Abstract
Objective
To find out whether dexamethasone induces an uncoupling of the endothelial nitric oxide synthase (eNOS).
Methods & Results
A major cause of eNOS uncoupling is a deficiency of its cofactor tetrahydrobiopterin (BH4). Treatment of human EA.hy 926 endothelial cells with dexamethasone decreased mRNA and protein expression of both BH4-synthesizing enzymes: GTP cyclohydrolase I and dihydrofolate reductase. Consistently, a concentration- and time-dependent reduction of BH4, dihydrobiopterin (BH2) as well as BH4: BH2 ratio was observed in dexamethasone-treated cells. Surprisingly, no evidence for eNOS uncoupling was found. We then analyzed the expression and phosphorylation of the eNOS enzyme. Dexamethasone treatment led to a down-regulation of eNOS protein and a reduction of eNOS phosphorylation at serine 1177. A reduction of eNOS expression may lead to a relatively normal BH4: eNOS molar ratio in dexamethasone-treated cells. Because the BH4-eNOS stoichiometry rather than the absolute BH4 amount is the key determinant of eNOS functionality (i.e., coupled or uncoupled), the down-regulation of eNOS may represent an explanation for the absence of eNOS uncoupling. Phosphorylation of eNOS at serine 1177 is needed for both the NO-producing activity of the coupled eNOS and the superoxide-producing activity of the uncoupled eNOS. Thus, a reduction of serine 1177 phosphorylation may render a potentially uncoupled eNOS hardly detectable.
Conclusions
Although dexamethasone reduces BH4 levels in endothelial cells, eNOS uncoupling is not evident. The reduction of NO production in dexamethasone-treated endothelial cells is mainly attributable to reduced eNOS expression and decreased eNOS phosphorylation at serine 1177.
Keywords: Dexamethasone, Endothelial cells, eNOS uncoupling, Nitric oxide synthase, Reactive oxygen species, Tetrahydrobiopterin
1. Introduction
Glucocorticoids are steroid hormones that are synthesized in the adrenal cortex. Glucocorticoids play an essential role in physiological processes like immune response, energy metabolism, electrolyte balance, and stress response. Chronic excessive activation of glucocorticoid receptor induces obesity, insulin resistance, glucose intolerance, dyslipidemia and hypertension.[1] Abnormalities of the hypothalamic-pituitary-adrenal axis and/or of tissue sensitivity to glucocorticoids are also associated with these cardiovascular risk factors in patients with the metabolic syndrome. Furthermore, glucocorticoids have direct effect on heart and blood vessels, mediated by both glucocorticoid and mineralocorticoid receptors.[2],[3] These effects influence vascular function, atherogenesis and vascular remodeling following intra-vascular injury or ischemia.
Synthetic glucocorticoids, such as dexamethasone (Dex), play a fundamental role in the treatment of inflammatory disease. One of the side effects of Dex treatment is an elevation of blood pressure. Our group has previously demonstrated that a major mechanism underlying the Dex-induced hypertension is a reduced nitric oxide (NO) production from the endothelium.[4]
Under physiological conditions, vascular NO is mainly produced by endothelial nitric oxide synthase (eNOS).[5] Endothelial NO relaxes blood vessels and reduces blood pressure. It diffuses from endothelial cells into the underlying smooth muscle cells and induces vasodilation by stimulating NO-sensitive guanylyl cyclase. NO produced by the endothelial eNOS also diffuses into the blood and inhibits platelet aggregation and adhesion. In addition to these antihypertensive and antithrombotic actions, eNOS-derived NO also possesses multiple anti-atherosclerotic properties, including prevention of leukocyte adhesion to vascular endothelium and leukocyte migration into the vascular wall; inhibition of low-density lipoprotein oxidation; and inhibition of vascular smooth muscle cell proliferation.[6],[7] Genetic depletion of eNOS elevates blood pressure,[8] and exacerbates diet-induced atherosclerosis in mouse models.[9],[10]
We have shown that Dex reduce eNOS mRNA and protein expression in human endothelial cells by transcriptional inhibition as well as mRNA destabilization.[4] The observed down-regulation of eNOS could be also demonstrated in vivo. Oral Dex treatment diminished eNOS mRNA level in several endothelium-rich organs in rats. Furthermore, plasma levels of NO2−/NO3− (NO oxidation products) were reduced as well as endothelium-dependent vasodilation. In addition, a significant increase in blood pressure could be observed in Dex-treated animals.[4] All these results suggest that Dex-induced blood pressure elevation is attributable to a reduced NO production by the eNOS enzyme. Consistent with this concept, our group further demonstrated that glucocorticoids had no effect on the blood pressure in eNOS−/− mice.[11]
Recent studies have shown that eNOS can be converted from a NO-producing enzyme to a molecule that generates superoxide under pathological conditions. This is referred to as eNOS uncoupling.[12],[13] Uncoupling of eNOS is known to be the major cause of endothelial dysfunction in a number of cardiovascular diseases, including hypertension, atherosclerosis, and diabetes mellitus.[12],[13]
Therefore, we conducted the present study to find out whether Dex treatment also causes eNOS uncoupling.
