Abstract
Background
Although radial access for drug-eluting stent (DES) combined with rotational atherectomy (RA) in patients with calcified coronary lesions may be associated with a lower risk of major bleeding complications and obtain favorable clinical results compared with femoral access, the long-term outcome data of this approach were limited in contemporary DES era.
Methods & Results
This retrospective study sought to compare in-hospital and long-term outcomes for patients undergoing RA via the transradial (TR) and transfemoral (TF) route in 126 consecutive patients (59 radial, 67 femoral) from 2009 to 2014. TR RA procedures were performed in 44/62 (71%) by the three TR operators, compared with 15/64 (23%) by the four TF operators in the present study. Significantly smaller diameter guide catheters and burrs (1.39 ± 0.16 mm vs. 1.53 ± 0.24 mm, P = 0.001) were used in the TR group. Procedural success rates were similar in both TR and TF groups. There was a significantly less major access site bleeding complications in favor of radial artery access (2% vs. 16%, P = 0.012). The incidence of in-hospital death or myocardial infarction was low in both groups. Although a trend of lower adverse event rate was demonstrated in the TR group compared with the TF one, no statistical significance (21% vs. 27%, P = 0.135) was detected.
Conclusions
Radial access, a useful alternative to femoral access for RA and DES, can be safely and successfully performed on up to 71% of the patients with heavily calcified coronary lesions needing RA by experienced TR operators.
Keywords: Calcified lesion, Drug-eluting stent, Rotablation, Transfemoral, Transradial
1. Introduction
Rotational atherectomy (RA) or rotablation can facilitate the delivery of stents in severely calcified coronary lesions by modifying plaque anatomy and smoothing inner vascular lumen.[1],[2] The implantation of bare-metal stent after RA in calcified lesions has a high success rate, with an acceptable incidence of complications and a clearly lower incidence of angiographic restenosis compared to plain angioplasty, but the restenosis rate and the need for revascularization remain high. Therefore, its use had been declined for a time.[3],[4] Recent reports have suggested that the use of RA in combination with drug-eluting stent (DES) implantation to treat heavily calcified coronary lesions can often achieve high procedural success accompanied by an acceptable restenosis rate.[5]–[12] Previous trials have also suggested that radial access for RA may be associated with a lower risk of major bleeding complications compared with femoral access.[13]–[15] However, the long-term data regarding efficacy and safety of DES combined with RA via radial access in patients with calcified coronary lesions were limited, partly because DES, especially the newer-generation DES, has been widely available worldwide only since 2007.
In view of the fact that transradial (TR) approach considerably reduces vascular access site complication and causes patients less discomfort, we have adopted this technique as a routine for percutaneous coronary intervention (PCI) in our institution since 1997, and radial artery access has become the default route for PCI since 2006 — by that time, all operators in our institution had already done TR PCI, individually, for over 500 cases. Currently, about 1500–1800 PCI procedures are performed in the hospital per year, and more than 85% of them, through the TR approach; as to the RA procedures, there was a temporal trend toward increasing use of RA and DES in the treatment of heavily calcified coronary lesions at out institution after 2007, when the publications of their favorable mid-tem and long-term results became available.[5],[6]
In this study, we retrospectively analyzed our real-world experience with TR versus transfemoral (TF) RA in contemporary DES era.
2. Methods
2.1. Study population
This study is a retrospectively observational analysis of 126 consecutive patients who underwent TR or TF RA in our hospital between January 2009 and January 2014. The RA procedure was performed in 59 (47%) of the 126 patients via radial access (TR group), and in 67 (53%) patients via the femoral approach (TF group). The ethical committee of the hospital approved the study protocol.
2.2. Operators
There are seven qualified and experienced operators for RA at our institution. However, although radial access is considered the default route for diagnostic cardiac catheterization and PCI at out institution, only three of them are “true” TR RA operators; that is, they use radial access as a preferred route for RA and reserve femoral artery access for those patients with a failed radial artery approach, an extraordinarily small radial artery palpated before the procedure, a planned use of a ≥ 1.75 mm burr or an 7/8 Fr guiding catheter, stage 4 or 5 chronic kidney disease, left-main disease, or hemodynamic instability. TR RA procedures were performed in 44/62 (71%) by the three TR operators; whereas, 15/64 (23%) by the other four non-TR operators in the present study.
