Abstract
The aim of this study was to prepare the rosiglitazone sodium enteric-coated tablets and investigate its release rate. The rosiglitazone sodium enteric-coated tablet was prepared by single punch tablet press using substituted hydroxypropyl cellulose and polyvinylpyrrolidone (PVP). The release rate from the enteric-coated tablet of rosiglitazone sodium was evaluated. The release rate study showed that few rosiglitazone sodium was released from enteric coated formulation within 2 h in simulated gastric juice, while it released more than 80% of the labeled amount in 30 min in simulated intestinal juice. The preparing method of rosiglitazone sodium enteric-coated tablets was simple and had a good reproducibility. The release condition and determined methods could be used for the routine determinations of rosiglitazone sodium enteric-coated tablets.
Keywords: Enteric-coated tablet, Release tests, Polyvinylpyrrolidone (PVP), Hydroxypropyl cellulose
1. Introduction
Diabetes has become a serious threat to human health after cardiovascular disease. Studies have shown that diabetics in the world are almost 400 million in 2014 and will increase to 472 million in 2030. China has the largest number of diabetics, the number of patients has continued to grow with 9.7 percent annual rate, which accounts for about 90 percent of type 2 diabetes (Yang et al., 2010). Several oral drugs are available for the treatment of type 2 diabetes mellitus which include the sulfonylureas, metformin, acarbose, thiazolidinediones (TZD) and DPP-VI inhibitors. TZD are peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists that improve peripheral insulin sensitivity by increasing adipose tissue lipogenesis and reducing hepatic fat content and hepatic glucose production antidiabetic agent (Tan et al., 2014; Julia et al., 2014; Soccio et al., 2014). Rosiglitazone (RSG) is an aminopyridylthiazolidinedione, and its chemical name is [(±)-5-[4-[2-[N-methyl-N(2-pyridyl)amino] ethoxy] benzyl]-2,4-dionethiozolidine] (Fig. 1). There are some reports about rosiglitazone maleate but there is few literature about rosiglitazone sodium, which is the only oral antidiabetic agent with independent intellectual property rights in China. It has been reported that the solubility of rosiglitazone sodium is 45 mg/ml in water or neutral physiological buffer at room temperature, which is much higher than that of rosiglitazone maleate which is 10 mg/ml in the same solutions (Li et al., 2005). Tailuo® is rosiglitazone sodium tablets on the market in China, and sells well. Chinese drug economist Zhu Cairong demonstrates that rosiglitazone sodium is the most cost-effective choice for type 2 diabetes patients for improving the quality of life and prolonging the life of patients with diabetes, and it also has great significance for the rational using of health resources and reducing the burden on patients and society (Zhu et al., 2006). Avandia® (rosiglitazone maleate) clinical use had been limited by FDA because of some clinical events on 2010 (Nissen and Wolski, 2007), but FDA requires removal of certain restrictions on the diabetes drug Avandia in 2013. Studies had shown that it did not increase the risk of heart attack and death (U.S. Food, 2013). It still has great prospects of markets (Wright et al., 2014).
Figure 1.
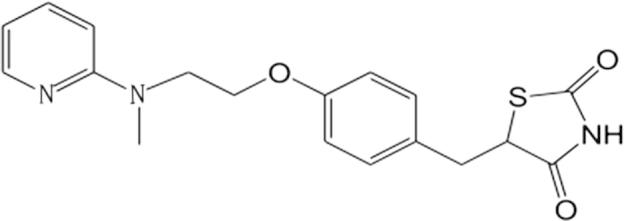
Chemical structure of rosiglitazone.
