Abstract
The motive behind present work was to formulate and evaluate gel containing microsponges of diclofenac diethylamine to provide prolonged release for proficient arthritis therapy. Quasi-emulsion solvent diffusion method was implied using Eudragit RS-100 and microsponges with varied drug–polymer ratios were prepared. For the sake of optimization, diverse factors affecting microparticles physical properties were too investigated. Microsponges were characterized by SEM, DSC, FT-IR, XRPD and particle size analysis, and evaluated for morphology, drug loading, in vitro drug release and ex vivo diffusion as well. There were no chemical interactions between drug and polymers used as revealed by compatibility studies outcomes. The drug polymer ratio reflected notable effect on drug content, encapsulation efficiency and particle size. SEM results revealed spherical microsponges with porous surface, and had 7.21 μm mean particle size. The microsponges were then incorporated in gel; which exhibited viscous modulus along with pseudoplastic behavior. In vitro drug release results depicted that microsponges with 1:2 drug–polymer ratio were more efficient to give extended drug release of 75.88% at the end of 8 h; while conventional formulation get exhausted incredibly earlier by releasing 81.11% drug at the end of 4 h only. Thus the formulated microsponge-based gel of diclofenac diethylamine would be a promising alternative to conventional therapy for safer and efficient treatment of arthritis and musculoskeletal disorders.
Keywords: Arthritis, Diclofenac diethylamine, Drug delivery, Extended release, Microsponges
1. Introduction
Diclofenac is a well recognized non-steroidal anti-inflammatory drug (NSAID) mostly used for alleviating swelling and pain allied with rheumatoid arthritis, juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, dysmenorrhea and other musculoskeletal ailments (Gan, 2010). Regrettably, diclofenac oral administration has limitations such as extensive first pass metabolism and gastrointestinal irritation. Even the intramuscular injection most of the time led to skin lesions. For these reasons, advance localized and transdermal delivery has gained a lot of importance in these days (Niethard et al., 2005, Kulkarni et al., 2011). Particularly, many researchers have attempted to develop novel transdermal formulations of diethylammonium salt of diclofenac i.e. diclofenac diethylamine (DDEA) owing to its lipophilic nature and other unique promising characteristics (Minghetti et al., 2007, Arora and Mukherjee, 2002). Diverse approaches intended for enhanced transdermal delivery of DDEA were incorporation in emulsion-based systems (Djordjevic et al., 2004, Kweon et al., 2004, Vucinic-Milankovic et al., 2007), niosomal entrapment (Manosroi et al., 2008), vehicle-chemical enhancer’s implication (Djordjevic et al., 2004, Khalil et al., 2000, Baboota et al., 2006, Zhao et al., 2009, Chaudhary et al., 2011), lyotropic liquid–crystal encapsulation (Yariv et al., 2010) and bicelles formation (Rubio et al., 2010), to list a few.
An efficient and secure topical formulation has noteworthy applications in treating varied disorders and clinical conditions owing to the facts; elimination of intravenous route allied acute toxicity, bypassing of gastrointestinal disturbances, diminished renal toxicity, no or shorten hospitalization, reduced dose frequency and increase patient satisfaction (Bhanu et al., 2011, Osmani et al., 2014, Shaikh and Pawar, 2010). But conventional dermatological products typically provide actives in relatively high concentrations but for a short duration of time; leading to a cycle of short term overmedication followed by long-term under medication along with rashes or some more serious side effects. The conventional emulgel formulation contains isopropyl alcohol for enhancing DDEA solubility; which is highly flammable, cause cutaneous and eye irritation and prolonged use led to dermal hypersensitivity and eczema. Furthermore, gel action is terminated swiftly as drug gets precipitated subsequent to water absorption by body tissues. So, a novel system necessitates which will increase the presence of active agents either on skin surface or within epidermis, concurrently reducing hasty transdermal penetration (Bhanu et al., 2011, Bregni et al., 2008).
Microsponge-based delivery systems (MDS) give assurance of drug localization on skin surface and within epidermis without entering in systemic circulation in greater extent; thereby reducing systemic and local cutaneous adversities. They also offer an advantage of programmable release and are biologically safe. Additionally, this technology presents quite a lot of benefits via drug entrapment by means of better formulation flexibility, abridged side effects, improved elegance and superior stability (D’souza and More, 2008, Vyas et al., 2010, Vyas and Khar, 2002, Won, 1987).
Thus, taking into consideration the advantages of microsponge drug delivery system, disadvantages of DDEA conventional topical dosage forms available in market, and non-availability of microsponge based delivery system of DDEA in the market; present research work was aimed to formulate and evaluate DDEA microsponge gel for its promising effects over extended period of time.
2. Materials
Materials required for the present work were procured from diverse sources. Diclofenac Diethylamine (DDEA) was procured from Aarati Drugs Ltd., Mumbai, India; while Eudragit RS 100 was provided as gift sample by Evonik Pharma, Mumbai, India. Sodium alginate and dibutyl phthalate were procured from Loba Chemie, Mumbai, India and Qualigens Fine Chemicals, Mumbai, India respectively. All the other ingredients used were of analytical grade, and were used as procured.
3. Methods
3.1. Characterization of pure drug
3.1.1. Melting point
Melting point of DDEA was noted by microcontrolled based melting point apparatus (SMP10/1, Stuart, UK). In a capillary tube having one end closed, sample was inserted. Then capillary was inserted in bath of silicone oil which was heated in controlled manner with the help of electric heating coil. The temperature at which drug sample started melting was noted as melting point temperature.
3.1.2. Differential scanning calorimetry (DSC)
To evaluate thermal behavior and thermotropic characteristics of drug, DSC study was implied. It is based on principle of measuring heat flow in and out of sample and reference for the period of controlled temperature cycle. Nearly 5 mg sample was sealed in aluminum pan followed by heating at rate of 10 °C/min over temperature range of 10–200 °C under nitrogen atmosphere of flow rate 10 ml/min and thermogram (Mettler-Toledo DSC 821e, Switzerland) was obtained.
3.1.3. FTIR spectroscopy
To check purity of drug and excipients, FTIR spectra were recorded (FTIR, A-410, Jasco, Japan) over wavelength range of 4000–400 cm−1 at resolution of 4 cm−1. Samples were dispersed in KBr and compressed in pellets by applying 5 tons pressure for 5 min using hydraulic press. Formed pellets were kept in light path and spectra were recorded.
3.1.4. UV spectroscopy
Calibration curve of DDEA was plotted using phosphate buffer solution (PBS) of pH 7.4 by keeping concentration range of 2–12 μg/ml. The drug was analyzed spectrophotometrically (Pharmaspec 1700, Shimadzu, Japan) at 276 nm (regression coefficient r2 = 0.999).
