Cardiac rehabilitation (CR) programs are well known to improve patients' functional status after cardiac surgery and are recommended by current guideline.[1] In fact, they promote not only structured physical exercises but also a complete secondary prevention determining an overall reduction in recurrent cardiac events and an improvement in functional, psychosocial status and survival.[2],[3]
Many studies have demonstrated a lower women's access to CR than men,[4]–[6] and also an important difference in their clinical profile and management.[7] CR seemed to be, unfortunately, underutilized, particularly in women,[7] and the reasons for this underuse might be linked to health system, socioeconomic and cultural status as well as patients-level factors. In fact, female patients often present a worse risk factors profile (in term of age, diabetes and hypertension) and this is probably the reason why physicians recommend CR more strongly in men than in women. The aim of this observational study was to analyse clinical and functional parameters differences at admission and at discharge in both sexes in a single Rehabilitation Centre after surgical procedure.
From April 2008 to April 2013, all consecutive patients, aged ≥ 18 years old, admitted to our Department for CR, were requested to enter this observational study after having obtained an informed consent. All patients underwent a complete clinical examination, plasma determination of brain natriuretic peptide (BNP) and other laboratory's tests (creatinine, haemoglobin) and a transthoracic echocardiogram. The neuropsychological status was examined using the mini mental state examination (MMSE),[8] the Hospital Anxiety and Depression Scale (HADS),[9] and the Geriatric Depression Scale (GDS).[10] A 6 min walking test (6MWT) and Barthel Index were also performed at admission and at discharge.
BNP was measured collecting blood sample by venopuncture and immediately analysed with the bedside Triage B type natriuretic fluorescence immunoassay (Biosite Diagnostics, La Jolla, CA, USA). The Triage Meter is used to measure BNP concentration by detecting a fluorescent emission that reproduces the amount of BNP in the blood. Two hundred and fifty microliter of whole blood was added to the disposable device. Next, the cells were filtered and divided from the plasma with BNP, which entered a reaction chamber, containing fluorescent BNP antibodies. After 2-min incubation, the BNP–antibody mixture migrated to an area containing immobilised antibodies and remained fixed there. The unbound fluorescent antibodies were washed away by the excess sample fluid. Then, the Triage Meter measured the fluorescent intensity of the BNP assay area. The assay results were complete in 15 min.
Echocardiograms were performed with a GE Vivid 7 Pro, according to the recommendations of the American Society of Echocardiography.[11] Two-dimensional apical 2- and 4-chamber views were used for volume measurements; left ventricular ejection fraction (LVEF) was calculated with a modified Simpson's method using biplane apical (2- and 4-chamber) views. Left ventricular systolic dysfunction was defined as an LVEF < 50%. All the echo examinations were performed by expert operators blinded to the results of BNP assay; the intra-observer variability was found to be < 5%. The medical therapy was normally assumed before the echocardiogram and the BNP measurement was obtained before the echo examination.
A comprehensive battery of psychological and neuropsychological tests measuring a broad range of cognitive functions was administered to each patient. The complete battery of tests consisted of the MMSE, the HADS, and the GDS. MMSE shows a good sensitivity in assessing a global cognitive deterioration (MMSE < 24). The results of MMSE score obtained were corrected according to age and years of school attendance. The HADS is a self-assessment scale and is designed to provide a screening device for anxiety and depression in a general hospital setting. The anxiety and depression subscales are valid measures of the severity of an emotional disorder: for each part, a score below 8 is in the normal range, 8–10 is borderline and above 10 indicates with good probability a mood disorder. The items of GDS are commonly used to determine self-reported symptoms of depression. In particular, GDS seems to be particularly useful in a population of ill patients and has been used in HF population. A score of 6 or greater is considered significant for the clinical presence of depression.
6MWT was performed at admission and discharge according to the ATS Statement of the American Thoracic Society.[12] Congestive heart failure patients able to walk underwent 6MWT if did not meet the exclusion criteria (unstable angina and myocardial infarction during the previous month, resting heart rate > 120 beats/min; systolic blood pressure > 180 mmHg or diastolic blood pressure > 100 mmHg).