2.3. Quantitative angiographic analysis and the indications for RA
Baseline and post-procedural coronary angiograms were digitally collected and assessed off-line in our catheterization laboratory with quantitative angiographic analysis (QCA) used a computer-assisted, automated edge-detection algorithm (Siemens AXIOM Artis and Zee™ Digital Cath Labs) by an independent experienced operator, totally unaware of the treatment allocation. Standard qualitative morphological criteria were recorded on the basis of their identifications from an un-foreshortened point of view. The external diameter of the contrast-filled non-tapered catheter tip was used for calibration standard, and the minimal lumen diameter at end diastole before intervention was measured from orthogonal projections; the results from the “worst” situation were recorded. Coronary calcium was angiographically graded as follows: none/mild; moderate (radiopacities noted only during the cardiac cycle before contrast injection); and severe (radiopacities noted without cardiac motion before contrast injection, generally compromising both sides of the arterial lumen).[16]
De-novo lesion in a native coronary artery with luminal diameter reduction of 70% to 99% by visual estimation and moderate to severe calcification of the target lesion were included in the present study. The indications for RA were uncrossable and/or undilatable calcified coronary lesions. The decision to perform RA was following failure to cross the heavily calcified coronary lesion with a balloon catheter or an intravascular ultrasound (IVUS) catheter, or failure to dilate the lesion with a balloon catheter at the rated burst pressure.
The performance of pre-intervention IVUS was not a general approach when calcifications were seen on fluoroscopy at or near the lesion, where the operator had already planned to perform rotablation. Intravascular ultrasound was used whenever disease severity permitted, to clarify the degree of calcification and extent of lesions, vessel caliber, location of calcification (superficial and/or deep), and involvement of bifurcations or ostia, as well as to assess the final result (confirmation of correct stent expansion and apposition). Calcified lesions, which underwent rotablation under IVUS guidance, were identified as subintimal (superficial) echo-dense structures producing external shadowing and with an arc ≥ 180°.
2.4. Procedure
All procedures were performed after written informed consents had been obtained. The choice between radial and femoral artery approach was at the discretion of the treating physicians. Six French guide catheters were the default strategy for TR procedures, unless it was anticipated that a ≥ 1.75 mm burr would be necessary. In the latter case, 7/8 French guide catheters were used.
RA was performed with the Rotablator® (Boston Scientific-Scimed Corporation, Natick, Massachusetts) using the smallest burr deemed necessary to modify the plaque and facilitate the passage of further devices, including balloons and stents. A dynaglide technique was used to advance the burr through the guiding catheter to reduce friction, and the atherectomy was performed using the pecking motion maneuver until completely through the lesion, with an initial ablation speed of 140,000–180,000 r/min. The duration of rotational atherectomy application was 15–20 s, with immediate cessation if the speed dropped by more than 5,000 r/min. The range of burr size used was 1.25–2.0 mm. During RA, intracoronary nitroglycerin and adenosine were injected to avoid coronary spasm and slow-flow. Temporary transvenous pacing is reserved for bradycardia refractory to atropine. All patients were preloaded with dual antiplatelet therapy, and all received intravenous unfractionated heparin (70 U/kg) during the procedure. For procedures lasting longer than 1 h, activated clotting time was measured, aiming for 250 s. Glycoprotein IIb/IIIa inhibitor use was at the operator's discretion.
One-hundred and thirteen of the 126 (90%) patients underwent stent implantation following RA, with pre-dilatation with a conventional balloon or a cutting balloon. Among them, 106/113 (94%) underwent stenting with DES. The stent length was chosen so as to cover the entire lesion, including the proximal and distal edges. In case of multiple stents, overlapping was performed. After stent deployment, post-dilatation for stent optimization under quantitative angiography and/or IVUS guidance was performed if residual in-stent stenosis was ≥ 20% of the vessel diameter.
In the radial group, the radial artery sheath was removed immediately following completion of the procedure and hemostasis was achieved using a hemostatic bandage (Stepy®P, Nichiban Co. Ltd., Tokyo, Japan). For femoral procedures, the femoral sheath was left in place for 2–4 h until the activated clotting time was < 180 s. Adequate external compression and further gauze pressure dressings with sand bag compression were applied for at least 6 h to achieve hemostasis. After the procedure, patients received dual-antiplatelet therapy with clopidogrel (75 mg/day) and aspirin (100 mg/day) for at least 3 months if they had received a bare metal stent (BMS); 12 months, if they had received a DES.
2.5. Study definitions and clinical follow-up
Coronary lesions were classified according to guidelines of the ACC/AHA Task Force on percutaneous transluminal coronary angioplasty. Procedural success was defined as having achieved a grade III thrombolysis in myocardial infarction (TIMI) flow and reduction of the target lesion to < 20% luminal diameter by visual angiographic assessment in the absence of mortality, myocardial infarction (MI) or stent thrombosis. Bleeding was classified as minor or major, depending on whether the bleeding was associated with hemodynamic compromise and/or blood transfusion. Vascular access site refers to any arterial or venous puncture site used for the procedure. Deaths were classified as either cardiac or non-cardiac. Deaths resulted from unascertained causes were categorized to be cardiac. Myocardial infarction was defined as clinical evidence of new myocardial ischemia, following the index procedure accompanied by new pathological Q-waves on the electrocardiography and/or an increase in creatinine phosphokinase-MB concentration of > 3 × 99th percentile. Post-procedural biomarkers were measured routinely, at 6 h and 12 h after the index procedure. Post-procedural myonecrosis was defined as having an increase in creatinine phosphokinase-MB concentration of 1 to 3 × 99th percentile. Target vessel revascularization (TVR) was defined as the need for a new revascularization, either percutaneous or surgical, of the vessel previously treated by RA and was clinically driven. Major adverse cardiovascular events (MACE) included death, recurrent non-fatal MI, recurrent non-fatal stroke, and TVR. Acute stent thrombosis was classified according to the Academic Research Consortium criteria. The primary outcome measure was defined as total MACE rates at the end of the follow-up period of July 2014.