2. Methods
2.1. Cell culture
Human EA.hy 926 endothelial cells were grown under 10 % CO2 in Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin and 1× HAT (hypaxanthine, aminopterin and thymidine) (Invitrogen).[14] Cells were treated with dexamethasone or its solvent dimethyl sulfoxide (DMSO) as control.
2.2. Real-time PCR for mRNA expression analyses
Gene expression at mRNA level was analyzed with quantitative real-time PCR (qPCR) using an iCycler IQ™ System (Bio-Rad Laboratories). Total RNA was isolated from cultured endothelial cells using peqGOLD TriFast (PEQLAB Biotechnologie GmbH). cDNA was generated using High-Capacity cDNA Reverse Transcription Kit (Life Technologies) and 40 ng of cDNA was used for qPCR analysis. The mRNA expression levels of the target genes were normalized to TATA box binding protein (TBP) mRNA.[14] The qPCR primer sequences were as follows: eNOS_forward: 5′-CAC CAG GAA GAA GAC CTT TAA AGA-3′, eNOS_reverse: 5′-TCA CTC GCT TCG CCA TCA C-3′; GCH1_forward: 5′-TCA GTG TGT GTC TGC AGA ACC A-3′, GCH1_reverse: 5′-TGT CTT CCA CCG TCA GTT CAT T-3′; DHFR_forward: 5′-TCC TCC CGC TGC TGT CA-3′, DHFR_reverse: 5′-GCC GAT GCC CAT GTT CTG-3′; TBP_forward: 5′-GCC GCC GGC TGT TTA ACT-3′, TBP_reverse: 5′-ACG CCA AGA AAC AGT GAT GCT-3′.
2.3. Western blotting for protein analyses
Confluent EA.hy 926 cells were incubated with dexamethasone and then total protein was isolated. Western blotting was performed using 30 µg of protein sample and antibodies against dihydrofloate reductase (DHFR, BD Biosciences), GTP cyclohydrolase I (GCH1, Abnova), eNOS (BD Biosciences), eNOS phospho-serine1177 (Cell Signaling) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Santa Cruz Biotechnology). Protein samples were separated on 10% SDS polyacrylamide gels and transferred onto nitrocellulose membranes. Blots were blocked for 1 h at room temperature with 5% powdered milk in TBS (10 mmo/L Tris HCl, pH 7.4, 150 mmo/L NaCl) with 0.1% Tween 20 and the incubated with the primary antibodies in 5 % powdered milk in TBS with 0.1% tween 20 overnight at 4°C. Blots were washed three times in TBS/Tween 20 (0.1 %) and then incubated with a horseradish peroxidase conjugated secondary antibody in 5% powdered milk and 0.1% Tween 20 in TBS for 1 h at room temperature.[15] After washing, immune complexes were developed using an enhanced horseradish peroxidase/luminol chemiluminescence reagent (PerkinElmer Life Science) according to the manufacturer's instructions.
2.4. Detection of biopterins with HPLC
EA.hy 926 cells were plated in 6-well plates and treated with Dex. After treatment, the cells were washed twice with ice-cold PBS+ (50 mL PBS + 50 µL 1,4-dithioerythritol + 10 µL Pefabloc®). Cells were scraped off the wells with ice-cold PBS++ (PBS + 5 nmol/L Neopterin) and identical samples were pooled. HPLC analyses were performed with phosphate buffer (50 mmol/L, pH 3.0) as eluent in appropriate columns (125/4 Nucleosil SA 100-5 mm, Macherey-Nagel) at 25°C and the flow rate of 1.2 mL/min. Equipment from Bischoff was used as well as the Hitachi fluorescence detector 8470 (excitation: 350 nm, emission: 450 nm). Evaluation was done with the help of MCDAcq Integrator software from Bischoff.[16],[17]
2.5. Detection of reactive oxygen species (ROS)
ROS levels were measured with H2DCFDA fluorescence,[18] and fluorescence-activated cell sorting (FACS) analyses.[19]