Currently, the formulations of Tailuo® (rosiglitazone sodium) on the market in China are mainly normal tablet or capsule. However, these types of general formulations have a common drawback. With the dissolution of rosiglitazone sodium from the normal tablets, the alkalinity of solution would increase. It will neutralize the gastric acid and cause gastric acid imbalance, and is not conducive for food digestion and absorption. Meanwhile, rosiglitazone sodium is rapidly converted into rosiglitazone or rosiglitazone hydrochloride in the gastric juice (Hu et al., 2012). It is not good for the absorption of rosiglitazone, since the solubility of rosiglitazone or rosiglitazone hydrochloride is less than that of rosiglitazone sodium in the aqueous medium and thiazolidinediones are absorbed in a non-dissociated molecular form (Kamila et al., 2008). The proportions of dissociation and non-dissociated form of thiazolidinediones are determined by the pKa of the drug and the pH of the absorption site, which can be expressed as Henderson–Hasselbalch equation: pKa–pH = lg (Ci/Cu). The proportion of non-dissociation drug and its absorption will increase with increasing pH value of the absorption site, according to Henderson–Hasselbalch equation.
The rosiglitazone sodium enteric-coated tablets, which have a special outer covering designed to dissolve and absorb in the small intestine were studied. This feature of enteric-coated tablets not only moderates the stomach irritation or neutralizes gastric acid aroused by the long-term use of rosiglitazone sodium, but also improves the interrelated food absorption and avoids some adverse reactions, such as nausea, vomiting and anorexia (Hu et al., 2012).
In this study, we prepared the rosiglitazone sodium enteric-coated tablet and investigated the drug release rate by HPLC (high performance liquid chromatography).
2. Materials and methods
2.1. Apparatus
The liquid chromatographic system consisted of an Agilent technologies 1100 series instrument equipped with a quaternary solvent delivery system and an Agilent UV detector. A Rheodyne syringe loading sample injector with a 20 μl sample loop was used for the injection of sample. Chromatographic data were collected and processed by Agilent Chemstation Plus software. The separation was performed at ambient temperature on a reversed phase lichrospher column (C18, 4.6 × 250 nm, 5 μm particle size). A dissolution system (Pharmatest type D-800L) complying with the USP Apparatus 2 specification was used in all the tests.
2.2. Chemicals
Rosiglitazone sodium, with the content of 99.3%, was made by Chongqing Medical University (Li et al., 2005). Rosiglitazone standard was obtained from Beijing Comens Chemical Company with a labeled purity of 99.7%. The HPLC grade methanol was obtained from Jiangshu Hanbang Technology Co., Ltd. (No. 20130938). Lactose, microcrystalline cellulose, hydroxypropyl cellulose, polyvinylpyrrolidone (PVP), magnesium stearate that were purchased from Huzhou Zhanwang pharmaceatical Co., Ltd. And acrylic resin that was purchased from Shanghai Chineway pharmaceatical Tech. Co., Ltd., were used for preparation of enteric-coated tablets. All other regents used were of analytical grade.
2.3. Formulation and preparation method
2.3.1. Formulation of rosiglitazone sodium enteric-coated tablets
Ingredients for 10,000 granules rosiglitazone sodium enteric-coated tablets: Rosiglitazone sodium 42.5 g (containing 4 rosiglitazone), lactose 600 (g), microcrystalline cellulose 300 g, hydroxypropyl cellulose 50 g, magnesium stearate 10 g,10% (w/v) polyvinylpyrrolidone (PVP) absolute alcohol solution, 5% (w/v) acrylic resin Abs. alcohol solution 2000 ml.
2.3.2. Preparation of rosiglitazone sodium enteric-coated tablets
42.5 g rosiglitazone sodium was initially pulverized and milled through 80 mesh sieves, then mixed with lactose and microcrystalline cellulose by equivalent additive and added into 10% polyvinylpyrrolidone Abs. alcohol solution. The wetted mass was granulated manually by passing it through a 12-mesh screen, dried at 50 °C and then passed through 16-mesh sieve to create granules of uniform size. Afterward, the granules were mixed with the magnesium stearate and compress into 105 mg tablets using 6 mm punches. The tablets are coated with 5% acrylic resin and the coating must take 10% weight of the tablet.