2.6. Statistical analysis
All patients who underwent RA were identified from the database of our catheterization laboratory, and the records were retrieved and reviewed in a retrospective fashion, with the information collected in a predesigned form. Data are expressed as mean ± SD for continuous variables and as percentages for categorical variables. Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL). Student's t-test was used to compare continuous variables and Chi-square test or Fisher's exact test was used to compare categorical variables. A P-value < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Patient characteristics
Baseline demographic and clinical characteristics of the 126 study patients are shown in Table 1. The patients in both groups were well matched for clinical characteristics. There was also no significant difference in the incidence of smoking, hypertension, diabetes, hyperlipidemia, chronic kidney disease (stages 4 and 5), previous MI and previous stroke between the two groups. However, significantly more male patients underwent RA via radial access, compared to those in the TF group (71% vs. 51%, P = 0.031), and those who underwent TR RA tended to be younger (69.3 ± 1.3 years vs. 72.9 ± 1.6 years, P = 0.082).
Table 1. Clinical characteristics of the 126 patients who underwent rotational atherectomy via radial or femoral access.
| Vascular access | TR (n = 59) | TF (n = 67) | P-value |
| Age, yr | 69.3 ± 1.3 | 72.9 ± 1.6 | 0.082 |
| Males | 42 (71%) | 34 (51%) | 0.031 |
| Body mass index, kg/m2 | 25.6 ± 6.5 | 25.1 ± 4.8 | 0.593 |
| Smoking | 6 (10%) | 8 (12%) | 0.975 |
| Hypertension | 43 (73%) | 56 (84%) | 0.214 |
| Diabetes | 30 (50%) | 38 (57%) | 0.631 |
| Hyperlipidemia | 35 (59%) | 41 (61%) | 0.975 |
| Previous cerebral vascular accident | 4 (7%) | 9 (13%) | 0.352 |
| Chronic kidney disease(Stage 4 and 5) | 14 (24%) | 19 (29%) | 0.699 |
| Previous myocardial infarction | 6 (10%) | 11 (16%) | 0.445 |
Data are expressed as mean ± SD or n (%). TF: transfemoral; TR: transradial.
3.2. Angiographic and procedural characteristics
Table 2 lists the angiographic and procedural characteristics in the TR and TF groups in this study. All of the 126 lesions attempted with RA were de novo lesions. In both groups, all of the lesions were ACC/AHA grade C lesions. The mean reference vessel size, the lesion length, and the mean diameter stenosis of the target lesions measured at baseline and after procedure by QCA were similar in both groups. Significantly smaller guide catheters were used in the radial compared with femoral group (P < 0.001). In the radial group, six French guide catheters were used in 59% of the cases, 7 French were used in 31% of the cases, and 8 French were used in 10% of the cases. The most commonly used guide catheter size was 7 French in femoral group; then, 8 French in 12% of the cases; and 6 French in 10%.
Table 2. Angiographic and procedural characteristics.
