Abstract
A new series of 3-(4-substituted phenyl)-1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1-substituted urea (5a–o) was synthesized by an effectual route via sulfonylcarbamates and explores the novel site for substitution in sulfonylurea as well as the way of thiazine can be prepared. The molecules were established by elemental analysis and spectroscopic viz. IR, 1H NMR, 13C NMR and MS techniques. All the fifteen derivatives were shown very prominent oral hypoglycemic effect at the dose of 40 mg/kg body weight (p.o.) in respect of standard drug glibenclamide and control. The hypoglycemic effect was studied using oral glucose tolerance test in normal and NIDDM in STZ-rat model. The compounds 5a, 5d, 5f, 5i, 5k and 5n were dominant out of fifteen derivatives for blood glucose lowering activity (more than 80%) when comparing with NIDDM control. These derivatives were either containing simply phenyl ring (5a, 5f and 5k) on to the second amine of sulfonylurea (R′ = H) or nitro group at the para position in compound 5d, 5i and 5n (R′ = NO2) to produce significant oral hypoglycemic effect. Other structural activity relationship is also observed regarding the heteroaromatic and substituted aromatic group at R and R′ position respectively.
Keywords: Sulfonylcarbamate, Heteroaromatic, Hypoglycemic, NIDDM
1. Introduction
Type 2 diabetes mellitus is the very common metabolic disease, which is illustrated by the blemish of insulin secretion as well as its sensitivity. Generally it is considered as sulfonylureas exert the hypoglycemic effect through promoting the insulin secretion from receptor of pancreatic β-cell (Kecskemeti et al., 2002). However some reports have been published to suggest that the sulfonylureas do not penetrate the β-cell of pancreas which results the binding of this pharmacophore in very specific sites of plasma membrane of β-cell (Flatt et al., 1994). It may be due to lower lipophilicity or due to ionized form of sulfonylureas. The second generation sulfonylurea is so potent stimulator of insulin secretion shown a great success for the treatment of type 2 diabetes, but due to exert hyperinsulinemia that causes the weight gain or hypoglycemia bears hindrance on their success (Hamaguchi et al., 2004). Other than hypoglycemic effect sulfonylurea are established for cytotoxicity (Jung et al., 1996), antimicrobial (Krajacić et al., 2005), vasodilator (Khelili et al., 1995) and antitubercular (Pan et al., 2012) all these consideration persuade the researcher to develop an effective oral hypoglycemic sulfonylurea derivative.
Sulfonylureas are generally undergone the chemical hydrolysis at ionizable hydrogen atom containing nitrogen which is situated between sulfonyl and carbonyl groups. The ionization leads the early cleavage of Sulfonylurea bridge, producing CO2 and the corresponding sulfonamide and amine (Zheng et al., 2008). Although the second generation sulfonylurea glibenclamide have longer duration of action but accumulates progressively in the β-cell (Kamp et al., 2003). The efficacy and penetration of sulfonylureas can be enhanced by decreasing the rate of ionizable metabolism. This article presents the synthesis of some new trisubstituted sulfonylurea derivatives containing substitution at ionizable nitrogen atom with some aromatic and heteroaromatic groups.
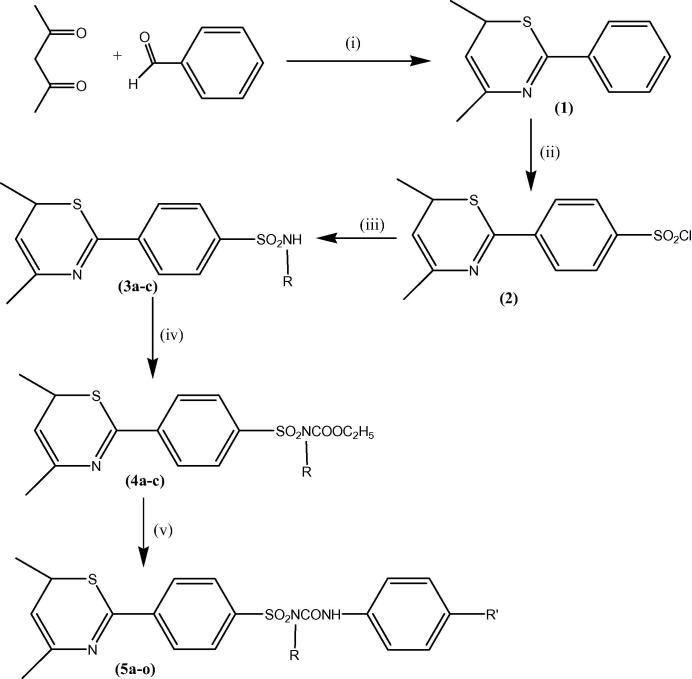
The synthesis of compounds was initiated by the preparation of unsubstituted phenyl ring containing thiazine heterocyclic compound (1). This compound was actually prepared from the condensation of acetylacetone with the ammonium thiocyanate and benzaldehyde under reflux. When it was further reacted with chlorosulfonic acid at the room temperature by using dioxane it results sulfonyl chloride group at the para position of the phenyl ring (2). Which on treatment with the primary amines were produced different sulfonamides (3) under mild acidic condition of acetic acid that further converted to sulfonylcarbamates (4) after treatment with the ethyl chloroformate in the presence of pyridine base. Finally the hydrolysis of ethyl ester of sulfonylcarbamates with primary amines results the different trisubstituted sulfonylurea derivatives (5a–o) (Scheme 1, Table 1).
Scheme 1.