3.2. Drug–excipient interaction study
Drug–excipient interactions were investigated by FTIR and DSC studies. IR spectra were recorded to check compatibility of drug with excipients, using FTIR spectrophotometer (FTIR, A-410, Jasco, Japan) over wavelength range of 4000–400 cm−1 at resolution of 4 cm−1. KBr dispersed samples were compressed in pellets by applying 5 tons pressure for 5 min using hydraulic press. Formed pellets were kept in light path and spectra were recorded.
DSC helps in assessing the physical properties of the sample nature (crystalline or amorphous) and indicates any probable interaction among drug and excipients. DDEA and physical mixture (DDEA and Eudragit RS 100) were subjected to thermal analysis. Indium standard was implied for calibrating DSC enthalpy and temperature scale. Samples were kept in aluminum pan hermetically and heated at constant rate of 10 °C/min over temperature range of 10–200 °C.
3.3. Preparation of DDEA microsponges
The microsponges enclosing DDEA were fabricated by quasi-emulsion solvent diffusion method (Tansel et al., 2002, Mine et al., 2006) using an inner phase comprising of Eudragit RS-100 and dibutyl phthalate (1% w/v) dissolved in 5 ml of dichloromethane. Dibutyl phthalate was added to improve the plasticity of polymer. Further DDEA was put in and dissolved through ultrasonication at 35 °C. Followed by dropwise addition of this mixture into aqueous solution of sodium alginate (outer phase) with stirring rate 500 rpm for 60 min. Subsequently, microsponges were formed due to dichloromethane removal from system by evaporation. Prepared microsponges were then filtered, washed with distilled water and subjected to drying at 40 °C for 12 h in hot air oven. Lastly microsponges obtained were weighed to determine production yield. Various formulation batches were prepared as per Table 1.
Table 1.
Composition of DDEA microsponges.
| Ingredients | Formulation batches |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| Diclofenac diethylamine: Eudragit RS 100 (mg) | 1:1 | 1:2 | 1:3 | 1:4 | 1:5 | 1:6 | 1:3 | 1:3 | 1:3 | 1:3 |
| Dichloromethane (ml) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dibutyl phthalate (% w/v) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sodium alginate (mg) | 50 | 50 | 50 | 50 | 50 | 50 | 30 | 40 | 60 | 70 |
| Water (ml) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
3.4. Evaluation of DDEA microsponges
3.4.1. Differential scanning calorimetry (DSC)
Thermogram of DDEA microsponge formulation was obtained using differential scanning calorimeter (Mettler-Toledo) DSC 821e outfitted with an intercooler. Indium standard was implied for calibrating DSC enthalpy and temperature scale. Microsponge samples were kept in aluminum pan hermetically and heated at constant rate of 10 °C/min over temperature range of 10–200 °C. By purging nitrogen with flow rate of 10 ml/min inert atmosphere was maintained.
3.4.2. Infrared spectroscopy
It was done using Fourier Transform Infrared Spectrophotometer (FTIR A-410, Jasco, Japan) using KBr pellet method. FTIR spectra of DDEA, Eudragit RS 100, physical mixtures of DDEA and Eudragit RS 100, and microsponge formulation were recorded in the wavelength range of 4000–400 cm−1.
3.4.3. Production yield
Microsponges production yield was determined by formula mentioned below (Kilicarslan and Baykara, 2003).
| (1) |
3.4.4. Actual drug content and encapsulation efficiency
Precisely weighed quantity (100 mg) of microsponges containing drug was kept in 100 ml phosphate buffer solution (pH 7.4) for 12 h with continuous stirring. Filtered samples (using 0.45 μm membrane filter) were further analyzed at 276 nm next to blank using UV spectrophotometer (Pharmaspec 1700, Shimadzu, Japan). Estimation of drug content and encapsulation efficiency for all batches were done using following expressions (Mine et al., 2006).
| (2) |
| (3) |
where Mact = actual DDEA content in weighed quantity of microsponges, Mms = weighed quantity of microsponges and Mthe = theoretical DDEA content in microsponges.
3.4.5. Scanning electron microscopy (SEM)
For assessing morphology and surface topography, prepared microsponges were examined under scanning electron microscope (LEO 440i, UK) operating at 5 kV. By means of double adhesive tape, samples were mounted on a metal stub and coating with platinum/palladium alloy under vacuum was done (Nokhodchi et al., 2007).
3.4.6. Particle size analysis
Particle size analysis of prepared microsponges was carried out using particle size analyzer (Malvern Mastersizer Hydro 2000, Malvern, UK). Microsponges were dispersed in double distilled water before running sample in instrument to ensure that light scattering signal (as indicated by particles count per second) is within the sensitivity range of instrument.
3.4.7. X-ray diffraction study
X-ray diffraction patterns were recorded by using X-ray diffractometer (Siemens, Model D5000, Germany) with Cu Kα radiation of wavelength 1.5405 Å and a crystal monochromator. The instrument was operated at voltage 45 mV and current 20 A. Diffraction patterns were run at 5–10 °C/min in terms of 2θ; crystal and physical state of DDEA was characterized.
3.5. Preparation of gel
For preparing DDEA microsponge gel, 0.5 g of Carbopol 940 was uniformly dispersed in beakers containing sufficient quantity of water and was allowed to hydrate overnight. Then it was mixed with 5 g of glycerin containing preservative to form paste. Later 95 ml of water was added slowly to paste under constant stirring, followed by dropwise triethanolamine addition to adjust pH to 6.5–7.5 (Maiti et al., 2011). The drug loaded microsponge gels were prepared by incorporation of microsponges containing drug equivalent to marketed formulation (Voltaren® Emulgel 1.16% w/w).
3.6. Evaluation of DDEA microsponge gels
3.6.1. Visual inspection
The organoleptic properties such as color, texture, consistency, homogeneity and physical appearance of gels containing microsponges were checked by visual observation.
3.6.2. pH measurement
Diverse gel formulations pH was recorded using digital pH meter. 5 g gel was dispersed in 45 ml distilled water at 27 °C and solution pH was measured (Purushothamrao et al., 2010, Jain and Singh, 2010).
3.6.3. Spreadability studies
One of the requisite qualities for an ideal gel is to encompass excellent spreadability. Spreadability is used to express extent of area of skin or affected part to which formulation readily spreads; which significantly affects therapeutic efficacy of formulation. Expression of spreadability is given in terms of time (in seconds) taken by two slides to slip off from gel placed in between under application of specific load. Better spreadability is indicated by minimum time required for slides separation. Mathematical expression used for spreadability calculation was:
| (4) |
where M = weight (in g) attached to upper slide, L = length (in cm) of glass slides, and T = time (in s) taken to separate the slides.
Wooden block-glass slide apparatus was used and by applying weight about 20 g, time for complete separation of upper slide (movable) from lower slide (fixed) was estimated.