| Vascular access | TR (n = 59) | TF (n = 67) | P-value |
| Baseline QCA | |||
| Reference vessel diameter, mm | 2.88 ± 0.40 | 2.91 ± 0.45 | 0.704 |
| Lesion length, mm | 21.8 ± 10.0 | 21.4 ± 10.1 | 0.787 |
| Minimal luminal diameter, mm | 0.56 ± 0.25 | 0.56 ± 0.17 | 0.861 |
| Diameter stenosis, % | 80.5 ± 5.78 | 80.8 ± 5.93 | 0.810 |
| Post-procedural QCA | |||
| Reference vessel diameter, mm | 2.91 ± 0.42 | 3.00 ± 0.42 | 0.261 |
| Minimal luminal diameter, mm | 2.68 ± 0.40 | 2.68 ± 0.47 | 0.961 |
| Diameter stenosis, % | 8.02 ± 5.25 | 10.2 ± 6.63 | 0.073 |
| Guide catheter diameter (Fr) | |||
| 6 Fr | 35 (59%) | 7 (10%) | < 0.001 |
| 7 Fr | 18 (31%) | 52 (78%) | < 0.001 |
| 8 Fr | 6 (10%) | 8 (12%) | 0.975 |
| Number of burr used | 1.25 ± 0.57 | 1.39 ± 0.57 | 0.235 |
| 1 burr | 47 (80%) | 40 (60%) | 0.026 |
| 2 burrs | 8 (14%) | 25 (37%) | 0.005 |
| 3 burrs | 4 (6%) | 2 (3%) | 0.562 |
| Largest size of burr use | |||
| 1.25 mm | 30 (51%) | 26 (39%) | 0.240 |
| 1.5 mm | 23 (39%) | 13 (19%) | 0.026 |
| 1.75 mm | 6 (10%) | 26 (39%) | < 0.001 |
| 2.0 mm | 0 (0%) | 2 (3%) | 0.533 |
| Mean of burr size, mm | 1.39 ± 0.16 | 1.53 ± 0.24 | 0.001 |
| Stent implantation | 53 (90%) | 60 (90%) | 0.809 |
| Bare metal stent | 2 (4%) | 5 (8%) | 0.544 |
| Drug-eluting stent | 51 (96%) | 55 (92%) | 0.673 |
| Stent diameter, mm | 3.0 ± 0.5 | 3.1 ± 0.3 | 0.812 |
| Stent length, mm | 24 ± 19 | 26 ± 21 | 0.789 |
| Procedural success rate | 57 (97%) | 66 (99%) | 0.911 |
Data are expressed as mean ± SD or n (%). TF: transfemoral; TR: transradial; QCA: quantitative angiographic analysis.
The mean numbers of the burr used were not significantly different in both groups. However, significantly less patients in the radial group than those in the femoral group (14% vs. 37%, P = 0.005) needed two burrs for a successful RA; meanwhile, mean burr size was significantly smaller in the radial group (1.39 ± 0.16 mm vs. 1.53 ± 0.24 mm, P = 0.001). As to the size of burr used, 1.25 mm and 1.5 mm burrs were more frequently used in TR group (51% and 39%, respectively) and 1.25 mm and 1.75 mm burrs, in TF (39% and 39%, respectively); that is, more burrs of less than 1.5 mm were used in TR group.
The use of stents following RA was high in both radial and femoral groups (90% vs. 90%, P = 0.809), most commonly involving DES (96% vs. 92%, P = 0.673). Both stent diameter and the total stent length were well matched in both groups.
Procedural complications included abrupt vessel closure (1 radial, 3 femoral), slow flow phenomenon (2 radial, 2 femoral), transient bradycardia or heart block (2 radial, 3 femoral), rota-wire disruption (0 radial, 1 femoral), and lodged burr (0 radial, 2 femoral). They were all successfully managed without the need of emergency surgery. There were no procedural deaths. Procedural success rate was similar in both radial and femoral cases (97% vs. 99%, P = 0.911).
3.3. In-hospital outcomes
In-hospital outcomes are displayed on Table 3. Mean hospital stay of the TR group was 5.1 ± 16.5 days and that of the TF group, 9.2 ± 20.6 days (P = 0.671). One (2%) patient in the TR group and two (3%) in the TF group suffered acute stent thrombosis requiring repeat PCI (P = 0.911). The three patients, having suffered from acute stent thrombosis, were presented with post-procedural MI. One of the two patients with acute stent thrombosis in TF group died of pneumonia with sepsis during hospitalization. The incidence rate of major vascular access site bleeding or hematoma needing transfusion was significantly lower in the TR group than that in TF (2% vs. 16%, P = 0.012). Ten patients in the TR group and 12 in TF (17% vs. 18%, P = 0.926) developed post-procedural myonecrosis.
Table 3. In-hospital and long-term outcomes.
| Vascular access | TR (n = 59) | TF (n = 67) | P value |
| Mean hospital stay (days) | 5.1 ± 16.5 | 9.2 ± 20.6 | 0.671 |
| In-hospital outcomes | |||
| Non-cardiac death | 0 (0%) | 1 (1%) | 0.883 |
| Acute stent thrombosis | 1 (2%) | 2 (3%) | 0.911 |
| Post-procedural myocardial infarction | 1 (2%) | 2 (3%) | 0.911 |
| Post-procedural myonecrosis | 10 (17%) | 12 (18%) | 0.926 |
| Major access site bleeding | 1 (2%) | 11 (16%) | 0.012 |
| Long-term outcomes | |||
| Non-cardiac death | 3 (5%) | 3 (4%) | 0.795 |
| Non-fatal myocardial infarction | 1 (2%) | 2 (3%) | 0.911 |
| Non-fatal stroke | 0 (0%) | 3 (4%) | 0.289 |
| Target vessel revascularization | 6 (10%) | 8 (12%) | 0.975 |
Data are expressed as mean ± SD or n (%). TF: transfemoral; TR: transradial.