For H2DCFDA fluorescence, cells were washed with Hanks balanced salt solution (HBSS) followed by a 30 min incubation step with the H2DCFDA (2.5 µmol/L) at 37°C. Cells were again washed with HBSS (37°C) and covered with pure HBSS or HBSS in combination with a substance like L-NAME for the measurement, depending on the experiment. ROS development was monitored up to 12 h within the plate reader (excitation: 485 nm, emission: 530 nm).[18]
For FACS analyses, the fluorescent dye H2DCFDA was used again. EA.hy 926 cells were seeded into 24-well plates to 80% confluence before treatment. After incubation with an experiment-specific substance, cells were washed with PBS. Subsequently, cells were incubated with H2DCFDA (2.5 µmol/L) in HBSS for 30 min at 37°C in the dark. Afterwards, cells were washed again with PBS and trypsinized. Medium was added to stop the enzymatic reaction and cells were collected in a 1.5 mL tube. After a 4-min centrifugation at 350 r/min and 4°C, the supernatant was discarded. The cell pellet was washed with cold PBS and centrifuged a second time. The cell pellet was resuspended in 300 µL PBS and transferred to a FACS-tube for analysis using the FACSCalibur™ flow cytometer (BD Biosciences).[19]
2.6. RFL-6 reporter cell assay for NO measurement
NO production by EA.hy 926 cells was bioassayed with RFL-6 rat lung fibroblasts as reporter cells as previously described.[20],[21] EA.hy 926 cells were grown to confluence and then incubated with Dex for 24 h or 48 h. For determination of NO production, EA.hy cells and confluent RFL-6 cells were washed twice with Locke's solution. Then EA.hy cells were incubated with Locke's solution containing 200 U/mL SOD and 100 µmol/L L-arginine, and RFL-6 cells with Locke's solution containing 0.6 mmol/L IBMX for 30 min. After the pre-incubation, EA.hy cells were incubated for 2 min at 37°C in 1 mL of Locke's solution containing 200 U/mL SOD, 0.3 mmol/L IBMX, 100 µmol/L L-arginine, and 10 µmol/L calcium ionophore A23187. Then the conditioned media containing the NO released from the EA.hy cells were transferred to the RFL-6 cells, and another incubation of 2 min at 37°C was performed. The reaction was stopped by aspiration of the solution, adding 1 mL of ice-cold 50 mmol/L sodium acetate, pH 4.0, and rapidly freezing the cells with liquid nitrogen. The cGMP content of the RFL-6 samples was determined by radioimmunoassay.[20],[21]
2.7. Statistics
Results are expressed as mean ± SE. Statistical differences between mean values were determined by analysis of variance (ANOVA) followed by Dunnett's test for multiple comparisons to a single control group. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Dexamethasone down-regulates the expression of GCH1 and DHFR
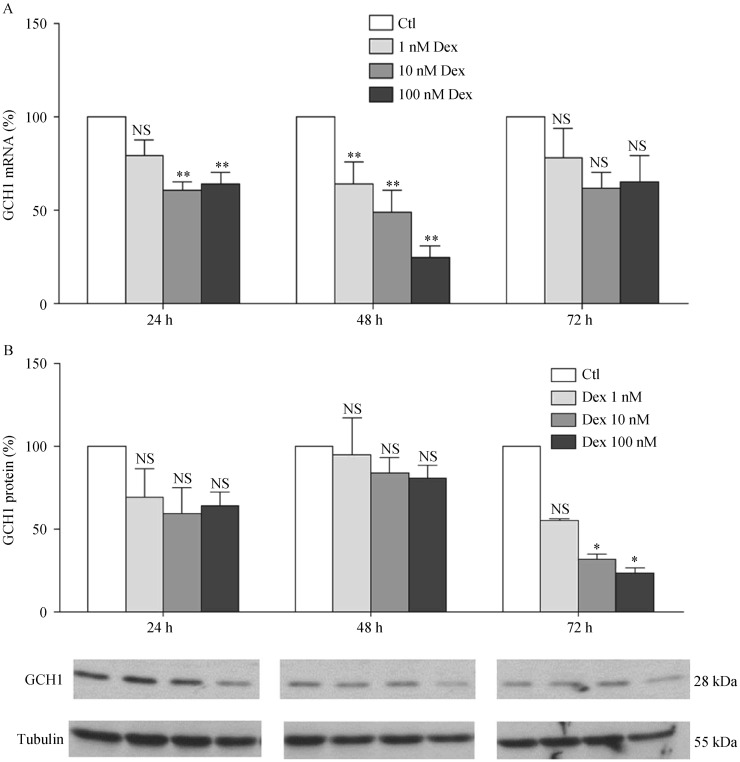
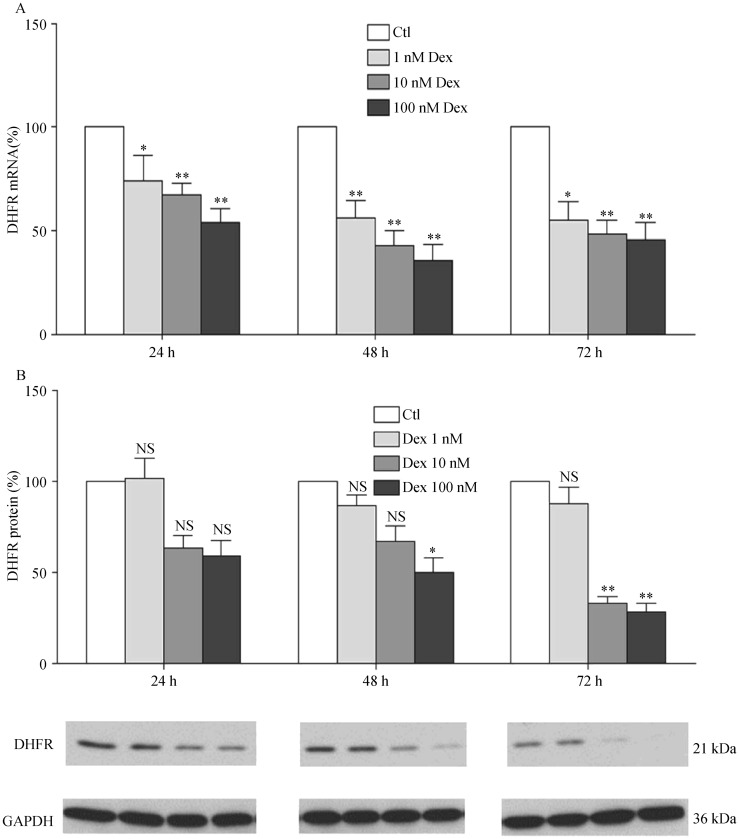
As the rate-limiting enzyme of the de novo pathway in the biosynthesis of BH4, GCH1 has a crucial function. Another important enzyme is DHFR, which can regenerate BH4 from BH2. To measure the impact of Dex on these two enzymes, human EA.hy 926 endothelial cells were treated with different concentrations of Dex (1, 10 or 100 nmol/L) for time periods up to 72 h. qPCR analyses showed a concentration- and time-dependent reduction of GCH1 mRNA expression in response to Dex treatment (Figure 1). GCH1 protein expression was found down-regulated after 72 h Dex treatment (Figure 1). Dex treatment also led to a down-regulation of DHFR expression at both mRNA and protein levels (Figure 2).