3.6.4. Tube extrudability
An ideal gel should possess good tube extrudability; so that when slight pressure is applied on tube, formulation should extrude out uniformly with an ease. Technique based upon percent quantity of gel extruded from tube on finger pressure application was adopted for examining extrudability. More the quantity extruded better the extrudability. Formulations were filled in clean, lacquered, collapsible aluminum tubes with 5 mm nasal tip opening and pressure was applied on tubes by means of first finger and thumb. Afterward tube extrudability was estimated in percentage by measuring amount of gel extruded through tip and compared with marketed formulation considering its extrudability as 100% (Purushothamrao et al., 2010).
3.6.5. Viscosity measurement
The viscosity of the gel formulation was measured with a Brookfield viscometer (Capcalc V2.2) using 1x model and cone number 01, with angular velocity of 5 rpm at 25 °C. An average of five readings was used for viscosity calculation.
3.6.6. Rheological study
Rheological investigation of gel formulations was performed using a controlled stress rheometer (Viscotech Rheometer, Rheologica Instruments AB, Lund, Sweden). Data analysis was done by Stress RheoLogic Basic software (version 5.0). Cone and plate geometry was used (with 25 mm diameter and a cone angle of 1.0°) operating in the oscillation and static mode. All measurements were taken at 25 °C using fresh samples every time. Relation among shear stress and shear rate was studied by subjecting samples to increasing stress (0.1–100 Pa). Oscillatory shear responses (G′ or elastic modulus, and G″ or loss/viscous modulus) were also determined at low strains over the frequency range 0.1–10 Hz. The linearity of viscoelastic properties was verified.
3.6.7. In vitro drug release
The in vitro release of gel formulations was studied using Franz diffusion cells. The cellophane membrane (0.45 μm) previously soaked overnight in dissolution medium was mounted onto Franz diffusion cell with 15 ml receptor compartment and effective diffusion area 2.84 cm2. PBS (pH 7.4) was used as receptor medium, and system was thermostated to 37 ± 1 °C under constant stirring. All batches of drug microsponge gels (F1–F10) and marketed formulation (F11) were assessed for the diffusion study. Aliquots of 1 ml volume were withdrawn at specific time intervals by maintaining sink condition. Withdrawn aliquots were then diluted using receptor medium and analyzed by UV spectrophotometer (Pharmaspec 1700, Shimadzu, Japan) at 276 nm against PBS pH 7.4. To reveal drug release mechanism and to contrast release profiles disparities among formulations, data obtained from timely drug release were used. Further, release data were analyzed by means of diverse mathematical models to know release kinetics (Zaki Rizkalla et al., 2011).
3.6.8. Ex vivo diffusion study
For ex vivo diffusion study by sacrificing male Wistar albino rats (7-9 weeks old, 200-250 g) full thickness skin was excised from abdomen, adhering subcutaneous fat was removed and were cut into pieces just larger than the effective surface area of diffusion cells. These skin pieces were immersed in normal saline briefly and then mounted between donor and receptor compartments of Franz diffusion cells. The study conditions and analytical methods were identical to in vitro release study as mentioned before (Rajan and Vasudevan, 2012). All experimental procedures including handling, were approved by the Institutional Animal Ethics Committee (Registration number: 144/2013) complied with the guidelines set out by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Animal Welfare Division, Ministry of Environment and Forests, Government of India, India.
3.6.9. Stability study
Optimized gel formulation was subjected to stability testing as per ICH norms. Gel was filled in clean, lacquered, collapsible aluminum tubes, and various replicates were kept at 40 °C and 75% RH in a humidity chamber. Gel was assessed for change in appearance, pH or in vitro release profile at an interval of 7, 15, 30, 60 and 90 days.
4. Results and discussions
4.1. Characterization of pure drug
4.1.1. Melting point
Melting point of DDEA was pragmatic in range of 148–154 °C (literature standard 154 °C). As experimental values were in good agreement with standard, procured drug was supposed to be pure.
4.1.2. Differential scanning calorimetry (DSC)
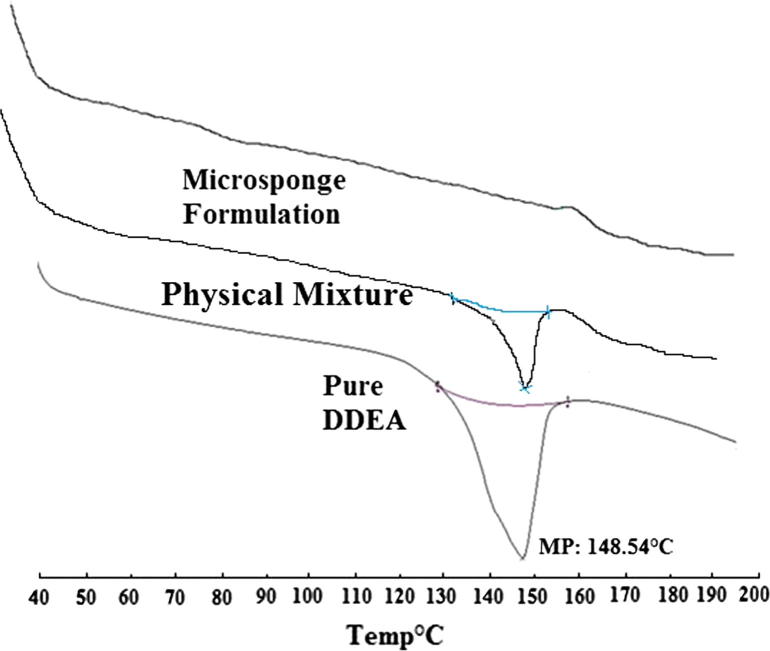
As reflected by DSC thermogram shown in Fig. 1, sharp endothermic peak was observed at 148.54 °C corresponding to melting point of drug in crystalline form; reflecting drug purity.
Figure 1.
DSC thermogram of DDEA, physical mixture and microsponge formulation.
4.1.3. FTIR spectroscopy
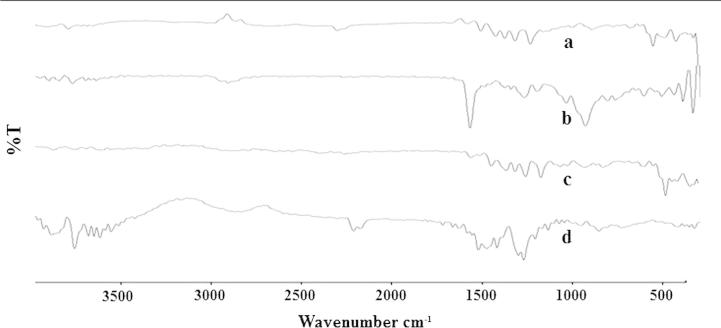
FTIR spectrum of procured DDEA was recorded (Fig. 2) and spectral interpretation was done. The characteristics IR absorption peaks of DDEA at 419.91–859.41 cm−1 (C—H bending), 741.10 cm−1 (C—Cl stretch), 1371.08 cm−1 (C—N stretch), 1697.49 cm−1 (C—O stretch), 2882.38–2992.40 cm−1 (CH2 group C—H stretch) and 3200.90 cm−1 (doublet NH group) were there in drug sample spectrum; which confirmed the purity of DDEA.