2.4. Release
2.4.1. Release condition
The release tests of rosiglitazone sodium enteric-coated tablets employed a calibrated dissolution apparatus (ChP 2010) with paddles at 75 rpm, the bath temperature was maintained at 37 ± 0.5 °C. For all the enteric-coated tablets, they must endure simulated gastric fluid (pH 1.2) in the first 2 h followed by 1 h in simulated intestinal fluid (pH 6.8) according to the standard method, since rosiglitazone sodium will hydrolyze quickly into rosiglitazone in aqueous solution, and rosiglitazone is insoluble in simulated intestinal fluid (pH 6.8). Therefore, we use simulated intestinal fluid (pH 6.8) that contained 0.25% sodium dodecyl sulphate (SDS) as release medium. The simulated gastric fluid was prepared by dissolving NaCl (3 g) in about 1450 (ml) of deionized water and then adjusted pH to 1.2 ± 0.1 with diluted HCl. The simulated intestinal fluid was prepared by the similar method: potassium phosphate monobasic (10.2 g) and SDS (3.75 g) were dissolved in a same 1000 ml of deionized water and then pH adjusted to 6.8 ± 0.1 with 1 N NaOH. Finally, the volume of each fluid was adjusted to 1500 (ml) with deionized water.
2.4.2. Chromatographic condition
The mobile phase consisted of 0.1 M sodium acetate buffer (adjusted pH to 6.2 with dilute potassium hydroxide) and methanol (30/70v/v). The mobile phase was filtered through a 0.45 μm membrane filter (Millipore®, Bedford, USA) and sonicated before use. Separation was achieved on a Lichrospher C18 column (4.6 mm × 250 mm. 5 μm), the flow rate of mobile phase was 1.0 ml/min and the sample injection volume was 20 μl. The detector was set at the wavelength of 247 nm. The column temperature was 40 °C (Onal, 2009). Theoretical plate number was not less than 5000 according to rosiglitazone. The resolution between rosiglitazone and the impurity was more than 1.5.
2.4.3. Tolerance test in simulated gastric fluid
Tolerance testing was carried out in 250 ml simulated gastric fluid (pH 1.2) with a paddle rotating was 75 rpm and with the bath temperature of 37 °C. Each sample was withdrawn after 2 h in the simulated gastric fluid and the simulated gastric fluid was collected at the same time; a 5 ml sample was removed from clay vessel using a glass syringe and filtered through a nylon filter (0.45 m, 25 mm) into labeled glass tubes. The solution was assayed by HPLC and release (%) was calculated with the following equation:
2.4.4. Release profile in simulated intestinal fluid
The release profiles of the enteric-coated tablets needed tolerance for 2 h in 250 ml of simulated gastric fluid, and following determine release amount in 250 ml of the simulated intestinal fluid. Samples were removed from the dissolution vessel at 10, 20, 30, 45 and 60 min during dissolution in simulated intestinal fluid, and the same amount of release medium was added immediately. Then the sample solutions were filtered through a nylon filter (0.45 m, 25 mm). The release was determined and calculated according to equation.
2.5. Method validation
The method was validated according to the Chinese pharmacopoeia category I requirements. The following validation characteristics were addressed: accuracy, precision, linearity, range and specificity.
2.6. Linearity and range
The linearity was obtained from five calibrators over a concentration range of 4–20 μg/ml (3.8841, 7.7683, 11.6521, 15.5362 and 19.4203) for rosiglitazone standard, that were dissolved and diluted with simulated intestinal fluid. Linearity and range were evaluated by linear regression analysis, which was calculated by the least square method.
2.7. Specificity
The specificity of the HPLC method was evaluated through the analysis of the solution of rosiglitazone sodium enteric-coated tablets and the solution of rosiglitazone standard and blank solution of rosiglitazone sodium enteric-coated tablets. All chromatograms were examined to determine if the active compound had any co-elution with the inactive ingredients peak from the release medium.