Figure 1. Dexamethasone down-regulates GCH1 expression.
(A): Human EA.hy 926 endothelial cells were treated with either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24, 48 or 72 h. mRNA expression of GCH1 was analyzed with qPCR; (B & C): protein expression was studied with Western blot analyses. β-tubulin was shown for normalization. Columns represent mean ± SE, n = 12–24. *P < 0.05, **P < 0.01 compared with Ctl. Ctl: control; Dex: dexamethasone; GCH1: GTP cyclohydrolase I; NS: not significant.
Figure 2. Dexamethasone down-regulates DHFR expression.
(A): EA.hy 926 endothelial cells were treated with either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24, 48 or 72 h. mRNA expression of DHFR was analyzed with qPCR; (B & C): protein expression was studied with Western blot analyses. GAPDH was shown for normalization. Columns represent mean ± SE, n = 12–24. *P < 0.05, **P < 0.01 compared with Ctl. Ctl: control; Dex: dexamethasone; DHFR: dihydrofolate reductase; NS: not significant.
3.2. Dexamethasone reduces tetrahydrobiopterin content
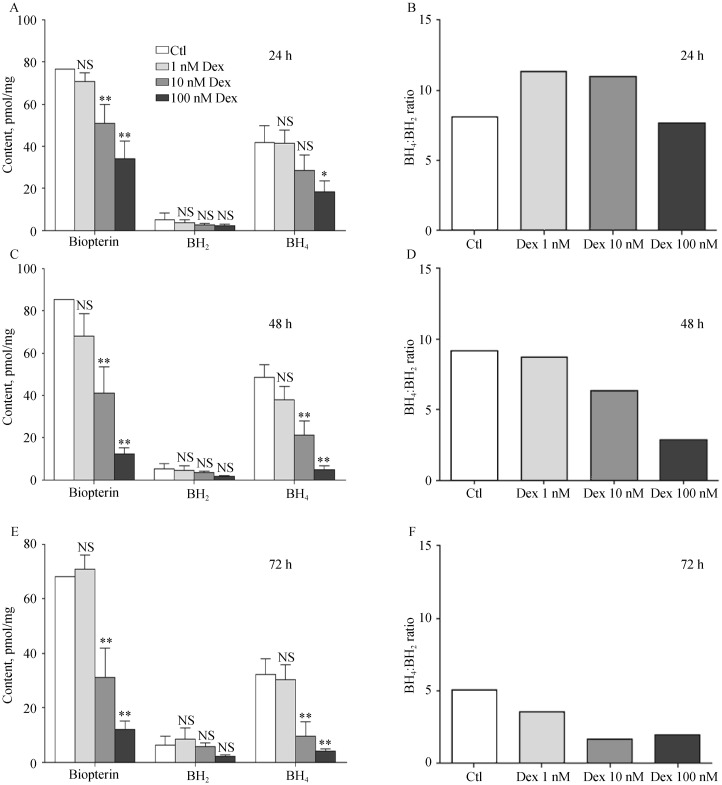
As a crucial cofactor for eNOS, BH4 plays an important role for eNOS functionality and coupling. A significant time- and concentration-dependent decrease in total biopterin, BH4 and BH2 after Dex treatment was observed (Figure 3). Since BH4: BH2 ratio is even more important than the absolute BH4 concentration for eNOS functionality, BH4: BH2 ratio was calculated. With increasing time and concentration of Dex treatment, the BH4: BH2 ratio declined (Figure 3).
Figure 3. Dexamethasone reduces biopterin content.
EA.hy 926 endothelial cells were treated with either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24 h (A&B), 48 h (C&D) or 72 h (E&F). Intercellular contents of total biopterin, BH4 and BH2 were analyzed with HPLC, and BH4: BH2 was calculated. Columns represent mean ± SE, n = 9. *P < 0.05, **P < 0.01 compared with Ctl. BH2: dihydrobiopterin; BH4: tetrahydrobiopterin; Ctl: control; Dex: dexamethasone; HPLC: high performance liquid chromatography; NS: not significant.