Barthel index was calculated both at admission and discharge. This is a scoring technique developed in 1965 and later modified by Granger, et al.[13] that measures patient's performance in 10 activities of daily life. The items can be divided into a group that is related to self-care (feeding, grooming, bathing, dressing, bowel and bladder care, and toilet use) and a group related to mobility (ambulation, transfers, and stair climbing). The maximal score is 100, if 5-point increments are used, indicating that the patient is fully independent in physical functioning. The lowest score is 0, representing a totally dependent bedridden state.
Continuous variables were expressed as mean ± SD. Categorical variables were analysed using the chi-square test or Fisher's exact test. For the comparisons between samples, we used the Mann-Whitney U-test and the Wilcoxon test. All probability values were two-tailed and differences were considered significant with a P value < 0.05. The 7.5 version of the SPSS software for Windows, release 12.0, SPSS Inc. Chicago USA was used.
Four-hundred and eighteen consecutive patients accepted to enter this observational study: 295 men (70.6%) and 123 women (29.4%) with a mean age of 68.7 ± 9.9 years. Patients were admitted to our Department for CR after coronary artery bypass (61%), after valve replacement (53.1%) or both procedures (26.8%) and in 12.7% after other procedures. The baseline characteristics of the population examined are summarized in Table 1. The CR sessions consisted in 90 min of physical rehabilitation twice a day supervised by a physiotherapist. According to the baseline status of patients, they underwent respiratory exercises, isotonic exercises for the upper and lower limbs (15 min; 5 min cool-down) and aerobic exercise using bicycle/treadmill training limiting to the 50%–70% of maximum heart frequency calculated by age and progressed according to the rating perceived exertion (Borg scale 11–14).
Table 1. Main characteristics and differences of the population examined.
| Males (n = 295) | Females (n = 123) | P | |
| Age, years | 67.9 ± 10.2 | 70.5 ± 9.2 | 0.01 |
| In-hospital stay, days | 15.3 ± 7.6 | 16.3 ± 8.1 | 0.25 |
| Creatinine, mg/dL | 1.2 ± 0.7 | 0.9 ± 0.4 | 0.004 |
| Haemoglobin, g/dL | 10.8 ± 1.4 | 10.7 ± 1.4 | 0.69 |
| LVEF, % | 47.1 ± 12.8 | 50.2 ± 13.4 | 0.03 |
| NYHA admission | 2.4 ± 0.7 | 2.6 ± 0.6 | 0.07 |
| NYHA discharge | 1.6 ± 0.6 | 1.8 ± 0.5 | 0.06 |
| BNP admission, pg/mL | 547.8 ± 598.5 | 467.1 ± 517.1 | 0.23 |
| BNP discharge, pg/mL | 365.6 ± 376.7 | 365.8 ± 436.0 | 0.9 |
| 6MWT admission, m | 327.2 ± 88.4 | 236.3 ± 80.4 | < 0.001 |
| 6MWT discharge, m | 414.6 ± 83.9 | 336.7 ± 75.9 | < 0.001 |
| Delta 6MWT, m | 87.4 | 100.4 | |
| Barthel index admission | 88.7 ± 21.2 | 80.7 ± 26.2 | 0.004 |
| Barthel index discharge | 94.8 ± 13.7 | 92.1 ± 16.9 | 0.135 |
| MMSE admission | 27.6 ± 2.8 | 27.1 ± 3.7 | 0.27 |
| MMSE discharge | 27.7 ± 2.8 | 27.2 ± 3.6 | 0.39 |
| HADS (A) admission | 5.1 ± 2.9 | 6.4 ± 3.6 | 0.007 |
| HADS (A) discharge | 4.5 ± 2.6 | 5.2 ± 2.8 | 0.08 |
| HADS (D) admission | 4.7 ± 3.3 | 6.0 ± 3.4 | 0.008 |
| HADS (D) discharge | 3.7 ± 2.8 | 5.3 ± 3.0 | 0.001 |
| GDS admission | 6.5 ± 2.8 | 7.1 ± 4.7 | 0.5 |
| GDS discharge | 5.6 ± 2.2 | 6.0 ± 2.9 | 0.6 |
BNP: brain natriuretic peptide; GDS: geriatric depression scale; HADS (A): hospital anxiety and depression scale (anxiety); HADS (D): hospital anxiety and depression scale (depression); LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; 6MWT: six minute walking test; MMSE: mini mental state examination.