Figure 2.
Overlain FTIR spectra of (a) DDEA, (b) Eudragit RS 100, (c) physical mixture and (d) optimized formulation.
4.2. Drug–excipient interaction study
To check out any possible interaction between drug and excipients used, compatibility study using DSC and FTIR was carried out. DSC results reflected similar thermal behavior of physical mixture as that of pure drug but with quite lower intensity. A sharp endothermic peak noted at 148.54 °C in case of DDEA, indicative of its melting point (Fig. 1). However, the melting endotherm of microsponge formulation was suppressed corresponding to partial protection of DDEA because of microsponge encapsulation. Also, significantly altered crystallinity of DDEA in microsponge formulation has been traced; confirming its dispersion in the system.
FTIR spectroscopic study results discovered no any new peak appearance or disappearance of existing peaks, discarding any chemical interaction probability among drug and polymer used. The characteristic ketone C O stretching vibration at 1741.01 cm−1, C—H bending from 416.21 to 746.42 cm−1, C—O stretching at 1647.94 cm−1, C—Cl stretch at 746.34 cm−1 and doublet N—H stretching around 3257.71 cm−1 were recognized in all spectra (Fig. 2). All characteristic peaks of DDEA were experiential in physical mixture and microsponge formulation spectrum. Thus, IR spectroscopy results depicted that DDEA was compatible with selected polymer, excipients and possess good stability in all microsponge formulations.
4.3. Evaluation of DDEA microsponges
4.3.1. Physical appearance
Microsponge particles with fairly white tincture were obtained by quasi-emulsion solvent diffusion method; with good flow properties than as compared with pure drug.
4.3.2. Production yield
The production yield of all batches of microsponges ranged from 24.55% to 79.24% (Table 2). The drug:polymer ratio and concentration of sodium alginate was found to affect production yield significantly. For drug:polymer ratio 1:1 (F1), production yield was very low i.e. 24.55% while for drug:polymer ratio 1:6 (F6), it was 79.24%. It reflected that higher the drug:polymer ratio, higher the productions yield. By way of low sodium alginate concentration (30 mg, F7), production yield was quite low i.e. 38.37% and as the concentration was increased (from 30 mg to 70 mg) the production yield was also found to be increased. This was due to abridged dichloromethane diffusion rate from concentrated solutions to aqueous phase at higher drug:polymer concentrations; which provide additional time for formation of droplet, thereby improving yield.
Table 2.
Actual drug content, encapsulation efficiency and production yield.
| Batch code | Drug:Polymer ratio | Theoretical drug content (%) | Actual drug content (%) ± SDa | Encapsulation efficiency (%) ± SDa | Production yield (%) ± SDa |
|---|---|---|---|---|---|
| F1 | 1:1 | 50 | 47.78 ± 0.01 | 95.56 ± 0.02 | 24.55 ± 0.26 |
| F2 | 1:2 | 33.33 | 31.04 ± 0.03 | 93.12 ± 0.02 | 38.8 ± 0.23 |
| F3 | 1:3 | 25 | 22.49 ± 0.01 | 89.96 ± 0.06 | 42.6 ± 0.42 |
| F4 | 1:4 | 20 | 16.36 ± 0.17 | 81.8 ± 0.20 | 59.2 ± 0.01 |
| F5 | 1:5 | 16.63 | 12.61 ± 0.02 | 75.82 ± 0.03 | 71.13 ± 0.08 |
| F6 | 1:6 | 14.28 | 9.75 ± 0.01 | 68.22 ± 0.04 | 79.24 ± 0.02 |
| F7 | 1:3 | 25 | 21.27 ± 0.01 | 85.08 ± 0.02 | 38.37 ± 0.23 |
| F8 | 1:3 | 25 | 21.83 ± 0.02 | 87.32 ± 0.01 | 40.52 ± 0.12 |
| F9 | 1:3 | 25 | 22.87 ± 0.01 | 91.48 ± 0.01 | 45.31 ± 0.18 |
| F10 | 1:3 | 25 | 23.13 ± 0.01 | 92.52 ± 0.03 | 47.84 ± 0.16 |
Standard deviation mean “n” = 3.
4.3.3. Actual drug content and encapsulation efficiency
The mean amount of drug entrapped in fabricated microsponges was found to be lesser than theoretical value for every drug:polymer ratio employed, in view of the fact that drug encapsulation efficiency did not attain 100%. This was for the reason that some drug gets dissolved in aqueous phase or solvent used. Encapsulation efficiency outcomes reflected that higher drug:polymer ratios led to superior drug loadings. Integration of higher sodium alginate amounts during microsponge preparation at elevated drug:polymer ratio caused slight increase in dispersed phase viscosity. Almost all of dispersed phase was transformed to solid microsponges on diffusion of solvents from inner phase, and alienated particles emerged. The utmost drug loading efficiencies for these formulations could be attributed to availability of maximum polymer amount to apiece drug unit in contrast to rest of formulations. The encapsulation efficiencies were in the range of 68.22–95.56% as quoted in Table 2.
4.3.4. Scanning electron microscopy (SEM)
For morphology and surface topography investigation, prepared microsponges were subjected to SEM analysis. The captured SEM image of microsponges is shown in Fig. 3. SEM results indicated that microsponges formed were highly porous, predominantly spherical and not much entire DDEA crystals were observed visually. Pores were induced by diffusion of solvent from surface of microsponges. Furthermore, it was exposed that the distinctive internal structure comprised of spherical cavity enclosing a stiff shell assembled of drug and polymer. The internal structure consisted of numerous annulled spaces and appearance of particles was such that they were perfect to term as microsponges.
Figure 3.
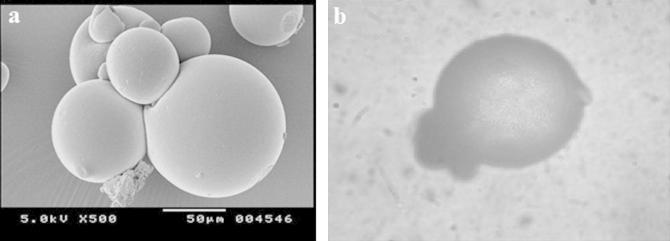
(a) SEM and (b) PPL microscopic images of microsponges.
Microsponges were also observed under binocular plane polarized light (PPL) microscope (Fig. 3), which revealed that formed microsponges were spherical as each single entity or in form of bunches and had porous nature.