3.3. No evidence for dexamethasone-induced eNOS uncoupling
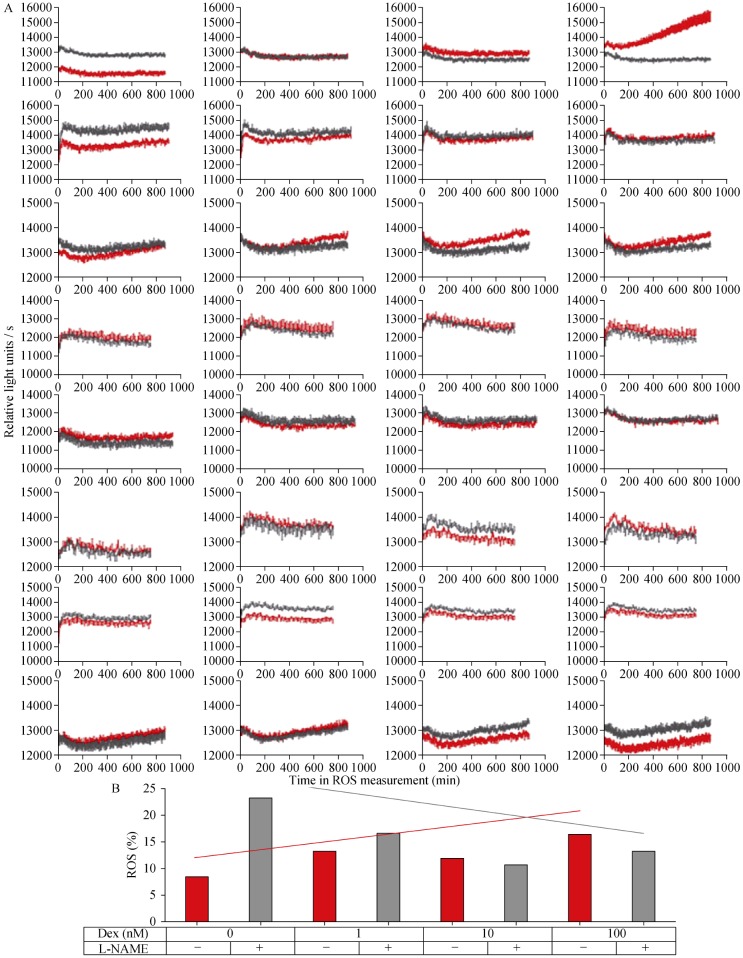
The results in ROS experiments were inconsistent. With the H2DCFDA fluorescence experiment as an example, we performed totally eight experiments and each experiment showed different results (Figure 4A). Generally, no significant increase in ROS was observed in EA.hy 926 endothelial cells in response to Dex treatment (Figure 4). The eNOS inhibitor L-NAME did not reduce ROS levels in Dex-treated cells, indicating that eNOS was not producing ROS, i.e., eNOS was not uncoupled (Figure 4). We also measured ROS production with L-012 chemiluminescence,[16] but found no evidence for eNOS uncoupling either (data not shown).
Figure 4. No evidence for dexamethasone-induced eNOS uncoupling.
(A): EA.hy 926 cells were treated for either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24 h. Then, cells were washed, and ROS measured with H2DCFDA fluorescence in presence (gray lines) or absence (red lines) of the eNOS inhibitor L-NAME (500 µmol/L). Totally 8 experiments (each row represents one experiment) were performed (n = 12 each), with no consistent results. (B): Cells were treated for 24 h and ROS measured with H2DCFDA FACS in presence (gray columns) or absence (red columns) of the eNOS inhibitor L-NAME (500 µmol/L). The graph shows representative results of one experiment. No significant differences were found. Integrated lines demonstrate the tendency of ROS development with increasing dexamethasone concentration in absence (red) or presence (gray) of L-NAME. Ctl: control; Dex: dexamethasone; ROS: reactive oxygen species.
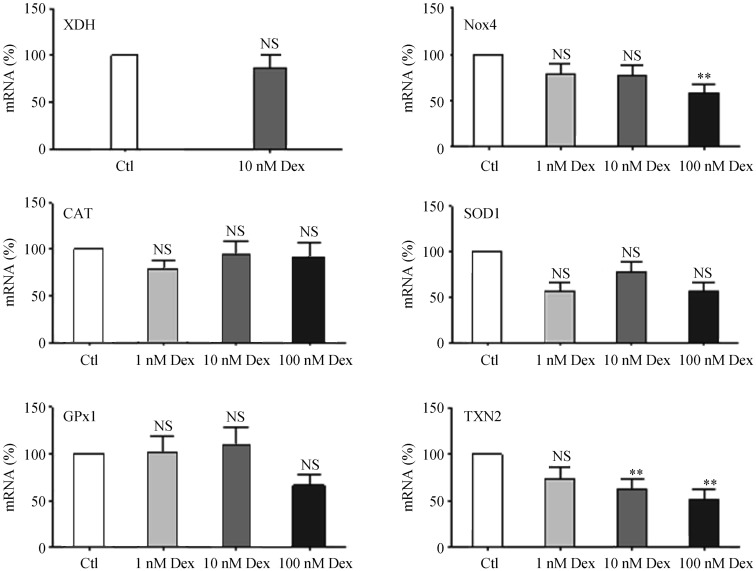
Treatment of EA.hy 926 cells with Dex led to a down-regulation of the ROS-producing enzyme NADPH oxidase 4 (Nox4) and a down-regulation of the antioxidant enzyme thioredoxin-2 (TNX2) (Figure 5). However, no significant effects of Dex were observed on redox enzymes such as xanthine dehydrogenase, catalase, superoxide dismutase 1, glutathione peroxidase 1 (GPx1) (Figure 5), the major redox enzymes in this cell type.[22]
Figure 5. Effects of dexamethasone on redox enzymes.