Reagents and reaction conditions: (i) Ammonium thiocyanate, gla. CH3COOH, reflux 3–4 h, (ii) Chlorosulfonic acid, 1,4 dioxane, stirring at r.t., (iii) Pyridine, aniline (3a), p-nitro aniline (3b), 2-amino pyridine (3c), reflux 3–4 h, (iv) Ethyl chloroformate, anhydrous K2CO3, dry acetone, reflux 18–20 h, (v) Substituted primary aromatic amines (R′NH2), toluene, reflux 3–4 h.
Table 1.
List of substitutions for different derivatives.
| Compounds | R | R′ | M.p. (°C) | Overall yield (%) |
|---|---|---|---|---|
| 5a | Phenyl | H | 224–226 | 80 |
| 5b | Phenyl | Cl | 254–258 | 84 |
| 5c | Phenyl | OH | 246–248 | 76 |
| 5d | Phenyl | NO2 | 241–245 | 79 |
| 5e | Phenyl | OCH3 | 252–253 | 68 |
| 5f | Phenyl-4-NO2 | H | 254–255 | 79 |
| 5g | Phenyl-4-NO2 | Cl | 257–259 | 76 |
| 5h | Phenyl-4-NO2 | OH | 259–260 | 86 |
| 5i | Phenyl-4-NO2 | NO2 | 258–260 | 84 |
| 5j | Phenyl-4-NO2 | OCH3 | 248–250 | 75 |
| 5k | Pyridin-2yl | H | 236–237 | 74 |
| 5l | Pyridin-2yl | Cl | 238–240 | 82 |
| 5m | Pyridin-2yl | OH | 258–259 | 72 |
| 5n | Pyridin-2yl | NO2 | 264–267 | 69 |
| 5o | Pyridin-2yl | OCH3 | 257–259 | 78 |
2. Result and discussion
The targeted trisubstituted sulfonylurea derivatives were prepared and confirmed by various spectroscopic and elemental analyses. Mass spectra and NMR data were helpful to conclude the molecular formula and weight as well as the chemical condition of atoms in the structure along with the data from the elemental analysis. The progress and completion of synthetic reactions were evaluated by the TLC. FTIR spectra were playing a crucial role in authenticating the presence functional group in the target structure and confirmation for the conversion of intermediates to next compound.
It can be seen into the FTIR spectra of compound 3a–c, the presence of peak for the secondary amine group (—NH—), were absent in the FTIR spectra of compound 4a–c, where the amine was substituted with ethylformate. This substitution made the amine to trisubstituted and the peak was become disappear due to the absence of N—H stretching. Further support for was made by the appearance of spectra for carbonyl group which given a hint for the presence of ethyl ester. The formation of sulfonylurea from the sulfonylcarbamate was confirmed by the FTIR spectra of compound 5a–o, where peak for —N—H— stretching was appeared again in terms of second amine group in sulfonylurea as it should be secondary amine.
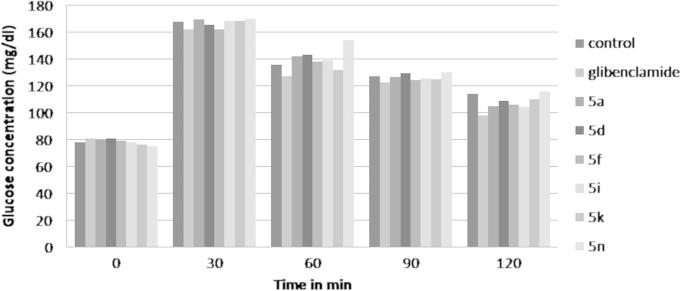
The type II diabetes (NIDDM) was induced by streptozotocin i.p. injection in rats successfully. The antidiabetic study was evaluated for fifteen derivatives of trisubstituted sulfonylurea (5a–o) by oral glucose tolerance (OGT) test and NIDDM rat model. The glucose lowering effect of test compounds is presented in Table 2 and the oral glucose tolerance test results are shown in the form of bar chart to compare the effects of most active derivative (Fig. 1).
Table 2.
Effect of sulfonylurea derivatives on blood glucose levels in NIDDM induced rats.
| Compounds | R/R′ | Blood glucose level (mg/dl) in STZ rats |
Percent inhibition in the rise of blood glucose level in STZ rats | |
|---|---|---|---|---|
| On fasting condition | After 90 min of fed | |||
| Control | – | 79.3 ± 3.4 | 116.2 ± 2.5 | – |
| Standard (Glibenclamide) | – | 78.7 ± 2.4 | 74.1 ± 4.8 | 112.4 |
| 5a | C6H5/H | 76.5 ± 3.7 | 82.3 ± 4.6 | 84.3 |
| 5b | C6H5/Cl | 72.3 ± 3.6 | 80.7 ± 2.9 | 77.3 |
| 5c | C6H5/OH | 73.6 ± 5.6 | 80.2 ± 3.8 | 82.1 |
| 5d | C6H5/NO2 | 77.4 ± 3.1 | 81.4 ± 2.4 | 89.2 |
| 5e | C6H5/OCH3 | 74.3 ± 4.7 | 83.9 ± 4.7 | 73.9 |
| 5f | 4-NO2C6H4/H | 78.5 ± 4.6 | 83.1 ± 5.1 | 87.5 |
| 5g | 4-NO2C6H4/Cl | 76.3 ± 3.4 | 82.4 ± 4.8 | 83.5 |
| 5h | 4-NO2C6H4/OH | 79.4 ± 5.4 | 86.4 ± 4.7 | 77.8 |
| 5i | 4-NO2C6H4/NO2 | 78.7 ± 3.8 | 82.0 ± 3.6 | 91.1 |
| 5j | 4-NO2C6H4/OCH3 | 77.2 ± 2.8 | 83.5 ± 5.1 | 83.0 |
| 5k | Pyridin-2yl/H | 78.2 ± 2.7 | 83.2 ± 4.7 | 89.7 |
| 5l | Pyridin-2yl/Cl | 74.7 ± 3.6 | 81.7 ± 4.5 | 81.0 |
| 5m | Pyridin-2yl/OH | 76.4 ± 4.2 | 83.9 ± 5.7 | 79.7 |
| 5n | Pyridin-2yl/NO2 | 76.2 ± 2.8 | 82.1 ± 5.3 | 84.0 |
| 5o | Pyridin-2yl/OCH3 | 74.2 ± 4.8 | 82.3 ± 5.5 | 78.1 |
Data are expressed as value of mean ± SEM, n = 6. The noninsulin dependent diabetes (NIDDM) was induced by streptozotocin (STZ) at 40 mg/kg body weight by i.p. injection.