4.3.5. Particle size analysis
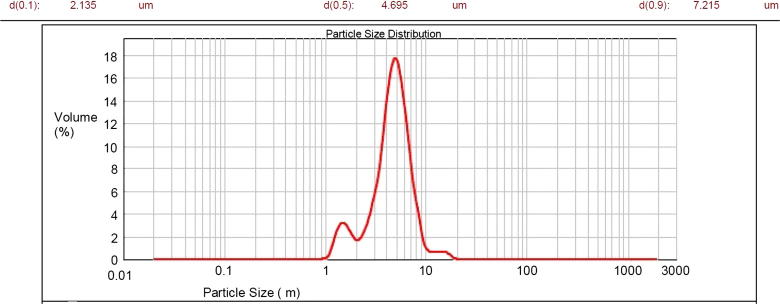
The average particle size of microsponge formulations should be in the range of 5–300 μm. Visual inspection results of all batches done using optical microscope for particle size discovered increased particle size with increase in drug:polymer ratio. It might be since polymer available at higher drug:polymer ratio was in more amount thereby increasing polymer wall thickness which led to larger size of microsponges. Besides, with increasing amount of sodium alginate particle size was found to be increased; attributed to rise in apparent viscosity at augmented sodium alginate concentrations. It results in the formation of bigger emulsion droplets and larger microsponge size. Optimized batch has been found with more percent of intact, uniform, spherical particles during optical microscopy; hence subjected to analysis using photon correlation spectroscopy (Malvern Mastersizer Hydro 2000, Malvern, UK). The results discovered particle size corresponding to 7.215 μm (Fig. 4).
Figure 4.
Particle size distribution curve of microsponges.
4.3.6. X-ray diffraction study
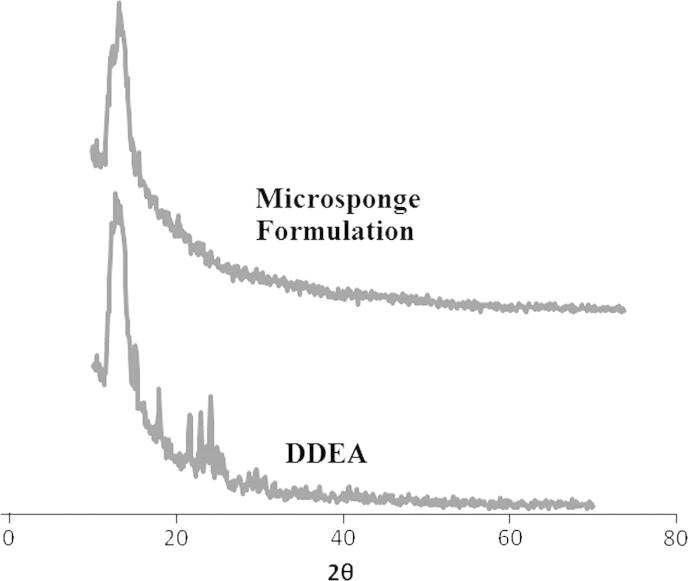
X-ray powder diffraction (XRPD) method was implied to evaluate physicochemical characteristics of prepared microsponges. Sharp peaks at diffraction angle (2θ) 13° were traced in X-ray diffractograms in case of both DDEA and its microsponge formulation (Fig. 5).
Figure 5.
XRPD pattern of DDEA and microsponge formulation.
For determination of occurrence of crystal habit modifications and polymorphs in drug crystals, XRPD is a valuable technique. In general when diffraction patterns are identical for two forms of crystals, they known to posses same internal structures and when patterns are unlike, crystals have diverse internal structures known as polymorphs. In a study at hand, samples depicted spectra with similar peak positions (2θ values). Consequently, no existence of polymorphs of DDEA in these samples was verified.
Additionally, for crystallinity determination comparison of some representative peak heights with those of a reference in diffraction patterns has been done. Final formulation of microsponges showed peaks at diffraction angle same as that of XRD pattern of DDEA but with some lower intensity, indicating its crystalline nature. The relative degree of crystallinity (RDC) value was found to be 1.26. So XPRD analysis revealed that the crystalline nature of drug was not completely lost and was found to remain thermally stable in the final formulation as well.
4.4. Evaluation of DDEA microsponge gel
4.4.1. Visual inspection
The prepared gel formulations of DDEA microsponges were inspected visually for their color, texture and appearance. All prepared formulations were pearl white, viscous in nature with smooth texture and of good homogeneity with no any lumps and syneresis.
4.4.2. pH measurement
The pH values of all prepared formulations were found in the range of 6.5–6.8, which are supposed to be suitable to pass up threat of nuisance on application to skin.
4.4.3. Spreadability study
The findings of spreadability depicted that formulated gels get easily spread on applying small amount of shear. Spreadability of gel containing unentrapped drug was found to be 3.52 g cm/s; while that of microsponge formulation it was 4.18 g cm/s; indicating that spreadability of drug loaded microsponge gel was good as compared to that of marketed one.
4.4.4. Extrudability study
The extrudability of prepared microsponge gel was found to be 96.63% when calculated by considering extrudability of unentrapped drug gel as 100%. Accordingly, the prepared formulation exhibited better extrudability which is one of the ideal characteristics.
4.4.5. Viscosity and rheological study
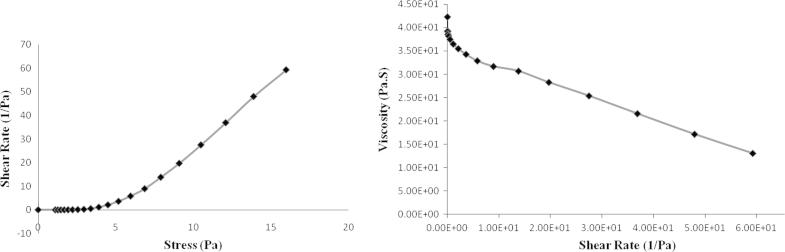
The viscosity of prepared DDEA microsponge gel was found to be 42,300 cPs. To investigate viscoelastic properties, rheological analysis of optimized gel formulation was done. It has been found that for gel system, viscous modulus (G″) was higher than elastic modulus (G′) indicating that the system was viscous. The formulation showed pseudoplastic rheology as evident by shear thinning and a decrease in viscosity with an increase in shear stress. The curved rheograms were resultant to shearing action onto long chain polymer molecules (Fig. 6). As shearing stress was raised, normally disarranged molecules start to line up their long axes in direction of flow. These orientations have abridged internal resistance and have permitted better shear rate on every consecutive shearing stress. The viscosity was found to be reliant on polymeric content of formulation.
Figure 6.
Rheograms of optimized formulation.