EA.hy 926 endothelial cells were treated with either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24 h. mRNA expression was analyzed with qPCR for XDH, Nox4, CAT, SOD1, GPx1 and TNX2. Columns represent mean + SE, n = 18–36. **P < 0.01, compared to Ctl. CAT: catalase; Ctl: control; Dex: dexamethasone; GPx1: glutathione peroxidase 1; Nox4: NADPH oxidase 4; NS: not significant; SOD1: superoxide dismutase 1; TNX2: thioredoxin-2; XDH: xanthine dehydrogenase.
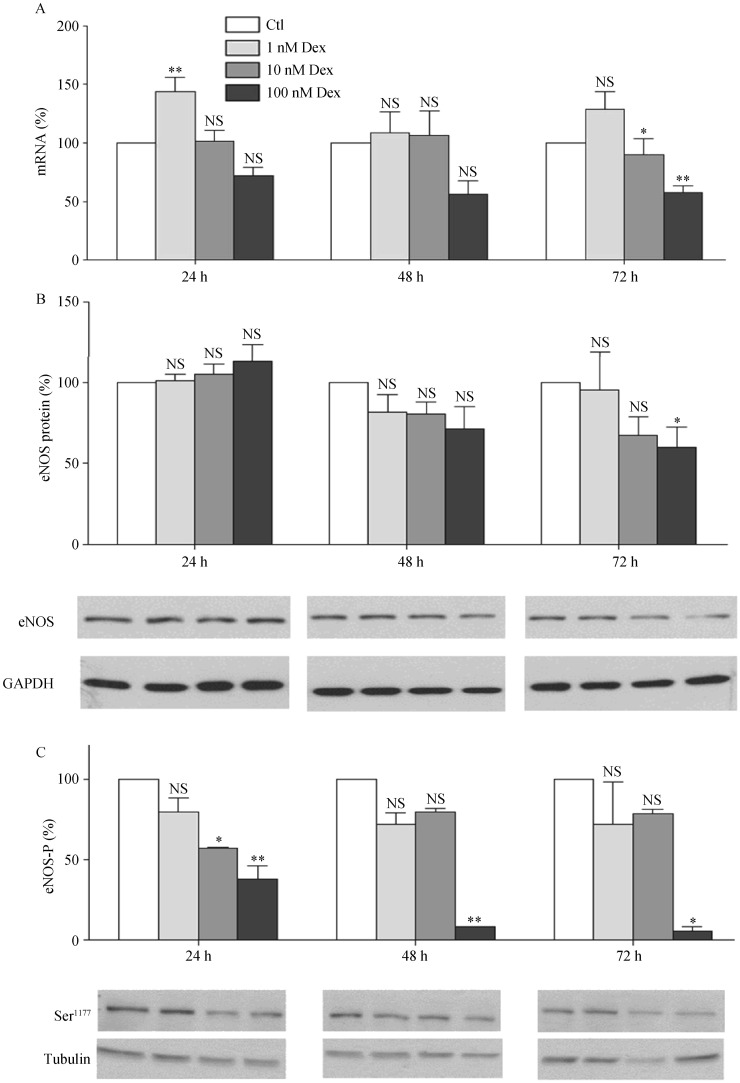
3.4. Dex down-regulates eNOS expression, decreases eNOS phosphorylation and reduces endothelial NO production
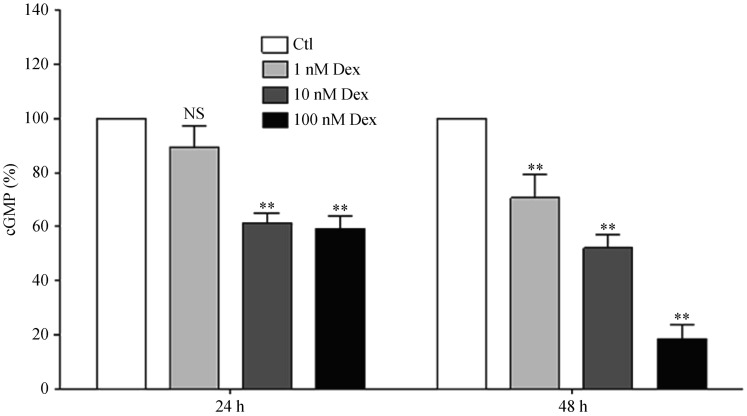
Treatment of EA.hy 926 endothelial cells led to a concentration- and time-dependent down-regulation of eNOS at both mRNA and protein levels (Figures 6A and 6B). A much greater effect was seen on eNOS phosphorylation, with a marked decrease in eNOS phosphorylation at serine 1177 (Figure 6C). The down-regulation of eNOS and reduction in eNOS phosphorylation were associated with a decrease in NO production (Figure 7).
Figure 6. Dexamethasone down-regulates eNOS expression and decreases eNOS phosphorylation.
Human EA.hy 926 endothelial cells were treated with either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24, 48 or 72 h. mRNA expression of eNOS was analyzed with qPCR (A); protein expression of eNOS (B) as well as eNOS-P at serine 1177 (C) were studied with Western blot analyses. GAPDH and β-tubulin were shown for normalization. Columns represent mean ± SE, n = 12–24. *P < 0.05, **P < 0.01 compared with Ctl. Ctl: control; Dex: dexamethasone; eNOS: endothelial nitric oxide synthase; eNOS-P: endothelial nitric oxide synthase phosphorylation; NS: not significant.