Figure 1.
Comparative chart of oral glucose tolerance test result of active derivatives. ∗Data expressed as the lowering of blood glucose level in mg/dl. n = 6, the normal rats were used for study at the dose of 2 g/kg body weight (p.o.) dose.
All test compounds were shown remarkable antidiabetic effect at 100 mg/kg body weight (p.o.). Out of these fifteen derivatives the compounds 5a, 5d, 5f, 5i, 5k and 5n were reduced the highest percentage of blood glucose level in diabetic rats. In glucose fed normal rats these compounds reduced the more than 80% of blood glucose as compared to control.
These active compounds either possessed simply phenyl ring (5a, 5f and 5k) on to the second amine of sulfonylurea (R′ = H) or nitro group at the para position in compound 5d, 5i and 5n (R′ = NO2) to produce significant blood glucose lowering activity. The para methoxy (R′ = OCH3) derivatives (5e, 5j, 5o) were given a little inferior activity compare to rest of the test compounds. While the chloro and hydroxyl derivatives were given considerable blood glucose lowering effect. Out of these six most active derivative, the compound containing nitro substituted phenyl ring at both the amine groups of sulfonylurea (5i) (R = R′ = NO2) is most effectively reduce the blood glucose level in both OGT test and NIDDM induced rats. The oral glucose tolerance test chart (Fig. 1) elucidates the effect of most active derivative on normal rat’s blood glucose level after glucose fed (2 g/kg b w p.o.) to assure the antihyperglycemic effect. So the conclusion for the SAR study of test compounds can be summarized as the tertiary nitrogen group containing sulfonylurea is useful for the antidiabetic drug development. The unsubstituted phenyl ring is favorable for activity and the presence of electron withdrawing nitro moiety in phenyl ring increase the activity very significantly.
3. Conclusion
From spectral and analytical data it can be easily concluded that the preparation of trisubstituted sulfonylurea is achieved successfully along with oral hypoglycemic activity. The structures suggested for synthesized compounds (5a–o) are well established by spectroscopic data and elemental analysis bearing thiazine containing trisubstituted sulfonylurea. All the synthesized compounds are active for oral hypoglycemic activity. Some of the compounds (5a, 5f and 5k) are showing strong glucose lowering effect in all test animals. The application was illustrated by the incorporation of various heterocyclic rings in the sulfonylurea pharmacophore which explore more directing site for the substitution than the previous synthesized sulfonylureas.
4. Experimental
All the chemicals used were procured from Qualigens, Fine Chemicals, Mumbai and CDH (P) Ltd., New Delhi. Melting point ranges of the newly synthesized compounds were determined by open capillary method and are uncorrected. Thin layer chromatography using Silica gel G (E. Merck) plates was used to assess the completion of reaction and purity of synthesized compounds by using combination of acetonitrile and carbontetrachloride (60:40) as mobile phase. Elemental analysis was obtained for all the newly synthesized compounds on Carlo Erba EA 1108 elemental analyzer. IR spectrum of compounds in KBr pellets were recorded on a FTIR spectrophotometer (JASCO) using KBr disk, 1H NMR spectra were recorded in DMSO on a Bruker Advance (400 MHz) NMR spectrophotometer using TMS as internal standard, 13C NMR were recorded at 75 MHz by using DMSO-d6 and mass spectra were taken by EIMS on SHIMADZU-2010 AT.
4.1. Procedure for synthesis of 4,6-dimethyl-2-phenyl-2H-1,3-thiazine (1) Pattan et al., 2009
In a 250 ml round bottom flask a mixture of 0.05 mol acetylacetone (5.0 g), 0.05 mol benzaldehyde (5.30 g) and 10 g of ammonium thiocyanate was refluxed with 15 ml of glacial acetic acid for 3–4 h using water condenser. The mixture was left for 12–14 h at room temperature and filtered. To this mixture around 200 ml of distilled water was added for precipitation of compound and the filtrate was neutralized with weak base hydroxide solution for further precipitation. Both the solids were combined and recrystallized from ethanol. Yield 89% and m.p. 144–46oC.
4.2. Procedure for synthesis of 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)benzene-1-sulfonyl chloride (2)
Two equimolar (25 mmol) solutions of chlorosulfonic acid and compound 1 were prepared separately by using 25 ml of 1,4-dioxane at room temperature. The exothermic solution of chlorosulfonic acid was added into the second solution with constant stirring to produce a homogenous solution. This solution was added into the crushed ice for precipitation of compound. The solid was filtered and recrystallized from ethanol. Yield 80% and m.p. 152–54 °C.
4.3. General procedure for synthesis of 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)-N-substituted benzenesulfonamide (3a–c)
An equimolar mixture of compound 2 (20 mmol) and substituted primary amine was prepared in 20 ml of pyridine base in a 250 ml round bottom flask and refluxed for 3–4 h. The solution was cooled and added into the acidified water with stirring to produce solid product which was filtered and recrystallized with ethanol. Yield 75–80% and m.p. 142–146 °C.