3.4. Long-term clinic outcomes
Table 3 also lists long-term clinic outcomes following RA. After the two cases with acute stent thrombosis and MI, and one with mortality during hospitalization had been excluded, 58 patients in the TR group and 65 in TF were evaluated. During a median follow-up of 29 months (IQR 30 months), there were no cardiac death in both study groups. However, three patients (5%) in the TR group and three in TF (4%) died of non-cardiac causes (P = 0.795). One patient (2%) in the TR group and 2 (3%) in TF suffered from recurrent non-fatal MI (P = 0.911). There was no non-fatal stroke in the TR group during follow-up but 3 (4%), in the TF group (P = 0.289). Six patients (10%) in the TR group and 8 (12%) in the TF group received TVR by either percutaneous or surgical intervention (P = 0.975). Although a trend of lower rate of total MACE was demonstrated in the TR group, there was no statistical significance (21% vs. 27%, P = 0.135). The Kaplan-Meier survival curve is showed in Figure 1.
Figure 1. The Kaplan-Meier survival curve of total MACE in the transradial group versus transfemoral group.
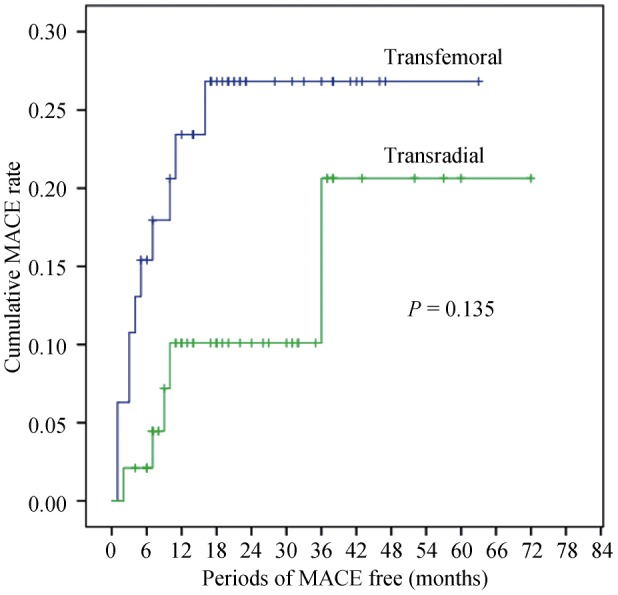
Log-rank test, P = 0.135. MACE: major adverse cardiovascular events.
4. Discussion
In the present study, we have clearly demonstrated that radial access is a feasible, safe, and effective way to perform RA for the treatment of heavily calcified coronary lesions. The inability to use larger burr sizes during TR RA does not compromise procedural success, which is comparable with a femoral approach. Moreover, radial access for RA, compared with femoral access, is associated with a significantly lower access site complication rate. Furthermore, even though it may not improve long-term outcomes, vessel modification with RA via radial approach followed by DES stenting can still be a therapeutic option in patients with heavily calcified lesions to improve immediate success.
For better stent appositions to improve clinical outcomes, debulking may be needed in heavily calcified coronary lesions prior to stent implantation. The rotablator is uniquely suited to these indications.[1],[2] Actually, rotablator is the only existing debulking device nowadays for lesion preparation prior to stent implantation.[1],[2] However, due to the complexity of the technique and the high restenosis rate of subsequent bare metal stenting in long calcified lesion, its use had declined for a time; in recent years, with the increasing use of DES and the aggressive treatment of long calcified lesions, the numbers of RA procedure performed have increased again significantly.[2],[12] DES reduces the risk of restenosis and represents an important advance in coronary intervention. Newer-generation DES with thin struts releasing limus-family drugs from durable or biodegradable polymers, compared with early-generation DES releasing sirolimus or paclitaxel, has further improved clinical outcomes; the risk of stent thrombosis becomes exceedingly low. In other words, the improved safety profile of newer DES comes without compromising their effectiveness. Therefore, DES is recommended in most clinical settings unless patients have showed contraindications to the use of dual antiplatelet therapy.[17] In this regard, a strategy combining the RA technique and DES implantation is judged to be a safe and effective treatment option for patients with complex lesions, including the heavily calcified lesions needing RA.[2],[5]–[12] Moreover, the resurgence of RA in the DES era also reflects the difficulties arising from the fact that older patients with more complex and calcified lesions require to be treated with contemporary clinical practice. Since the proportion of patients with coronary calcified lesions expects to increase as the population ages, RA with DES is thus considered an essential technique and made available in all catheterization laboratories.