Figure 7. Dexamethasone reduces endothelial NO production.
Human EA.hy 926 endothelial cells were treated with either Dex (1, 10 or 100 nmol/L) or with the solvent control DMSO for 24 or 48 h and NO production bioassayed with RFL-6 reporter cells. EA.hy 926 cells were washed and subsequently stimulated with 10 µmol/L calcium ionophore A23187 for 2 min before the transfer of conditioned media to RFL-6 reporter cells. The cGMP content in the RFL-6 cells was measured by radioimmunoassay. Columns represent mean + SE, n = 3. **P < 0.01 compared with Ctl. Ctl: control; Dex: dexamethasone; NS: not significant.
4. Discussion
In the present study, we demonstrate that treatment of endothelial cells with Dex leads to a down-regulation of BH4-synthesizing enzymes and a reduction of intercellular BH4 content. At the same time, Dex decreases eNOS expression, eNOS phosphorylation at serine 1177, and NO production. An uncoupling of eNOS, however, is not evident.
In our previous studies,[4],[11] we have provided evidence that a reduction in endothelial NO production represents a major mechanism for Dex-induced hypertension. In these studies, we proposed that the reason for the reduction NO production is a down-regulation of the eNOS enzyme.[4],[11] The objective of the present study is to test the hypothesis that the decreased NO production in response to Dex treatment may also involve eNOS uncoupling, a common mechanism seen in many pathological conditions including hypertension, atherosclerosis and diabetes.[12],[13] Uncoupling of eNOS is a crucial mechanism contributing significantly to endothelial dysfunction. It not only reduces NO production, but also potentiates the pre-existing oxidative stress. The overproduction of reactive oxygen species (e.g., superoxide and subsequently peroxynitrite) by uncoupled eNOS in turn enhances oxidation of BH4, leading to BH4 deficiency, and thus creating a vicious circle.[12],[13]
A number of mechanisms are implicated in eNOS uncoupling.[7],[23] Among these, BH4 deficiency is likely to represent a major cause of eNOS uncoupling. BH4 is biosynthesized from GTP with GCH1 acting as the rate-limiting enzyme.[24] Under conditions associated with oxidative stress, superoxide can modestly and peroxynitrite strongly oxidize BH4 to BH2 leading to BH4 deficiency.[25] BH2 can be reduced back to BH4 by the enzyme DHFR.[26],[27] Thus, BH4 deficiency can be caused by enhanced oxidation due to oxidative stress, by reduced de novo synthesis due to downregulation/inhibition of GCH1 or by reduced recycling from BH2 due to down-regulation of DHFR.[28]
An important mechanism of eNOS uncoupling in response to cardiovascular risk factors is oxidative stress caused by NADPH oxidases, the major ROS-producing enzymes in the vasculature.[29] An upregulation of NADPH oxidase expression and/or activity may cause BH4 oxidation and eNOS uncoupling. The predominant NADPH oxidase isoform in human endothelial cells (EA.hy 926 cells as well as human umbilical vein endothelial cells) is Nox4.[30] Dex treatment reduces Nox4 expression in EA.hy 926 cells (Figure 5). This is likely to be the reason why Dex does not induce oxidative stress and BH4 oxidation.
We did, however, observe a reduced BH4 level in EA.hy 926 cells after Dex treatment, which can be explained by the down-regulation of BH4-synthesizing enzymes GCH1 and DHFR. Recent studies have shown that BH4 and BH2 have similar Km for eNOS, but BH2 is without eNOS cofactor activity. BH2 may compete with BH4 for binding to eNOS, or even displace BH4 prebound to eNOS, thus promoting eNOS uncoupling and superoxide production.[26],[31],[32] Therefore, BH4: BH2 ratio rather than the absolute amount of BH4 is the key determinant eNOS functionality (i.e., NO or superoxide production by eNOS).[32] As shown in Figure 3, the calculated BH4: BH2 ratio was reduced by Dex treatment as well.
Despite the reduced intercellular BH4 content and BH4: BH2 ratio, we found no evidence for eNOS uncoupling in Dex-treated endothelial cells. We propose that two mechanisms may be responsible for this paradoxical phenomenon: eNOS-BH4 stoichiometry and eNOS phosphorylation.
Overexpression of eNOS in apolipoprotein E-knockout (eNOS-Tg/ApoE-KO) mice paradoxically results in increased vascular superoxide production at least in part from uncoupled eNOS.[33] The superoxide production induced by eNOS overexpression can be prevented by BH4 supplementation or by endothelium-specific GCH1 overexpression.[34] These data suggest that eNOS: BH4 ratio determines eNOS function. Indeed, in eNOS-overexpressing (eNOS-Tg) mice where BH4 levels are limiting (eNOS-Tg/ApoE-KO), superoxide production is markedly elevated compared with wild type and eNOS-Tg littermates, whereas eNOS is not uncoupled in the presence of saturating levels of BH4 (eNOS-Tg and eNOS/GCH-Tg).[35] Recently, the quantitative regulation of eNOS coupling by BH4-eNOS stoichiometry has been elegantly demonstrated in a model cell system.[32] Quantitative analysis of cellular BH4 and eNOS levels has revealed a striking linear relationship between eNOS protein and cellular BH4 stoichiometry, with eNOS uncoupling at eNOS: BH4 molar ratio > 1.[32]
In the present study, Dex treatment led to a reduction of BH4 and BH4: BH2 ratio (Figure 3). Meanwhile, we observed a down-regulation of eNOS protein (Figure 7). Although the precise quantitative stoichiometry of eNOS and BH4 could not be measured in our study, it is possible that the low BH4 level after Dex treatment is still sufficient for the eNOS enzyme, whose expression level is also reduced. This may be one reason for the absence of eNOS uncoupling in Dex-treated endothelial cells.