4.3.1. 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)-N-phenyl benzenesulfonamide (3a)
IR vmax (KBr, in cm−1): 1432–44 (Ar—C C—), 1193 (C—N), 2356 (C—S—C), 1380–76 (C—CH3), 1356 and 1174 (SO2N), 3282 (—NH—), 3086 (Ar—C—H); 1H NMR (300 MHz, DMSO-d6, δ): 5.60 (1H, d, C—H of thiazine), 3.46 (1H, m, CH—S of thiazine), 1.53 (3H, d, CH3 of thiazine) 1.72 (3H, s, CH3 of thiazine), 7.94–8.14 (4H, m, Ar—SO2), 4.04 (1H, s, NH), 6.41-7.15 (5H, m, Ar—N); 13C NMR (75 MHz, DMSO-d6, d, ppm): 24.2 and 27.5 (CH3), 30.8 (CH3— CH—S of thiazine), 120.1 (CH3—CH— CH3 of thiazine) 167.1 (N—C—S of thiazine), 142.4 (C—SO2), 115.0–135.8 (C of Ar); FAB-MS (m/z): 358 [M+].
4.3.2. 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)-N-(4-nitrophenyl)benzenesulfonamide (3b)
IR vmax (KBr, in cm−1): 1441–54 (Ar—C C—), 1205 (C—N), 2346 (C—S—C), 1378–82 (C—CH3), 1352 and 1164 (SO2N), 3312 (—NH—), 3074 (Ar—C—H), 1536 and 1310 (—NO2),; 1H NMR (300 MHz, DMSO-d6, δ): 5.46 (1H, d, C—H of thiazine), 3.32 (1H, m, CH—S of thiazine), 1.68 (3H, d, CH3 of thiazine) 1.77 (3H, s, CH3 of thiazine), 7.93–8.14 (4H, m, Ar—SO2), 4.12 (1H, s, NH), 6.34–7.19 (4H, d, Ar—N); FAB-MS (m/z): 403 [M+].
4.3.3. 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)-N-(pyridine-2-yl)benzenesulfonamide (3c)
IR vmax (KBr, in cm−1): 1431–52 (Ar—C C—), 1225 (C—N), 2340 (C—S—C), 1365–78 (C—CH3), 1341 and 1159 (SO2N), 3346 (—NH—), 3092 (Ar—C—H); 1H NMR (300 MHz, DMSO-d6, δ): 5.46 (1H, d, C—H of thiazine), 3.43 (1H, m, CH—S of thiazine), 1.47 (3H, d, CH3 of thiazine) 1.78 (3H, s, CH3 of thiazine), 7.87–8.02 (4H, m, Ar—SO2), 4.03 (1H, s, NH), 6.47–7.91 (4H, d, pyridin); FAB-MS (m/z): 359 [M+].
4.4. General procedure for the synthesis of ethyl 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl(substituted) carbamate (4a–c) Rathish et al., 2009
The corresponding sulfonamide (3a–c) (20 mmol) was mixed with the solution of ethyl chloroformate (26 mmol) and anhydrous potassium carbonate (3 g) in dry acetone (250–300 ml) and was refluxed for 18–20 h. Acetone was removed by distillation under reduced pressure. The solution was left overnight and added in water (100–150 ml) and neutralized with acetic acid. The solid product was filtered and washed with distilled water. The product was dried and recrystallized from ethanol. Yield 85–92% and m.p. 142–158 °C.
4.4.1. Ethyl 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl(phenyl)carbamate (4a)
IR vmax (KBr, in cm−1): 1440–46 (Ar—C C—), 1190 (C—N), 2345 (C—S—C), 1387–81(C—CH3), 1351 and 1172 (SO2N), 1751 (—C O), 1274 and 1095 (—C—O—, str.), 3029 (Ar—C—H), 2980 (C—H, alkyl); 1H NMR (300 MHz, DMSO-d6, δ): 5.61 (1H, d, C—H of thiazine), 3.33 (1H, m, CH—S of thiazine), 1.67 (3H, d, CH3 of thiazine) 1.86 (3H, s, CH3 of thiazine), 1.42 (3H, t, CH3 of ethyl ester), 4.22 (2H, m, CH2 of ethyl ester), 7.81–8.03 (4H, m, Ar—SO2), 6.36–7.05 (5H, m, Ar—N); 13C NMR (75 MHz, DMSO-d6, d, ppm): 24.3 and 28.2 (CH3), 30.4 (CH3— CH—S of thiazine), 120.6 (CH3—CH— CH3 of thiazine) 167.7 (N—C—S of thiazine), 142.4 (C—SO2), 154.8 (C O), 14.4 (CH3 of ethyl), 58.2 (CH2 of ethyl) 121.2–129.4 (C of Ar); FAB-MS (m/z): 430 [M+].
4.4.2. Ethyl4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl(4-itrophenyl)carbamate (4b)
IR vmax (KBr, in cm−1): 1432–36 (Ar—C C—), 1178 (C—N), 2359 (C—S—C), 1388–80(C—CH3), 1360 and 1168 (SO2N), 1748 (—C O), 1287 and 1086 (—C—O—, str.), 3041 (Ar—C—H), 2907 (C—H, alkyl), 1536 and 1302 (—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.59 (1H, d, C—H of thiazine), 3.57 (1H, m, CH—S of thiazine), 1.54 (3H, d, CH3 of thiazine) 1.78 (3H, s, CH3 of thiazine), 1.32 (3H, t, CH3 of ethyl ester), 4.17 (2H, m, CH2 of ethyl ester), 7.95–8.14 (4H, m, Ar—SO2), 7.72–7.86 (4H, m, Ar—N); FAB-MS (m/z): 475 [M+].