Traditionally, femoral access has been the preferred approach for RA because of the requirement for larger calibre guide catheters. Since mounting evidences support its benefits, such as, reduction in bleeding complications and mortality, many interventional cardiologists worldwide are driven to predominant use of the radial access approach and to adopt the idea that radial access becomes the default strategy in most cases.[18]–[20] Previous trials have also suggested that radial access for RA may be an useful alternative to femoral access for RA, which is associated with excellent procedural success and a low rate of complications, allowing the range of devices available to the TR operators to be expanded.[13]–[15] Radial access for RA may have other advantages over femoral access, e.g., the radial route is particularly useful in patients with peripheral artery disease or in others with a high risk of femoral access site complications, such as, elderly or obese patients.[15] Other studies have shown that TR PCI is associated with early ambulation and a reduced length of hospital stay compared with TF access.[21] Therefore, a TR approach may facilitate RA procedures for selected patients. However, the long-term data regarding efficacy and safety of DES combined with RA via radial access in patients with calcified coronary lesions were limited. This is partly because DES, especially the newer-generation DES, has only become widely available worldwide since 2007. Moreover, calcified coronary lesions are a well-known risk factors of short- and long-term poor outcomes, not only for BMS but for DES also.[22]–[26] Hence, the reappraisal of using TR RA in combination of DES in the treatment of heavily calcified coronary lesions had not been cared much by interventional cardiologists until the publications of its favorable mid- and long-term results appeared in 2007.[5],[6]
In the present study, about 70% RA procedures were accomplished transradially by experienced TR operators. There is no indication from our data to suggest that patients in the radial group had less severe disease or lesion severity than those in the femoral group. According to the recommendations of the manufacturer, a burr size of 1.25 mm, 1.5–1.75 mm, 2.0 mm, and > 2.0 mm is compatible with a 5 Fr, 6 Fr, 7 Fr, or 8 Fr guide catheter, respectively. Moreover, the use of 7 Fr and 8 Fr guiding catheters may be feasible for TR PCI in as many as 71.5% and 44.9% of the male patients, and 40.3% and 24.0% of the female patients.[27] In fact, we have found that 7Fr sheath and guiding catheters were compatible with the radial artery diameter in about 70% of the cases and 8Fr in 13% at our institution. Further, there has been a decreasing need for large burr sizes as RA has evolved to become a prelude to stent implantation.[1],[2] According to our findings, 80% of patients in the TR group and 60% of patients in the TF group needed only one burr for RA; the largest size of burr used were < 1.75 mm in 90% of the TR cases and 58% in TF; and all but two patients needed a burr size of 2 mm. That is, although smaller burrs were used in the radial group, more often than not, that is all required to satisfactorily debulk a lesion to enable successful stent delivery and deployment; therefore, radial access proffers itself as an attractive option for interventional cardiologists, and TR RA should theoretically be performed in even greater numbers of patients. But why has the TR RA not been more widely used so far? One of the possible reasons is that TR RA is a demanding technique, which requires much training and experience to perform.[2],[18],[28]–[30] Secondly, a concern for procedure-related and device specific complications exists.[28],[29] Accordingly, many operators are reluctant to use TR RA more extensively. This selection bias may prevent operators from getting the experiences to use the radial access for RA effectively, and exercise a negative impact on the procedure outcomes.[28],[29] It is believed that proctorships and training courses should improve the results and acceptance of TR RA in the future.[30] Technologic progress of materials and increased operators' experiences to obtain predictable results from improved technique may enable operators to proceed with RA via radial approach for cases having previously led to a conversion to femoral access. The other possible solution to this problem will be to maintain referral centers of excellence for RA of complex calcified coronary lesions.[2]
The present study shows a significant number of patients, even treated by the three TR operators, requiring further treatment by or crossed over to the TF approach. In our institution, we avoided performing TR percutaneous coronary intervention on those patients with a feeble or absent radial pulsation, an abnormal Allen's test, a small sized radial artery which precluded the use of a 6F introducer, or an abnormality of artery which causes coronary cannulation failure. If the patient was under chronic hemodialysis or had severe (stages 4 and 5) chronic kidney disease and considered a candidate for future hemodialysis, we avoided the TR approach too, so as to preserve the radial artery for future radiocephalic arteriovenous fistula. Older age, female gender, hemodynamic instability, and the presence of severe chronic kidney disease or end-stage renal disease were significantly related to the choosing of TF approach.[31] In the present study, male gender was significantly related to the use of the radial approach, and those who underwent TF RA tended to be older; that is, to some extent, the operators' decisions reflect the real-world practice of physician preference in selecting the TF RA approach for patients because of the difficulties in obtaining vascular access due to the smaller size of the radial artery in female patients and in manipulating guiding catheters in the tortuous brachio-subclavian arteries in the elderly. Among the 18 of 62 (29%) patients underwent TF RA by the three TR operators, the femoral approach was used in two patients with poor or absent radial pulse, and six patients were under chronic hemodialysis or had severe chronic kidney disease, thus considered candidates for future hemodialysis. For the five patients with hemodynamic instability that required a second vascular access site for transvenous temporary pacing or intra-aortic balloon pumping, operators also chose the TF approach in the first place. Three patients had radial attempt that failed due to the need of using a larger burr and two others with marked tortuosities of brachio-subclabvian artery that caused 7 Fr/8 Fr guide catheter for coronary cannulation failure, were switched to the femoral approach. Evidently choosing an appropriate route for RA is not a matter concerning the establishment of a predetermined choice but the selection of the most suitable arterial access, when the genuine needs of the patients are taken into consideration. If the operator of one particular patient considers not that TR and TF interventions are equally feasible, whichever arterial access the operator considers safer and more effective for the patient should be applied.