The second reason may be the reduced eNOS phosphorylation at serine 1177. Phosphorylation is an important mechanism regulating eNOS enzymatic activity.[36] The activation of eNOS catalytic function by serine 1177 phosphorylation is due to inhibition of calmodulin dissociation from eNOS and enhancement of the internal rate of eNOS electron transfer.[37] This phosphorylation is required for both the NO-producing activity of the coupled eNOS and the superoxide-producing activity of the uncoupled eNOS. Therefore, even if the reduction of BH4 is sufficient to induce eNOS uncoupling, the massive dephosphorylation at serine 1177 may inhibit the activity of the uncoupled eNOS enzyme, thus rendering the superoxide-producing activity of eNOS undetectable.
In conclusion, we found no evidence of eNOS uncoupling in endothelial cells treated with Dex despite a down-regulation of BH4-producing enzymes and a reduction of BH4 levels. The reduced NO production in Dex-treated endothelial cells is attributable to a down-regulation of the eNOS protein and eNOS dephosphorylation at serine 1177.
Acknowledgments
Tobias S and Siuda D were members of the Neuroscience Graduate School at the Johannes Gutenberg University of Mainz supported by the DFG (Deutsche Forschungsgemeinschaft) Research Training Group (GRK 1044). Siuda D was also supported by the Center for Thrombosis and Hemostasis (CTH, funded by the Federal Ministry of Education and Research, BMBF 01EO1003) of Johannes Gutenberg University Medical Center, Mainz, Germany.
References
- 1.Moghadam-Kia S, Werth VP. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol. 2010;49:239–248. doi: 10.1111/j.1365-4632.2009.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buttgereit F, Burmester GR, Lipworth BJ. Inflammation, glucocorticoids and risk of cardiovascular disease. Nat Clin Pract Rheumatol. 2009;5:18–19. doi: 10.1038/ncprheum0963. [DOI] [PubMed] [Google Scholar]
- 3.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157:545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 4.Wallerath T, Witte K, Schafer SC, et al. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci U S A. 1999;96:13357–13362. doi: 10.1073/pnas.96.23.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia N, Forstermann U, Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Forstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr Pharm Des. 2009;15:3133–3145. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 8.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlencordt PJ, Gyurko R, Han F, et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 10.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallerath T, Godecke A, Molojavyi A, et al. Dexamethasone lacks effect on blood pressure in mice with a disrupted endothelial NO synthase gene. Nitric Oxide. 2004;10:36–41. doi: 10.1016/j.niox.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Forstermann U. Pharmacological prevention of eNOS uncoupling. Curr Pharm Des. 2014;20:3595–3606. doi: 10.2174/13816128113196660749. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol. 2013;13:161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Siuda D, Zechner U, El Hajj N, et al. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res Cardiol. 2012;107:283. doi: 10.1007/s00395-012-0283-3. [DOI] [PubMed] [Google Scholar]
- 15.Siuda D, Tobias S, Rus A, et al. Dexamethasone upregulates Nox1 expression in vascular smooth muscle cells. Pharmacology. 2014;94:13–20. doi: 10.1159/000365932. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Witte K, August M, et al. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–2544. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 17.Xia N, Daiber A, Habermeier A, et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Junk P, Huwiler A, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 19.Witte I, Altenhofer S, Wilgenbus P, et al. Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells. Cell Death Dis. 2011;2:e112. doi: 10.1038/cddis.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Forstermann U. Structure-activity relationship of staurosporine analogs in regulating expression of endothelial nitric-oxide synthase gene. Mol Pharmacol. 2000;57:427–435. doi: 10.1124/mol.57.3.427. [DOI] [PubMed] [Google Scholar]
- 21.Xia N, Strand S, Schlufter F, et al. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Spanier G, Xu H, Xia N, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):S111–S116. [PubMed] [Google Scholar]
- 23.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 25.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabtree MJ, Tatham AL, Hale AB, et al. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284:28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Horke S, Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Goettsch C, Xia N, et al. Differential roles of PKCalpha and PKCepsilon in controlling the gene expression of Nox4 in human endothelial cells. Free Radic Biol Med. 2008;44:1656–1667. doi: 10.1016/j.freeradbiomed.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Vasquez-Vivar J, Martasek P, Whitsett J, et al. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crabtree MJ, Tatham AL, Al-Wakeel Y, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki M, Kawashima S, Yamashita T, et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alp NJ, McAteer MA, Khoo J, et al. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 35.Bendall JK, Alp NJ, Warrick N, et al. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 36.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 37.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]