4.4.3. Ethyl4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl(4-methoxyphenyl)carbamate (4c)
IR vmax (KBr, in cm−1): 1443–47 (Ar—C C—), 1165 (C—N), 2361 (C—S—C), 1382–78 (C—CH3), 1350 and 1161 (SO2N), 1695 (—C O), 1282 and 1092 (—C—O—, str.), 3029 (Ar—C—H), 2914 (C—H, alkyl), 1540 and 1311 (—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.71 (1H, d, C—H of thiazine), 3.34 (1H, m, CH—S of thiazine), 1.56 (3H, d, CH3 of thiazine) 1.60 (3H, s, CH3 of thiazine), 1.34 (3H, t, CH3 of ethyl ester), 4.20 (2H, m, CH2 of ethyl ester), 7.82–8.07 (4H, m, Ar—SO2), 6.57–7.72 (4H, m, Ar—N), 3.63 (1H, s, —OCH3); FAB-MS (m/z): 460 [M+].
4.5. General procedure for the synthesis of 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-substituted-1-(substituted)urea (5a–o) Rathish et al., 2009
The specified carbamate derivative (4a–c) was dissolved in hot toluene (30 ml). In the above solution the corresponding primary aromatic amine was added slowly and refluxed for 3–4 h. On cooling the refluxed solution, precipitation of desired product was occurred. After filtration solid was recrystallized from toluene and methanol.
4.5.1. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1,3-diphenylurea (5a)
IR vmax (KBr, in cm−1): 1178 (C—N), 2355 (C—S—C), 1349 and 1180 (SO2N), 1697 (—C O), 3029 (Ar—C—H), 3346 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.68 (1H, d, C—H of thiazine), 3.31 (1H, m, CH—S of thiazine), 1.55 (3H, d, CH3 of thiazine) 1.73 (3H, s, CH3 of thiazine), 7.87–8.02 (4H, m, Ar—SO2), 7.01–7.70 (10H, m, Ar), 6.02 (1H, s, —NH— of ureido); 13C NMR (75 MHz, DMSO-d6, d, ppm): 24.1 and 28.7 (CH3), 30.8 (CH of thiazine), 168.1 (N—C—S of thiazine), 141.9 (C—SO2), 154.3 (—C O), 121.0–129.8 (C of Ar); FAB-MS (m/z): 477 [M+]; Anal. Calcd for C25H23N3O3S2: C, 62.87; H, 4.85; N, 8.80; O, 10.05; S, 13.43 Found: C, 62.82; H, 4.89; N, 8.75; O, 10.06, S, 13.37.
4.5.2. 3-(4-chlorophenyl)-1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1-phenylurea (5b)
IR vmax (KBr, in cm−1): 1159 (C—N), 2358 (C—S—C), 1356 and 1191 (SO2N), 1707 (—C O), 3020 (Ar—C—H), 3341 (—NH—), 745 (C—Cl); 1H NMR (300 MHz, DMSO-d6, δ): 5.61 (1H, d, C—H of thiazine), 3.35 (1H, m, CH—S of thiazine), 1.54 (3H, d, CH3 of thiazine) 1.74 (3H, s, CH3 of thiazine), 7.78–8.01 (4H, m, Ar—SO2) 7.0–7.65 (9H, m, Ar), 6.07 (1H, s, —NH— of ureido); FAB-MS (m/z): 511 [M+]; Anal. Calcd for C25H22ClN3O3S2: C, 58.64; H, 4.33; N, 8.21; O, 9.37; S, 12.52. Found: C, 58.58; H, 4.37; N, 8.18; O, 9.32; S, 12.56.
4.5.3. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphenyl)-1-phenylurea (5c)
IR vmax (KBr, in cm−1): 1152 (C—N), 2343 (C—S—C), 1341 and 1175 (SO2N), 1710 (—C O), 3021 (Ar—C—H), 3324 (—NH—), 3421 (—OH); 1H NMR (300 MHz, DMSO-d6, δ): 5.62 (1H, d, C—H of thiazine), 3.33 (1H, m, CH—S of thiazine), 1.57 (3H, d, CH3 of thiazine) 1.74 (3H, s, CH3 of thiazine), 7.87–8.00 (4H, m, Ar—SO2) 7.03–7.74 (9H, m, Ar), 6.05 (1H, s, —NH— of ureido), 5.01 (—OH); FAB-MS (m/z): 493 [M+]. Anal. Calcd for C25H23N3O4S2: C, 60.83; H, 4.70; N, 8.51; O, 12.97; S, 12.99. Found: C, 60.78; H, 4.72; N, 8.54; O, 12.90; S, 12.94.
4.5.4. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-nitrophenyl)-1-phenylurea (5d)
IR vmax (KBr, in cm−1): 1141 (C—N), 2334 (C—S—C), 1344 and 1187 (SO2N), 1707 (—C O), 3028 (Ar—C—H), 3332 (—NH—), 1537 and 1334 (C—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.60 (1H, d, C—H of thiazine), 3.30 (1H, m, CH—S of thiazine), 1.52 (3H, d, CH3 of thiazine) 1.70 (3H, s, CH3 of thiazine), 7.90–8.03 (4H, m, Ar—SO2) 7.00–7.71 (9H, m, Ar), 6.03 (1H, s, —NH— of ureido); FAB-MS (m/z): 522 [M+]. Anal. Calcd for C25H22N4O5S2: C, 57.46; H, 4.24; N, 10.72; O, 15.31, S, 12.27. Found: 57.46; H, 4.24; N, 10.72; O, 15.31, S, 12.27.