4.4.6. In vitro drug release
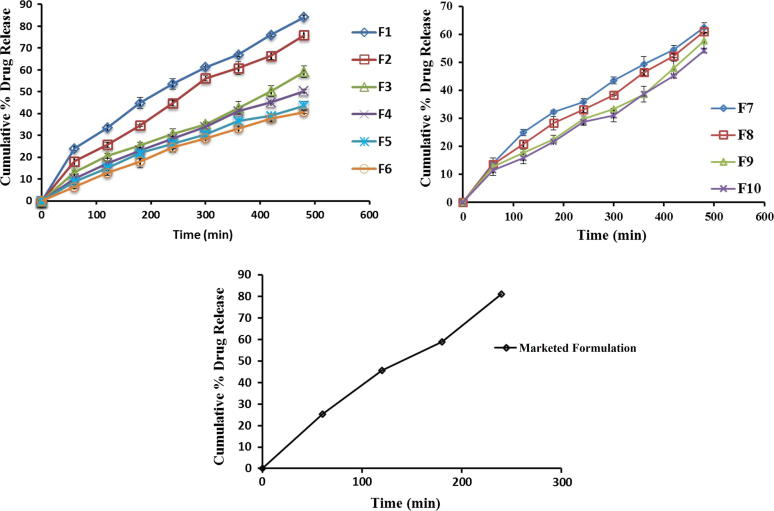
The drug release was observed to decline within range of 84.18–40.69% with respect to rise in drug:polymer ratio from 1:1 to 1:6. The reason behind is as drug:polymer ratio has increased; in each microsponge, to encapsulate drug, the polymer amount available was more. It led to thickening of polymer matrix wall, thus extended diffusion path and ultimately lesser drug release. The highest drug release i.e. 84.18% was found for the formulation F1, while the lowest 40.69% for F6. Initial burst release observed in case of formulations F1 and F2 can be allocated to existence of non-encapsulated drug near surface or on the exterior of microsponges. Graphical presentation for comparative drug release of all batches F1–F6 and F7–F10 is shown in Fig. 7.
Figure 7.
Comparative drug release profile of F1–F10 and marketed formulation.
It has been reported that with increasing amount of sodium alginate from batches F7 to F10, the drug release went on decreasing. It might be due to fact that the polymer matrix releases drug after complete swelling and the time required for swelling of polymer is directly proportional to stabilizer concentration. The declined release rate with increased amount of sodium alginate for formulations F7–F10 was from 62.51% to 54.26%.
4.4.7. Release profile of unentrapped DDEA gel
Drug release study for gel containing unentrapped DDEA i.e. marketed formulation (Voltaren® Emulgel 1.16% w/w) was carried out, release profile of which was as depicted in Fig. 7. The gel got exhausted by releasing 81.11% of drug at the end of 4 h only. In contrast microsponge-based gel exhibited sustained drug release up to 8 h thereby minimizing the side effects, skin irritation and hypersensitivity reactions. As the F2 formulation exhibited 75.88% drug release after completion of 8 h, and also found superior in terms of percent intact porous microsponges, other physical parameters and drug release. So, F2 formulation has been considered as best and more efficient to give an extended drug release among the all.
4.4.8. Drug release kinetics
The in vitro release data were subjected to various release models namely, zero order, first order, Higuchi, Peppas and Korsmeyer–Peppas, and by highest r2 value best fit model was decided. The in-vitro drug release showed highest regression value for the Peppas model (0.998 for F6). Peppas model was found to be best fit for most of the formulations, on the basis of maximum regression values (Table 3). By putting release data in Korsmeyer equation drug release mechanism for all formulations was investigated. For formulations F1–F10, n values were found in range 0.6095–0.8926. The n value for Korsmeyer–Peppas model was seen to be in the range 0.5–1, which is indicative of non-Fickian diffusion.
Table 3.
Release kinetic data of microsponge formulations.
| Batch code | Zero order | First order | Higuchi | Peppas | Korsmeyer Peppas parameters |
Best fit model | |
|---|---|---|---|---|---|---|---|
| na | ka | ||||||
| F1 | 0.964 | 0.940 | 0.994 | 0.991 | 0.6095 | 1.9044 | Higuchi |
| F2 | 0.980 | 0.940 | 0.984 | 0.989 | 0.7108 | 0.9187 | Peppas |
| F3 | 0.983 | 0.967 | 0.953 | 0.982 | 0.6992 | 0.7078 | Zero |
| F4 | 0.989 | 0.934 | 0.988 | 0.992 | 0.7816 | 0.3993 | Peppas |
| F5 | 0.981 | 0.899 | 0.992 | 0.990 | 0.7723 | 0.3777 | Higuchi |
| F6 | 0.990 | 0.942 | 0.995 | 0.998 | 0.8926 | 0.3268 | Peppas |
| F7 | 0.971 | 0.905 | 0.992 | 0.992 | 0.6866 | 0.8781 | Higuchi and Peppas |
| F8 | 0.986 | 0.953 | 0.967 | 0.990 | 0.7089 | 0.7147 | Peppas |
| F9 | 0.987 | 0.983 | 0.927 | 0.965 | 0.7115 | 0.6222 | Zero |
| F10 | 0.979 | 0.972 | 0.949 | 0.976 | 0.7432 | 0.4917 | Zero |
n-kinetic constant, k-release rate constant.
4.4.9. Ex vivo diffusion study
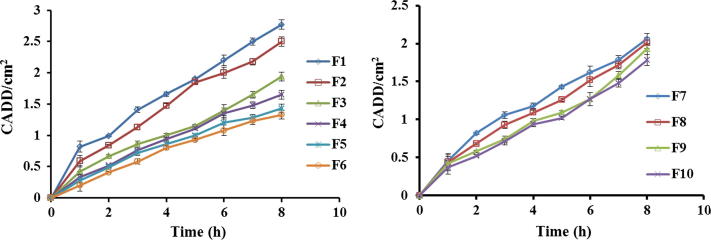
The ex vivo diffusion studies were completed for all formulations on excised rat skin with use of PBS (pH 7.4). It has been observed that the formulation F1 exhibited higher drug diffusion on completion of 8 h while that of F6 it was contradictory. Similarly in case of formulations F7–F10, there was a slight decrease in amount of drug diffused from F7 to F10 respectively. Study results indicated that the Q value (collective sum of drug permeated per unit surface area of skin) was decreased at higher ratio due to an increase in Eudragit RS 100 concentration, and also it was found to decrease at high sodium alginate concentrations. The cumulative amount of drug diffused per unit surface area of skin (CADD) from microsponge containing gel formulations was plotted against time as shown in Fig. 8. The slopes of linear portions of obtained permeation profiles were estimated as steady state flux (J) of drug from gel formulations. As compared to F1–F3 the flux of drug was found to be relatively sluggish for batches F4–F6; reflecting slow discharge of entrapped drug from these microsponges. The sum of drug permeated through unit area subsequent to 8 h was also found to be lower for batches F4–F6.
Figure 8.
Drug diffusion profiles of F1–F10 formulations.
The drug diffusion rate for first 1 h was found to be elevated compare to next 7 h in case of all formulations. This was possibly being attributable to the release of free unentrapped DDEA which affected the flux at first that goes slowly decreased for the next 7 h. Thus, retarded release of entrapped drug from microsponges was established by slower flux values.