2.8. Accuracy and precision
The accuracy of the methods was determined through the recovery test. The recovery study was finished by testing 2, 3.2 and 4 mg rosiglitazone standard samples add to the blank of rosiglitazone enteric-coated tablets according to 50%, 80% and 100% of the declared amount 4 mg of rosiglitazone enteric-coated tablets. Then the amounts were determined according to the release method in simulated intestinal fluid. Repeatability and intermediate precision were used to assess the precision of the method. Repeatability was evaluated through relative standard deviation (RSD) of the data of 100% recovery by injecting the samples 9 times repeatedly. The intermediate precision was evaluated through the RSD of the inter-day repeated measurement.
2.9. Stability of solution
Stability of solution focused on the stability of sample solution. The evaluated method of the stability of solution was that the sample solution was placed at 40 °C in 4 h and determined in 0, 0.5, 1, 2 and 4 h, respectively.
2.10. The method of release determination
The method of release determination of rosiglitazone sodium enteric-coated tablets is from ChP 2010 apparatus XI. The rosiglitazone sodium enteric-coated tablets were added to the 250 ml 0.01 mol/l hydrochloric acid (9 → 1000), maintained at 37 ± 0.5 °C and stirred for 2 h at 75 rpm and then the hydrochloric acid solution was discarded. The test specimen should not be crack, melted or disaggregate. Another 250 ml solution at 37 ± 0.5 °C of phosphate buffer (pH = 6.8, containing 0.25% SDS) was added immediately. The test condition was same as before and maintained, and 5 ml aliquots solution was withdrawn at 30 min, filtered with 0.45 um nylon filter. This solution is the sample solution. The 20 μg/ml standard solutions were prepared in volumetric flasks and filtered with the same filters listed earlier. All the sample solutions and standard solutions were analyzed by HPLC method.
3. Results and discussion
3.1. Release conditions
The discriminatory power of the dissolution method depends on the method ability to detect changes in the drugs. Drugs solubility and solution stability were important properties to be considered when selecting the dissolution medium. The solubility tests showed that the rosiglitazone sodium was soluble in 0.01 and 0.1 M hydrochloric acid, insoluble in water of pH 6.8. However, there had been reported in some literatures that the rosiglitazone may be soluble in phosphate buffer (pH 6.8) containing 0.25% SDS. So the solution (pH 1.2) of NaCl and hydrochloric acid as well as the phosphate buffered solution containing SDS were selected as the release media.
3.2. Release rate in simulated gastric fluid
In vitro release studies of rosiglitazone sodium from enteric-coated tablets were performed in simulated gastric fluid (pH 1.2) for 2 h. Table 1 showed that the percentage of the residual content of rosiglitazone sodium in enteric-coated tablets were calculated with respect to its initial amount. The data showed that few rosiglitazone sodium was released from enteric-coated tablets formulation within 2 h of agitation. Moreover, it was observed that there was no indication that the disaggregation, cracking or melting occurs at the surface of the enteric-coated tablets in the simulated gastric fluid solution. The rosiglitazone sodium enteric-coated tablets showed satisfactory acid resistance in the stomach.
Table 1.
The percentage of the residual content of rosiglitazone sodium enteric-coated tablets after agitation in simulated gastric fluid (pH 1.2) for 2 h.