4.5.5. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-methoxyphenyl)-1-phenylurea (5e)
IR vmax (KBr, in cm−1): 1147 (C—N), 2340 (C—S—C), 1347 and 1176 (SO2N), 1687 (—C O), 3031 (Ar—C—H), 3346 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.70 (1H, d, C—H of thiazine), 3.34 (1H, m, CH—S of thiazine), 1.56 (3H, d, CH3 of thiazine) 1.78 (3H, s, CH3 of thiazine), 7.86–8.00 (4H, m, Ar—SO2), 7.12–7.76 (9H, m, Ar), 6.08 (1H, s, —NH— of ureido), 5.01 (3H, s, —OCH3); FAB-MS (m/z): 507 [M+]; Anal. Calcd for C26H25N3O4S2: C, 61.52; H, 4.96; N, 8.28; O, 12.61, S, 12.63. Found: C, 61.57; H, 4.90; N, 8.21; O, 12.67, S, 12.62.
4.5.6. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1-(4-nitrophenyl)-3-phenylurea (5f)
IR vmax (KBr, in cm−1): 1148 (C—N), 2312 (C—S—C), 1336 and 1128 (SO2N), 1706 (—C O), 3029 (Ar—C—H), 3403 (—NH—), 1547 and 1348 (C—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.72 (1H, d, C—H of thiazine), 3.29 (1H, m, CH—S of thiazine), 1.55 (3H, d, CH3 of thiazine) 1.72 (3H, s, CH3 of thiazine), 7.91–8.18 (8H, m, Ar—SO2 and Ar—NO2), 7.04–7.61 (5H, m, Ar), 6.12 (1H, s, —NH— of ureido); FAB-MS (m/z): 522 [M+]; Anal. Calcd for C25H22N4O5S2: C, 57.46; H, 4.24; N, 10.72; O, 15.31; S, 12.27. Found: C, 57.41; H, 4.19; N, 10.78; O, 15.24; S, 12.21.
4.5.7. 3-(4-chlorophenyl)-1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1-(4-nitrophenyl) urea (5g)
IR vmax (KBr, in cm−1): 1154 (C—N), 2321 (C—S—C), 1331 and 1135 (SO2N), 1710 (—C O), 3018 (Ar—C—H), 3308 (—NH—), 1539 and 1352 (C—NO2), 754 (C—Cl); 1H NMR (300 MHz, DMSO-d6, δ): 5.75 (1H, d, C—H of thiazine), 3.31 (1H, m, CH—S of thiazine), 1.62 (3H, d, CH3 of thiazine) 1.69 (3H, s, CH3 of thiazine), 7.84–8.14 (8H, m, Ar—SO2 and Ar—NO2), 7.25–7.51 (4H, m, Ar), 6.08 (1H, s, —NH— of ureido); FAB-MS (m/z): 556 [M+]; Anal. Calcd for C25H21ClN4O5S2: C, 53.90; H, 3.80; N, 10.06; O, 14.36; S, 11.51. Found: C, 53.92; H, 3.87; N, 10.09; O, 14.28; S, 11.
4.5.8. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphenyl)-1-(4- nitrophenyl)urea (5h)
IR vmax (KBr, in cm−1): 1148 (C—N), 2321 (C—S—C), 1339 and 1141 (SO2N), 1697 (—C O), 3010 (Ar—C—H), 3315 (—NH—), 1542 and 1357 (C—NO2), 3414 (C—OH); 1H NMR (300 MHz, DMSO-d6, δ): 5.64 (1H, d, C—H of thiazine), 3.27 (1H, m, CH—S of thiazine), 1.58 (3H, d, CH3 of thiazine) 1.74 (3H, s, CH3 of thiazine), 7.93–8.18 (8H, m, Ar—SO2 and Ar—NO2), 6.14–7.32 (4H, m, Ar), 6.04 (1H, s, —NH— of ureido), 5.02 (1H, s, OH); FAB-MS (m/z): 538 [M+]; Anal. Calcd for C25H22N4O6S2: C, 52.75; H, 3.73; N, 12.34; O, 19.73; S, 11.30. Found: C, 52.71; H, 3.64; N, 12.27; O, 19.76; S, 11.36.
4.5.9. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1,3-bis(4-nitrophenylurea) (5i)
IR vmax (KBr, in cm−1): 1142 (C—N), 2324 (C—S—C), 1336 and 1151 (SO2N), 1687 (—C O), 3021 (Ar—C—H), 3325 (—NH—), 1547 and 1346 (C—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.68 (1H, d, C—H of thiazine), 3.30 (1H, m, CH—S of thiazine), 1.61 (3H, d, CH3 of thiazine) 1.78 (3H, s, CH3 of thiazine), 7.91–8.08 (12H, m, Ar—SO2 and Ar—NO2), 6.09 (1H, s, —NH— of ureido); FAB-MS (m/z): 567 [M+]; Anal. Calcd for C25H21N4O7S2: C, 55.75; H, 4.12; N, 10.40; O, 17.82; S, 11.91. Found: C, 55.70; H, 4.09; N, 10.34; O, 17.87; S, 11.85.
4.5.10. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-methoxyphenyl)-1- (4-nitrophenyl)urea (5j)
IR vmax (KBr, in cm−1): 1151 (C—N), 2331 (C—S—C), 1332 and 1148 (SO2N), 1701 (—C O), 3030 (Ar—C—H), 3321 (—NH—), 1546 and 1339 (C—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.59 (1H, d, C—H of thiazine), 3.27 (1H, m, CH—S of thiazine), 1.63 (3H, d, CH3 of thiazine) 1.75 (3H, s, CH3 of thiazine), 7.90–8.12 (8H, m, Ar—SO2 and Ar—NO2), 6.32–7.50 (4H, m, Ar), 6.02 (1H, s, —NH— of ureido), 3.35 (3H, s, —OCH3); FAB-MS (m/z): 552 [M+]; Anal. Calcd for C26H24N4O6S2: C, 56.51; H, 4.38; N, 10.14; O, 17.37; S, 11.60. Found: C, 56.53; H, 4.32; N, 10.17; O, 17.32; S, 11.57.
