result from thiopurine and/or anti-TNF antibody exposure10,11,12, such contribution among IL- 10R-deficient subjects has been described as remote since tumors have developed in patients not exposed to immune suppression7.
In conclusion, this case highlights the critical importance of pursuing a diagnosis of IL-10R deficiency in any patient presenting as an infant with severe colitis in association with perianal disease. A missed diagnosis can obviously delay appropriate curative treatment for the intestinal inflammation but also places the patient at great risk of developing large B cell lymphoma. This case also emphasizes that IL-10R deficiency can present in children from unrelated parents and the diagnosis can remain elusive even in adolescence. Clinicians should be aware of this complication and diagnosis and pursue HSCT early in disease course. Autologous HSCT as a consolidation therapy for patients with chemotherapy-refractory B cell lymphoma should only be offered in patients with concomitant IBD after IL-10R deficiency has been excluded. If HSCT is not feasible, surveillance steps should be implemented at a young age for early detection of such tumors. Gene replacement or gene therapy approaches, as this field evolves, may be warranted for such patients.
Acknowledgments
D.S.S is a recipient of a Research Fellowship Award Grant from the Crohn's and Colitis Foundation of America. S.B.S is supported by NIH Grants HL59561, DK034854, and AI50950, the Helmsley Charitable Trust, and the Wolpow Family Chair in IBD Treatment and Research.
References
- 1.Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, et al. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol. 2014;122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40(5):706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361(21):2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143(2):347–355. doi: 10.1053/j.gastro.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt KR, Shah N, Faizura-Yeop I, Kocacik Uygun DF, Frede N, Muise AM, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. The Journal of allergy and clinical immunology. 2013;131(3):825–830. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Pigneur B, Escher J, Elawad M, Lima R, Buderus S, Kierkus J, et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: a survey of the Genius Working Group. Inflammatory bowel diseases. 2013;19(13):2820–2828. doi: 10.1097/01.MIB.0000435439.22484.d3. [DOI] [PubMed] [Google Scholar]
- 7.Neven B, Mamessier E, Bruneau J, Kaltenbach S, Kotlarz D, Suarez F, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood. 2013;122(23):3713–3722. doi: 10.1182/blood-2013-06-508267. [DOI] [PubMed] [Google Scholar]
- 8.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer cell. 2011;20(6):781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Kumanovics A, Perkins SL, Gilbert H, Cessna MH, Augustine NH, Hill HR. Diffuse large B cell lymphoma in hyper-IgE syndrome due to STAT3 mutation. Journal of clinical immunology. 2010;30(6):886–893. doi: 10.1007/s10875-010-9452-z. [DOI] [PubMed] [Google Scholar]
- 10.Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, et al. Risk of Lymphoma in Patients with Inflammatory Bowel Disease Treated with Azathioprine and 6-Mercaptopurine: a Meta-Analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014 doi: 10.1016/j.cgh.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-alpha therapy in inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2014;39(5):447–458. doi: 10.1111/apt.12624. [DOI] [PubMed] [Google Scholar]
- 12.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lemann M, Cosnes J, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]