| Batch No. | Times (%) |
Average (%) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 140,301 | 94.7 | 103.2 | 93.1 | 98.7 | 106.2 | 95.4 | 98.6 |
| 140,302 | 99.3 | 92.1 | 104.4 | 97.8 | 99.7 | 91.9 | 97.5 |
| 140,303 | 101.9 | 92.6 | 99.9 | 107.2 | 96.1 | 94.8 | 98.8 |
3.3. Release profiles
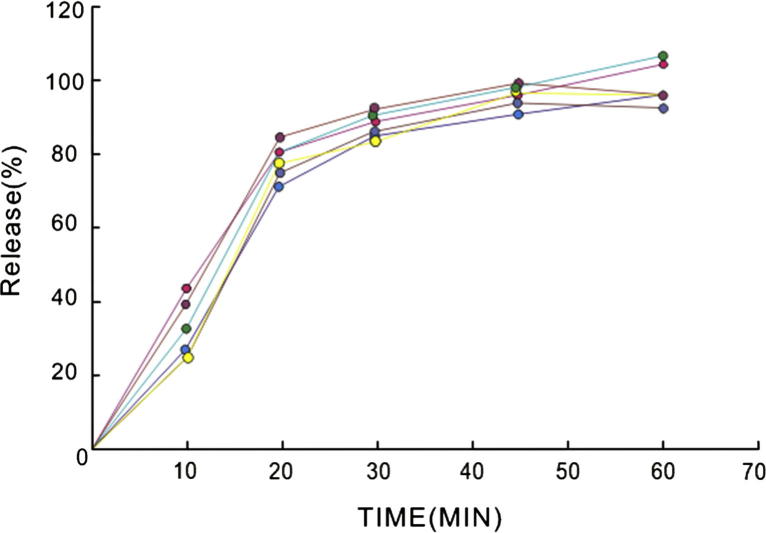
Release profiles of rosiglitazone sodium from enteric coated formulation over the period of 60 min in simulated intestinal fluid (pH 6.8) are shown in Fig. 2. From the image, it was found that initial 30 min was the most important period for release and more than 80% of the total rosiglitazone sodium content within enteric coated formulation was released from enteric coated tablets within 30 min, and release rate of formulation decreased after the time gradually. The release did not significantly change after 45 min. Rosiglitazone sodium was rapidly released in the simulated intestinal fluid, suggesting that the enteric coating layer dissolved in the phosphate buffer (pH = 6.8, containing 0.25% SDS) rapidly. There are small standard deviations in every time of determined of the release curves indicate the repeatability of the method and the uniformity of the enteric-coated tablets.
Figure 2.
Release profiles of rosiglitazone sodium from enteric-coated tablets performed in simulated intestinal fluid (pH = 6.8) for 60 min with rotation at 75 rpm at 37 °C.
3.4. Method validation
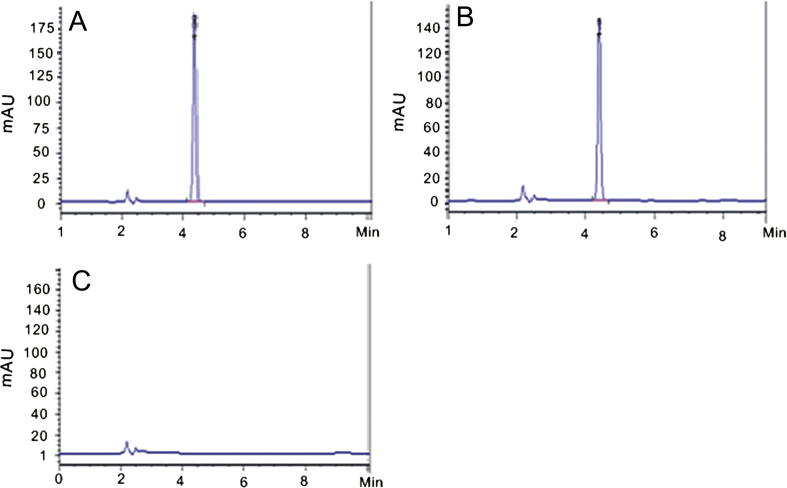
The performance parameters evaluated in these methods were: Linearity, specificity, precision and accuracy. The specificity analysis revealed that the HPLC method employed did not obtain interference from the ingredients, since there was no other peak in the retention time of roglitazone sodium (about 5.0 min) (Fig. 3). The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution. The chromatographic peak purity applied for rosiglitazone peak, demonstrated that there were no impurities on the peak too. Thus, the HPLC method is useful to quantify rosiglitazone sodium in pharmaceutical formulation from the release tests. Each solution was prepared in triplicate. The linearity was evaluated by linear regression analysis which was calculated by the least square regression method. The method demonstrated to be a good linear one in the range of 4–20 μg/ml with a correlation coefficient of 0.999.
Figure 3.