4.4.10. Effect of formulation variables
4.4.10.1. Effect of composition of external phase
Composition of external phase was altered for formulations F7–F10 by changing concentration of sodium alginate from 30 to 70 mg. It has been observed that on increasing the amount of sodium alginate, production yield, encapsulation efficiency and particle size were increased while slight decrease in drug release was noticed (Table 4).
Table 4.
Effect of composition of external phase.
| Batch code | Sodium alginate concentration (mg) | Production yield (%) ± SDa | Encapsulation efficiency (%) ± SDa | % CDR ± SDa |
|---|---|---|---|---|
| F7 | 30 | 38.37 ± 0.23 | 85.08 ± 0.02 | 62.51 ± 0.76 |
| F8 | 40 | 40.52 ± 0.12 | 87.32 ± 0.01 | 61.08 ± 0.59 |
| F3 | 50 | 42.6 ± 0.42 | 89.96 ± 0.06 | 59.12 ± 0.53 |
| F9 | 60 | 45.31 ± 0.18 | 91.48 ± 0.01 | 57.81 ± 0.57 |
| F10 | 70 | 47.84 ± 0.16 | 92.52 ± 0.03 | 54.26 ± 0.62 |
Standard deviation mean “n” = 3.
4.4.10.2. Effect of drug:polymer ratio
Increase in drug:polymer ratio has been found to result as increase in production yield; while drug content, encapsulation efficiency and percent drug release were found to be decreased (Table 5). The reason behind that is as drug:polymer ratio went on increasing, the polymer amount available for each microsponge to encapsulate the drug was more, thus rising polymer matrix wall thickness which led to extended diffusion path and ultimately to lesser drug release. Consequently the amount of drug diffused and flux of the formulations were decreased at higher drug:polymer ratio.
Table 5.
Effect of drug–polymer ratio.
| Batches | Drug:polymer ratio | Production yield (%) ± SDa | Drug content (%) ± SDa | Encapsulation efficiency (%) ± SDa | % CDR ± SDa | Flux (mg/cm2 h) |
|---|---|---|---|---|---|---|
| F1 | 1:1 | 24.55 ± 0.26 | 47.78 ± 0.01 | 95.56 ± 0.02 | 84.18 ± 0.01 | 0.3181 |
| F2 | 1:2 | 38.8 ± 0.23 | 31.04 ± 0.03 | 93.12 ± 0.02 | 75.88 ± 0.03 | 0.2968 |
| F3 | 1:3 | 42.6 ± 0.42 | 22.49 ± 0.01 | 89.96 ± 0.06 | 59.12 ± 0.02 | 0.2207 |
| F4 | 1:4 | 59.2 ± 0.01 | 16.36 ± 0.17 | 81.8 ± 0.20 | 50.27 ± 0.05 | 0.1999 |
| F5 | 1:5 | 71.13 ± 0.08 | 12.61 ± 0.02 | 75.82 ± 0.03 | 43.66 ± 0.01 | 0.1742 |
| F6 | 1:6 | 79.24 ± 0.02 | 9.75 ± 0.01 | 68.22 ± 0.04 | 40.69 ± 0.01 | 0.1621 |
Standard deviation mean “n” = 3.
4.4.11. Stability study
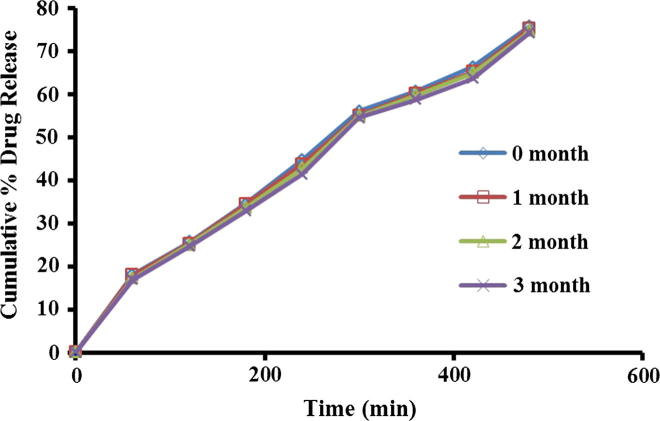
During stability studies formulation appearance was found pearl white, homogenous, smooth and no significant deviation in pH was seen. It was also noted from outcomes that there were no considerable changes in drug content as well as percent drug release. Therefore no evidence of degradation of drug was observed.
After comparative assessment of optimized formulation (F2) drug release profiles prior and after 3 months stability study, similarity factor (f2) was calculated (Fig. 9). Similarity factor was found to be f2 = 82.38 (>50). As similarity factor greater than 50 indicates good stability of the product, in view of this it has been concluded that the formulation was stable over the period of 3 months.
Figure 9.
Drug release profile of microsponge gel during stability study.
5. Conclusions
Controlled drug delivery via the polymer based systems has been proposed to be prevailing both in present and in future; as having numerous potential advantages for scientific as well as economic reasons. The thought behind developing polymeric microsponge delivery system was to deliver DDEA in a continual manner for extensive time period to reduce application frequency, hypersensitive reactions and to improve bioavailability, safety than marketed conventional formulation. The method implemented was quasi-emulsion solvent diffusion; found to be simple, reproducible and rapid. Formed microsponges were spherical shape, have high porosity and good flow. Varied drug–polymer ratio reflected remarkable effect on particle size, drug content and encapsulation efficiency. Microsponge-based gel showed viscous modulus along with pseudoplastic behavior and in vitro drug release reflected highest regression value for Peppas model. Formulation with 1:2 drug–polymer ratio was found more efficient to give extended drug release (75.88% at 8 h); while conventional formulation exhausted extremely earlier (81.11% at 4 h). Thus, gel containing microsponges prepared in this study was found to be promising as new-fangled delivery system offering prolonged release of DDEA in treating rheumatoid arthritis, juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis and other musculoskeletal ailments.
Acknowledgments
The authors express deep sense of gratitude toward Aarati Drugs Ltd., Mumbai (India) and Evonik Pharma, Mumbai (India) for providing the gift samples of DDEA and Eudragit RS 100 respectively.
Footnotes
Peer review under responsibility of King Saud University.