The main limitation of the present study is its retrospective nature. Smaller differences and confounders may exist between two groups, and may affect the success of either approach if examined in a prospective randomized manner. Certain degrees of the operators' biases based on their experiences with TR or TF procedures cannot be eliminated thoroughly either. Secondly, the small poll of the study has certainly reduced the power to detect significant differences. Thirdly, the absence of angiographic follow-up may underestimate the event rates. Nevertheless, there were no adverse trends in the radial group. Due to economic considerations furthermore, we have seldom used closure devices after the TF approach at our institution for they are not reimbursed by the health insurance system in Taiwan; otherwise, previous reports have clearly stated that it can significantly reduce major access bleeding complications.[32] Finally, it is worth-noting that the present study was performed by those operators having substantial TR RA experiences, so no major technical limitations would pose problem in their PCI via the TR route; in other words, the benefits of this technique may be less apparent with inexperienced operators.
In conclusion, we have demonstrated that radial access is a useful alternative to femoral access for RA and DES. This approach is associated with excellent procedural success, a lower rate of vascular access complications, and comparable long-term outcomes compared to femoral approach. If increasing numbers of operators are able to obtain predictable results from improved technique, if more favorable data are obtained from randomized trials, if the RA equipment becomes more user-friendly, and if its cost becomes more competitive, then TR RA in combination with DES deserves to be established as a major tool in the treatment of heavily calcified coronary lesions.
References
- 1.Camnitz WM, Keeley EC. Heavily Calcified Coronary Arteries: The Bane of an Interventionalist's Existence. J Interv Cardiol. 2010;23:254–255. doi: 10.1111/j.1540-8183.2010.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv. 2014;7:345–353. doi: 10.1016/j.jcin.2013.12.196. [DOI] [PubMed] [Google Scholar]
- 3.Mauri L, Reisman M, Buchbinder M, et al. Comparison of rotational atherectomy with conventional balloon angioplasty in the prevention of restenosis of small coronary arteries: results of the Dilatation vs Ablation Revascularization Trial Targeting Restenosis (DART) Am Heart J. 2003;145:847–854. doi: 10.1016/S0002-8703(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 4.Moussa I, Di Mario C, Moses J, et al. Coronary stenting after rotational atherectomy in calcified and complex lesions. Angiographic and clinical follow-up results. Circulation. 1997;96:128–136. doi: 10.1161/01.cir.96.1.128. [DOI] [PubMed] [Google Scholar]
- 5.Schlüter M, Cosgrave J, Tübler T, et al. Rotational atherectomy to enable sirolimus-eluting stent implantation in calcified, nondilatable de novo coronary artery lesions: Mid-term clinical and angiographic outcomes. Vasc Dis Manag. 2007;4:63–69. [Google Scholar]
- 6.Khattab AA, Otto A, Hochadel M, et al. Drug-eluting stents versus bare metal stents following rotational atherectomy for heavily calcified coronary lesions: Late angiographic and clinical follow-up results. J Interv Cardiol. 2007;20:100–106. doi: 10.1111/j.1540-8183.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 7.Furuichi S, Sangiorgi GM, Godino C, et al. Rotational atherectomy followed by drug-eluting stent implantation in calcified coronary lesions. EuroIntervention. 2009;5:370–374. doi: 10.4244/v5i3a58. [DOI] [PubMed] [Google Scholar]
- 8.García de Lara J, Pinar E, Ramón Gimeno J, et al. Percutaneous coronary intervention in heavily calcified lesions using rotational atherectomy and paclitaxel-eluting stents: outcomes at one year. Rev Esp Cardiol. 2010;63:107–110. doi: 10.1016/s1885-5857(10)70016-5. [DOI] [PubMed] [Google Scholar]
- 9.Mezilis N, Dardas P, Ninios V, et al. Rotablation in the drug eluting era: immediate and long-term results from a single center experience. J Interv Cardiol. 2010;23:249–253. doi: 10.1111/j.1540-8183.2010.00542.x. [DOI] [PubMed] [Google Scholar]
- 10.Benezet J, Díaz de la Llera LS, Cubero JM, et al. Drug-eluting stents following rotational atherectomy for heavily calcified coronary lesions: long-term clinical outcomes. J Invasive Cardiol. 2011;23:28–32. [PubMed] [Google Scholar]
- 11.Dardas P, Mezilis N, Ninios V, et al. The use of rotational atherectomy and drug-eluting stents in the treatment of heavily calcified coronary lesions. Hellenic J Cardiol. 2011;52:399–406. [PubMed] [Google Scholar]