HPLC chromatograms of control solution (A), sample solution (B) and blank solution (C).
The representative equation for the linearity was A = 65.277C + 89.766. For the precision, the repeatability demonstrated RSD 0.74% for each day was analyzed. The RSD for intermediate precision was 1.45%. These results can demonstrate the good precision of the method for dissolution test. The accuracy of the proposed method was evaluated by recovery experiments, using the rosiglitazone sodium reference substance addition technique. Three different concentrations of rosiglitazone sodium reference substance were added to placebo sample to test, as shown in Table 2. The mean recovery was found to be 99.3%, indicating the high accuracy of this method.
Table 2.
The determination results of recovery (n = 3).
| No. | Added amount (mg) | Determined amount (mg) | Recovery rate (%) | Mean recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 2.0096 | 1.9878 | 98.9 | 98.9 | 1.68 |
| 2 | 2.0096 | 2.0231 | 100.7 | ||
| 3 | 2.0096 | 1.9528 | 97.2 | ||
| 1 | 3.2154 | 3.1193 | 97.0 | 99.3 | |
| 2 | 3.2154 | 3.2207 | 100.2 | ||
| 3 | 3.2154 | 3.2358 | 100.9 | ||
| 1 | 4.0192 | 3.9065 | 97.2 | 98.0 | |
| 2 | 4.0192 | 4.0068 | 99.7 | ||
| 3 | 4.0192 | 3.9032 | 97.1 |
In order to guarantee the solution stability during all the analysis time, the stability of the sample solution for release test was evaluated by the peak area of rosiglitazone and degradation products at different times at 40 °C. The result showed that the solutions remained stable in simulated intestinal fluid in 4 h, the peak area of RSD was 1.79 and no degradation products were observed in any chromatogram. So, it is possible to guarantee the integrity of the drug during all the analysis time. The sample solution is therefore considered stable for 4 h at 40 °C.
3.5. Drug product evaluation
The release of three batches of rosiglitazone sodium enteric-coated tablets was performed in compliance with the method mentioned in application of the method to sample determination, the results is given in Table 3. Acceptance criteria in ChP for enteric-coated tablets were not or almost not released in simulated gastric fluid and immediate released in simulated intestinal fluid. The releases in simulated intestinal fluid from the tablets are usually more than 80%. These criteria including test times are usually established on the study in simulated gastric fluid and basis of an evaluation of the released profile data in simulated intestinal fluid. In this article, it was observed that this product was not released in the simulated gastric fluid and 80% released in the simulated intestinal fluid in 30 min. So, this acceptance criterion was suitable for the product.
Table 3.
The release amount of rosiglitazone enteric-coated tablets.
| Batch No. |
Times (%) |
RSD (%) |
|||||
|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
||
| 140,301 | 88 | 83 | 86 | 81 | 89 | 84 | 3.6 |
| 140,302 | 83 | 86 | 91 | 85 | 85 | 87 | 3.2 |
| 140,303 | 89 | 90 | 84 | 88 | 82 | 85 | 3.6 |
3.6. Discussion
Rosiglitazone sodium is soluble in water, but it could be easily hydrolyzed into rosiglitazone and sodium hydroxide. In order to prevent the hydrolysis of rosiglitazone sodium during the preparation of enteric-coated tablets, it was better to adopt the method of dry-granulation or ethanol-granulation. Furthermore, for the properties of hydrolysis into rosiglitazone, it was unstable under alkaline conditions or within a certain pH range. The solubility of rosiglitazone sodium in aqueous solution would drop dramatically with pH increased. When phosphate buffer (pH 6.8) was used directly as release medium, rosiglitazone sodium will precipitate, so the phosphate buffer solution (pH 6.8) containing 0.25% SDS as surfactant was used. But this solution was unstable and easy to precipitate at room temperature or at lower temperature. The medium must be maintained at above 37 °C, and the precipitation of SDS was quickly dissolved. In addition, the filtered samples should be measured or stored timely at 40 °C, that was the reason for the stability of the solution studied at 40 °C. The relative standard deviation of the release of three batches of samples under this release medium was less than 5%.