References
- Arora P., Mukherjee B. Design, development, physicochemical, and in-vitro and in-vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J. Pharm. Sci. 2002;91:2089–2096. doi: 10.1002/jps.10200. [DOI] [PubMed] [Google Scholar]
- Baboota S., Shakeel F., Kohli K. Formulation and evaluation of once-a-day transdermal gels of diclofenac diethylamine. Methods Find. Exp. Clin. Pharmacol. 2006;28:109–114. doi: 10.1358/mf.2006.28.2.977842. [DOI] [PubMed] [Google Scholar]
- Bhanu V.P., Shanmugam V., Lakshmi P.K. Development and optimization of novel diclofenac emulgel for topical drug delivery. Int. J. Comp. Pharm. 2011;2:1–4. [Google Scholar]
- Bregni C., Chiappetta D., Faiden N., Carlucci A., Garcia R., Pasquali R. Release study of diclofenac from new carbomer gels. Pak. J. Pharm. Sci. 2008;21:12–16. [PubMed] [Google Scholar]
- Chaudhary H., Kohli K., Amin S., Rathee P., Kumar V. Optimization and formulation design of gels of diclofenac and curcumin for transdermal drug delivery by Box-Behnken statistical design. J. Pharm. Sci. 2011;100:580–593. doi: 10.1002/jps.22292. [DOI] [PubMed] [Google Scholar]
- D’souza J.I., More H.N. Topical anti-inflammatory gels of fluocinolone acetonide entrapped in eudragit based microsponge delivery system. Res. J. Pharm. Technol. 2008;1:502–506. [Google Scholar]
- Djordjevic L., Primorac M., Stupar M., Krajisnik D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int. J. Pharm. 2004;271:9–11. doi: 10.1016/j.ijpharm.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Gan T.J. Diclofenac: an update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010;26:1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- Jain V., Singh R. Development and characterization of Eudragit RS 100 loaded microsponges and its colonic delivery using natural polysaccharides. Acta Pol. Pharm. Drug Res. 2010;67:407–415. [PubMed] [Google Scholar]
- Khalil E., Najjar S., Sallam A. Aqueous solubility of diclofenac diethylamine in the presence of pharmaceutical additives: a comparative study with diclofenac sodium. Drug Dev. Ind. Pharm. 2000;26:375–381. doi: 10.1081/ddc-100101243. [DOI] [PubMed] [Google Scholar]
- Kilicarslan M., Baykara T. The effect of the drug/polymer ratio on the properties of verapamil hydrochloride loaded microspheres. Int. J. Pharm. 2003;252:99–109. doi: 10.1016/s0378-5173(02)00630-0. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.V., Wagh Y.J., Setty C.M., Sa B. Development and characterization of sodium alginate hydroxypropyl methylcellulose-polyester multilayered hydrogel membranes for drug delivery through skin. Polym.-Plast. Technol. Eng. 2011;50:490–497. [Google Scholar]
- Kweon J.H., Chi S.C., Park E.S. Transdermal delivery of diclofenac using microemulsions. Arch. Pharm. Res. 2004;27:351–356. doi: 10.1007/BF02980072. [DOI] [PubMed] [Google Scholar]
- Maiti S., Ray S., Kaity S. Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm. 2011;61:257–270. doi: 10.2478/v10007-011-0022-6. [DOI] [PubMed] [Google Scholar]
- Manosroi A., Jantrawut P., Manosroi J. Anti-inflammatory activity of gel containing novel elastic niosomes entrapped with diclofenac diethylammonium. Int. J. Pharm. 2008;360:156–163. doi: 10.1016/j.ijpharm.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Mine O., Erdal C., Ahmet A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int. J. Pharm. 2006;318:103–117. doi: 10.1016/j.ijpharm.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Minghetti P., Cilurzo F., Casiraghi A., Montanari L., Fini A. Ex-vivo study of transdermal permeation of four diclofenac salts from different vehicles. J. Pharm. Sci. 2007;96:814–823. doi: 10.1002/jps.20770. [DOI] [PubMed] [Google Scholar]
- Niethard F.U., Gold M.S., Solomon G.S., Liu J.M., Unkauf M., Albrecht H.H., Elkik F. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J. Rheumatol. 2005;32:2384–2392. [PubMed] [Google Scholar]
- Nokhodchi A., Jelvehgari M., Reza S.M., Reza M.M. Factors affecting the morphology of benzoyl peroxide microsponges. Micron. 2007;38:834–840. doi: 10.1016/j.micron.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Osmani R.A., Aloorkar N.H., Kulkarni A.S., Harkare B.R., Bhosale R.R. A new cornucopia in topical drug delivery: microsponge technology. Asian J. Pharm. Sci. Technol. 2014;4:48–60. [Google Scholar]
- Purushothamrao K., Khaliq K., Sagare P., Patil S.K., Kharat S.S., Alpana K. Formulation and evaluation of vanishing cream for scalp psoriasis. Int. J. Pharm. Sci. Technol. 2010;4:32–41. [Google Scholar]
- Rajan R., Vasudevan D.T. Effect of permeation enhancers on the penetration mechanism of transferosomal gel of ketoconazole. J. Adv. Pharm. Technol. Res. 2012;3:112–116. doi: 10.4103/2231-4040.97286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio L., Alonso C., Rodriguez G., Barbosa-Barros L., Cordech L., De la Maza A., Parra J.L., Lopez O. Bicellar systems for in-vitro percutaneous absorption of diclofenac. Int. J. Pharm. 2010;386:108–113. doi: 10.1016/j.ijpharm.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Shaikh K.S., Pawar A.P. Liposomal delivery enhances cutaneous availability of ciclopiroxolamine. Latin Am. J. Pharm. 2010;29:763–770. [Google Scholar]
- Tansel C., Omoglu C.T., Baykara T. The effects of pressure and direct compression on tabletting of microsponges. Int. J. Pharm. 2002;242:191–195. doi: 10.1016/s0378-5173(02)00155-2. [DOI] [PubMed] [Google Scholar]
- Vucinic-Milankovic N., Savic S., Vuleta G., Vucinic S. The physicochemical characterization and in-vitro/in-vivo evaluation of natural surfactants based emulsions as vehicles for diclofenac diethylamine. Drug Dev. Ind. Pharm. 2007;33:221–234. doi: 10.1080/03639040601150179. [DOI] [PubMed] [Google Scholar]
- Vyas L.K., Tapar K.K., Laddha B.H., Lahoti A.O., Nema R.K. Formulation and development of anti-blemish preparations using microsponge technology. J. Chem. Pharm. Res. 2010;2:562–571. [Google Scholar]
- Vyas S.P., Khar R.K. First ed. CBS Publication; New Delhi: 2002. Targeted and Controlled Drug Delivery-Novel Carrier System. [Google Scholar]
- Won, R. 1987. Method for delivering an active ingredient by controlled time release utilizing a novel delivery vehicle which can be prepared by a process utilizing the active ingredient as a porogen. U.S. Patent 4,690,825.
- Yariv D., Efrat R., Libster D., Aserin A., Garti N. In-vitro permeation of diclofenac salts from lyotropic liquid crystalline systems. Colloids Surf. B: Biointerfaces. 2010;78:185–192. doi: 10.1016/j.colsurfb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Zaki Rizkalla C.M., Latif Aziz R., Soliman I.I. In-vitro and in-vivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS Pharm. Sci. Technol. 2011;12:989–1001. doi: 10.1208/s12249-011-9663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Li Y., Fang L., Ren C., Xu Y., He Z. Effect of O-acylmenthol and salt formation on the skin permeation of diclofenac acid. Drug Dev. Ind. Pharm. 2009;35:814–826. doi: 10.1080/03639040802623933. [DOI] [PubMed] [Google Scholar]