4.5.11. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-phenyl-1-(pyridin-2-yl) urea (5k)
IR vmax (KBr, in cm−1): 1146 (C—N), 2341 (C—S—C), 1328 and 1124 (SO2N), 1712 (—C O), 3027 (Ar—C—H), 3326 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.67 (1H, d, C—H of thiazine), 3.34 (1H, m, CH—S of thiazine), 1.72 (3H, d, CH3 of thiazine) 1.55 (3H, s, CH3 of thiazine), 7.87–8.02 (9H, m, Ar), 6.62–8.10 (4H, m of pyridine), 6.05 (1H, s, —NH— of ureido); FAB-MS (m/z): 478 [M+]; Anal. Calcd for C24H22N4O3S2: C, 60.23; H, 4.63; N, 11.71; O, 10.03; S, 13.40. Found: C, 60.18; H, 4.65; N, 11.68; O, 10.07; S, 13.44.
4.5.12. 3-(4-chlorophenyl)-1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-1-(pyridin-2-yl) urea (5l)
IR vmax (KBr, in cm−1): 1149 (C—N), 2354 (C—S—C), 1322 and 1131 (SO2N), 1707 (—C O), 3022 (Ar—C—H), 3314 (—NH—), 1528 and 1329 (C—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.76 (1H, d, C—H of thiazine), 3.32 (1H, m, CH—S of thiazine), 1.68 (3H, d, CH3 of thiazine) 1.51 (3H, s, CH3 of thiazine), 7.87–8.02 (8H, m, Ar), 6.62–8.10 (4H, m of pyridine), 6.05 (1H, s, —NH— of ureido); FAB-MS (m/z): 512 [M+]; Anal. Calcd for C24H21ClN4O3S2: C, 56.19; H, 4.13; N, 10.92; O, 9.36; S, 12.50. Found: C, 56.15; H, 4.11; N, 10.88; O, 9.31; S, 12.45.
4.5.13. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphenyl)-1-(pyridin-2-yl)urea (5m)
IR vmax (KBr, in cm−1): 1142 (C—N), 2337 (C—S—C), 1327 and 1126 (SO2N), 1713 (—C O), 3027 (Ar—C—H), 3323 (—NH—), 3420 (C—OH); 1H NMR (300 MHz, DMSO-d6, δ): 5.70 (1H, d, C—H of thiazine), 3.33 (1H, m, CH—S of thiazine), 1.71 (3H, d, CH3 of thiazine) 1.53 (3H, s, CH3 of thiazine), 6.87–8.02 (8H, m, Ar), 6.66–8.17 (4H, m of pyridine), 6.01 (1H, s, —NH— of ureido), 5.02 (1H, s, —OH); FAB-MS (m/z): 494 [M+]; Anal. Calcd for C24H22N4O4S2: C, 58.28; H, 4.48; N, 11.33; O, 12.94; S, 12.97. Found: C, 58.22; H, 4.43; N, 11.26; O, 12.90; S, 12.94.
4.5.14. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-nitrophenyl)-1-(pyridin-2-yl)urea (5n)
IR vmax (KBr, in cm−1): 1147 (C—N), 2324 (C—S—C), 1322 and 1124 (SO2N), 1709 (—C O), 3025 (Ar—C—H), 3319 (—NH—), 1537 and 1347 (C—NO2); 1H NMR (300 MHz, DMSO-d6, δ): 5.73 (1H, d, C—H of thiazine), 3.30 (1H, m, CH—S of thiazine), 1.69 (3H, d, CH3 of thiazine) 1.52 (3H, s, CH3 of thiazine), 7.87–8.12 (8H, m, Ar), 6.68–8.08 (4H, m of pyridine), 6.05 (1H, s, —NH— of ureido); FAB-MS (m/z): 523 [M+]; Anal. Calcd for C24H21N5O5S2: C, 55.05; H, 4.04; N, 13.38; O, 15.28; S, 12.25. Found: C, 55.09; H, 4.08; N, 13.31; O, 15.22; S, 12.21.
4.5.15. 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-methoxyphenyl)-1-(pyridin-2-yl)urea (5o)
IR vmax (KBr, in cm−1): 1140 (C—N), 2337 (C—S—C), 1335 and 1131 (SO2N), 1702 (—C O), 3034 (Ar—C—H), 3329 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.70 (1H, d, C—H of thiazine), 3.32 (1H, m, CH—S of thiazine), 1.72 (3H, d, CH3 of thiazine) 1.49 (3H, s, CH3 of thiazine), 6.77–8.03 (8H, m, Ar), 6.88–8.13 (4H, m of pyridine), 6.02 (1H, s, —NH— of ureido), 3.71 (3H, s, of OCH3); FAB-MS (m/z): 508 [M+]; Anal. Calcd for C25H24N4O4S2: C, 59.04; H, 4.76; N, 11.02; O, 12.58; S, 12.61. Found: C, 59.10; H, 4.72; N, 11.07; O, 12.51; S, 12.54.
4.6. Blood glucose lowering studies
In the present study effects of oral administration of synthesized trisubstituted sulfonylurea compounds on glucose tolerance in normal and streptozotocin induced type II diabetic (NIDDM) rats have been investigated. The animal study was performed under the supervision of expert following all the guidelines of CPCSEA in the approved laboratory (Reg no. 1171/c/08/CPCSEA) under the controlled condition with the proposal no. 35/14/IAEC/SPS/SOA.