- 12.Chen CC, Hsieh IC. Application of rotational atherectomy in the drug-eluting stent era. J Geriatr Cardiol. 2013;10:213–216. doi: 10.3969/j.issn.1671-5411.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gioia G, Comito C, Moreyra AE. Coronary rotational atherectomy via transradial approach: A study using radial artery intravascular ultrasound. Catheter Cardiovasc Interv. 2000;51:234–238. doi: 10.1002/1522-726x(200010)51:2<234::aid-ccd22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Egred M, Andron M, Alahmar A, et al. High-speed rotational atherectomy during transradial percutaneous coronary intervention. J Invasive Cardiol. 2008;20:219–221. [PubMed] [Google Scholar]
- 15.Watt J, Oldroyd KG. Radial versus femoral approach for high-speed rotational atherectomy. Catheter Cardiovasc Interv. 2009;74:550–554. doi: 10.1002/ccd.22066. [DOI] [PubMed] [Google Scholar]
- 16.Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease: a statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–65. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- 17.Stefanini GG, Holmes DR., Jr Drug-eluting coronary-artery stent. N Engl J Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Yoon J. Transradial approach as a default route in coronary artery interventions. Korean Circ J. 2011;41:1–8. doi: 10.4070/kcj.2011.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan S, Rao SV. Radial versus femoral access for percutaneous coronary intervention: implications for vascular complications and bleeding. Curr Cardiol Rep. 2012;14:502–509. doi: 10.1007/s11886-012-0287-5. [DOI] [PubMed] [Google Scholar]
- 20.Dangoisse V, Guédès A, Gabriel L, et al. Full conversion from transfemoral to transradial approach for percutaneous coronary interventions results in a similar success rate and a rapid reduction of in-hospital cardiac and vascular major events. EuroIntervention. 2013;9:345–352. doi: 10.4244/EIJV9I3A56. [DOI] [PubMed] [Google Scholar]
- 21.Small A, Klinke P, Della Siega A, et al. Day procedure intervention is safe and complication free in higher risk patients undergoing transradial angioplasty and stenting. The discharge study. Catheter Cardiovasc Interv. 2007;70:907–912. doi: 10.1002/ccd.21277. [DOI] [PubMed] [Google Scholar]
- 22.Tan K, Sulke N, Taub N, et al. Clinical and lesion morphologic determinants of coronary angioplasty success and complications: current experience. J Am Coll Cardiol. 1995;25:855–865. doi: 10.1016/0735-1097(94)00462-Y. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann R, Mintz GS, Popma JJ, et al. Treatment of calcified coronary lesions with Palmaz-Schatz stents. An intravascular ultrasound study. Eur Heart J. 1998;19:1224–1231. doi: 10.1053/euhj.1998.1028. [DOI] [PubMed] [Google Scholar]
- 24.Moussa I, Ellis SG, Jones M, et al. Impact of coronary culprit lesion calcium in patients undergoing paclitaxel-eluting stent implantation (a TAXUS-IV sub study) Am J Cardiol. 2005;96:1242–1247. doi: 10.1016/j.amjcard.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Berenguer A, Mainar V, Bordes P, et al. Incidence and predictors of restenosis after sirolimus-eluting stent implantation in high-risk patients. Am Heart J. 2005;150:536–542. doi: 10.1016/j.ahj.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Migliorini A, Shehu M, Carrabba N, et al. Predictors of outcome after sirolimus-eluting stent implantation for complex in-stent restenosis. Am J Cardiol. 2005;96:1110–1112. doi: 10.1016/j.amjcard.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Ikei H, Hosokawa G, et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 1999;46:173–178. doi: 10.1002/(SICI)1522-726X(199902)46:2<173::AID-CCD12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Whitlow PL. Is rotational atherectomy here to stay? Heart. 1997;78(Suppl. 2):35–36. doi: 10.1136/hrt.78.suppl_2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsdale DR, Morris JL. If rotablator is useful, why don't we use it? Heart. 1997;78(Suppl. 2):S36–S37. doi: 10.1136/hrt.78.suppl_2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonardi RA, Townsend JC, Bonnema DD, et al. Comparison of percutaneous coronary intervention safety before and during the establishment of a transradial program at a teaching hospital. Am J Cardiol. 2012;109:1154–1159. doi: 10.1016/j.amjcard.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jen HL, Yin WH, Chen KC, et al. Transradial approach in myocardial infarction. Acta Cardiol. 2011;66:239–245. doi: 10.1080/ac.66.2.2071257. [DOI] [PubMed] [Google Scholar]
- 32.Sheth RA, Walker TG, Saad WE, et al. Quality improvement guidelines for vascular access and closure device use. J Vasc Interv Radiol. 2014;25:73–84. doi: 10.1016/j.jvir.2013.08.011. [DOI] [PubMed] [Google Scholar]