Several chromatographic conditions and detectors were tested during the preliminary phases of this study. Weak alkaline sodium acetate buffer solution and methanol were used as the mobile phase, so as to inhibit the ionization of rosiglitazone and various kinds of salts of rosiglitazone. Employing this mobile phase, the retention time was short, the theoretical plate number was high and chromatographic peak was sharp and well-separated. Determination of rosiglitazone may choose common UV detectors. In the study of maximum absorption wavelength of rosiglitazon sodium, the maximum absorption wavelengths were changed within several nanometers with the changes of solvent or pH values of the solution. The detection wavelengths of rosiglitazone were often between 244 and 248 nm, but 247 nm was selected in most of the literatures. So 247 nm was also selected as the detection wavelength in this article. The results of release studies display that few rosiglitazone sodium was released from enteric coated formulation within 2 h in simulated gastric juice. However, under simulated enteric juice, rosiglitazone can totally release within 60 min. These results could explain that the enteric-coated tablet was protected in the acid environment of stomach and the hydrolysis speed of rosiglitazone sodium into rosiglitazone slows down in the weak alkaline environment of intestine.
4. Conclusion
In this study, rosiglitazone sodium was prepared as enteric-coated tablets for sustained release dosage in intestinal fluid. The release of rosiglitazone sodium from enteric-coated tablets was determined in simulated gastric juice (pH 1.2) and simulated intestinal juice (pH 6.8). The results suggested that the enteric-coated tablets provided potential advantages by reducing undesirable gastro-damages of rosiglitazone sodium. The parameters of validation demonstrate good precision and accuracy, which proves the reliability of HPLC method which could be used for the routine determinations of rosiglitazone sodium enteric-coated tablets form. In conclusion, the study demonstrates that the enteric-coated tablet of rosiglitazone sodium is a promising clinical formulation for the long-term treatments.
Acknowledgements
This work was supported by Natural Science Foundation of Chongqing (CSTC, 2013BB5286). We also thank the Chongqing Medical University for partial financial support of this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Hu, X.N., Gan, Y.J., Wu, L.C. et al., 2012. Preparative methods of the thiazolidinedione metallic salts enteric pharmaceutical preparations CN101327198.
- Julia T., Li W.J., Preeti K. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2014;53(6):1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- Kamila M.M., Mondal N., Ghosh L., Gupta B.K. Drug dissolution studies and determination of rosiglitazone maleate in tablets and polymeric microspheres by a rapid, validated RP-HPLC method. J. Liq. Chromatogr. Relat. Technol. 2008;31(16):2503–2516. [Google Scholar]
- Li, S., Xie, Y.D., Zhang, T., 2005. Thiazolidine derivatives and medicinal application there of CN1253136.
- Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Onal A. Spectrophotometric and HPLC determinations of anti-diabetic drugs, rosiglitazone maleate and metformin hydrochloride, in pure form and in pharmaceutical preparations. Eur. J. Med. Chem. 2009;44(12):4998–5005. doi: 10.1016/j.ejmech.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.B., Shi l.l., Zhou Z.X. Peroxisome proliferator-activated receptor-related drug development progress. Chin. Pharm. J. 2014;48(6):406–409. [Google Scholar]
- U.S. Food and Drug Administration News Release (November 25), 2013. FDA requires removal of certain restrictions on the diabetes drug Avandia.
- Wright M.B., Bortolini M., Tadayyon M., Bopst M. Minireview: challenges and opportunities in development of PPAR agonists. Mol. Endocrinol. 2014;28(11):1756–1768. doi: 10.1210/me.2013-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.Y., Lu J., Weng J.P., Jia W.P., Ji L. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- Zhu C., Liu Z.H., Ni Z.Z. Cost-effectiveness analysis of rosiglitazone sodium in type 2 diabetes mellitus patients. Mod. Prev. Med. 2006;33(5):698–700. [Google Scholar]