4.6.1. Study of streptozotocin induced non-insulin dependent diabetes mellitus (NIDDM) in rats
Healthy adult wistar rats (160–200 g) were housed in standard conditions and fasted overnight. To induce acute NIDDM, streptozotocin (STZ) 40 mg/kg body weight was freshly dissolved in citrate buffer solution (pH 4.5) was administered intraperitoneally. The rats were kept separately with food and water ad libitum. The blood glucose level was checked for fasting glucose levels. When the animals showing fasting glucose levels > 140 mg/dl and 200 mg/dl 2 h after the normal fed.
Diabetes induced rats were classified into groups of six animals each. Group I was fed with the control vehicle (1% tween 20 in distilled water) in a volume of 10 ml/kg. The reference drug glibenclamide (20 mg/kg) and synthesized compounds (5a–o) in the dose of 100 mg/kg suspended in vehicle were administered per oral in volume of 10 ml/kg to respective groups. The rats were fed with their normal diet after 15 min. of dosing. The blood was collected by retro orbital prexus technique just prior to and 90 min after the normal fed loading then serum was separated. The Blood glucose changes were measured in blood serum by semiauto biochemical analyzer (Erba Mannheim chem 5×) using glucose oxidase peroxidase (GOD/POD) kit of erba Mannheim.
4.6.2. Oral glucose tolerance (OGT) test in normal rats
Standard experimental procedure was followed for the OGT test; normal rats were used for the experiment. In short, the base line blood glucose level was measured. The respective groups of rats were administered the respective compounds (i.e. control, standard and test compounds) in specified dose used in previous procedure of induced NIDDM. All the animals were given glucose (2 g/kg p.o.) 15 min after dosing. Blood samples were collected prior to and after 0, 30, 60, 90, 120 min. of the glucose loading then the glucose levels were measured by semi-auto biochemical analyzer.
Data of antidiabetic study were presented in the form of ±SEM and percent reduction in blood glucose level by test drug. The percent reduction was calculated by taking difference of blood glucose levels of animals at fasting and at 90 min. after fed of derivatives and comparing it with the data of control at 90 min. after fed. 0% reduction indicates there is no reduction in the level of blood sugar.
Acknowledgments
We would like to convey our thanks to school of pharmaceutical science, SOA university, Bhubaneswar (Odisha) for their contribution in my analytical work and next to ISER Bhopal for the spectroscopical study such as NMR and mass. I also want to thank my colleagues for their support in different aspects of this project.
Footnotes
Peer review under responsibility of King Saud University.
References
- Flatt P.R., Shibier O., Szecowka J., Berggren P.O. New perspectives on the actions of sulphonylureas and hyperglycaemic sulphonamides on the pancreatic beta-cell. Diabete. Metab. 1994;20:157–162. [PubMed] [Google Scholar]
- Hamaguchi T., Hirose T., Asakawa K., Itoh Y., Kamado K., Tokunaga K., Tomita K., Masuda H., Watanabe N., Namba M. Short term effects of l-carnitine on serum lipids in STZ-induced diabetic rats. Diabetes Res. Clin. Pract. 2004;66:129–132. doi: 10.1016/j.diabres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Jung S., Song J., Lee H., Choi S., Lee C. Synthesis and evaluation of cytotoxic activity of novel arylsulfonylimidazolidinones. Bioorg. Med. Chem. Lett. 1996;6:2553–2558. [Google Scholar]
- Kamp F., Kizilbash N., Barbara E.C., Berggren P., Hamilton J.A. Sulfonylureas rapidly cross phospholipid bilayer membranes by a free-diffusion mechanism. Diabetes. 2003;52:2526–2531. doi: 10.2337/diabetes.52.10.2526. [DOI] [PubMed] [Google Scholar]
- Kecskemeti V., Bagi Z., Posa I., Koesis E., Koltai M.I. Synthesis, characterization and in vitro antimicrobial activity of novel sulfonylureas of 15-membered azalides. Curr. Med. Chem. 2002;9:53–71. [Google Scholar]
- Khelili S., Leclerc G., Faury G., Verdetti J. Synthesis and vasodilator effect of 3- and 7-sulfonylurea-1,2,4-berzthiazin-1,1-dioxides of rat aorta. Bioorg. Med. Chem. 1995;3:495–503. doi: 10.1016/0968-0896(95)00040-n. [DOI] [PubMed] [Google Scholar]
- Krajacić M.B., Kujundzić N., Dumić M., Cindrić M., Brajsa K., Metelko B., Novak P. Synthesis, characterization and in vitro antimicrobial activity of novel sulfonylureas of 15-membered azalides. J. Antibiot. 2005;58:380–389. doi: 10.1038/ja.2005.48. [DOI] [PubMed] [Google Scholar]
- Pan L., Jiang Y., Liu Z., Liu X., Liu Z., Wang G., Li Z., Wang D. Synthesis and evaluation of novel monosubstituted sulfonylurea derivatives as antituberculosis agents. Eur. J. Med. Chem. 2012;50:18–26. doi: 10.1016/j.ejmech.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Pattan S.R., Bukitagar A.A., Pattan J.S., Kapadnin B.P., Jadhav S.G. Synthesis and evaluation of some new substituted phenylthiazole derivatives and their atnitubercular activity. Indian J. Chem. 2009;48B:1033–1037. [Google Scholar]
- Rathish I.G., Javed K., Bano S., Ahmad S., Alam M.S., Pillai K.K. Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur. J. Med. Chem. 2009;44:2673–2678. doi: 10.1016/j.ejmech.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Zheng V., Yates S.R., Papiernik S.K. Transformation kinetics and mechanism of the sulfonylurea herbicides pyrazosulfuron ethyl and halosulfuron methyl in aqueous solutions. J. Agric. Food Chem. 2008;56:7367–7372. doi: 10.1021/jf800899e. [DOI] [PubMed] [Google Scholar]