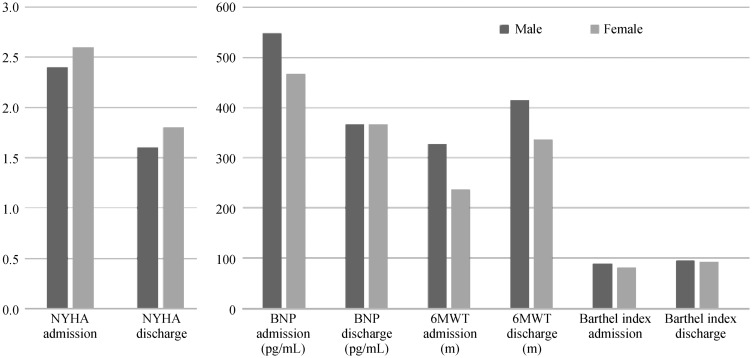
Women were older than men (70.5 ± 9.2 years vs. 67.9 ± 10.2 years; P = 0.01) with a better renal function (0.9 ± 0.4 mg/dL vs. 1.2 ± 0.7 mg/dL; P = 0.004) and LVEF (50.2% ± 13.4% vs. 47.1% ± 12.8%; P = 0.03). The mean NYHA class at admission was similar in the two sexes (2.4 ± 0.7 in males and 2.6 ± 0.6 in females; P = 0.07) and improved at discharge (1.6 ± 0.6 vs. 1.8 ± 0.5, respectively; P = 0.06; delta NYHA = −0.8 in each gender). Also mean BNP at admission was similar (547.8 ± 598.5 pg/mL in males vs. 467.1 ± 517.1 pg/mL in females; P = 0.23) and at discharge it proved to be reduced both in men and women (365.6 ± 376.7 pg/mL vs. 365.8 ± 436.0 pg/mL; P = 0.9; delta BNP: 182.2 pg/mL vs. −101.3 pg/mL) (Table 1). Women demonstrated a worse Barthel Index score at admission (80.7 ± 26.2 in females vs. 88.7 ± 21.2; P = 0.004) that ameliorated at discharge both in males and females (92.1 ± 16.9 in females vs. 94.8 ± 13.7; P = 0.135; delta Barthel 6.1 vs. 11.4). The 6MWT [performed in 305 patients (215 males)] demonstrated a better performance in males (327.2 ± 88.4 m vs. 236.3 ± 80.4 m at admission; P < 0.001) and ameliorated before discharging in both sexes (414.6 ± 83.9 m in males vs. 336.7 ± 75.9 m in females at admission; P < 0.001; delta 6MWT: 87.4 m for males and 100.4 m for females). These results are briefly reported in Figure 1.
Figure 1. Gender differences in the efficacy of cardiovascular rehabilitation.
BNP: brain natriuretic peptide; NYHA: New York Heart Association; 6MWT: six minute walking test;
The mean MMSE was similar in both sexes and found no cognitive impairment (27.6 ± 2.8 in males vs. 27.1 ± 3.7 in females; P = 0.27) whereas women demonstrated to be a little more anxious and depressed than men both at admission and discharge [anxiety at admission HADS(A): 5.1 ± 2.9 in men vs. 6.4 ± 3.6; P = 0.007]. Depression at admission HADS(D): 4.7 ± 3.3 in men vs. 6.0 ± 3.4; P = 0.008. Depression at discharge HADS(D): 3.7 ± 2.8 in men vs. 5.3 ± 3.0; P = 0.001; delta −1 vs. −0.7. No differences were found at GDS score between men and women (6.5 ± 2.8 vs. 7.1 ± 4.7, P = 0.5 at admission; 5.6 ± 2.2 vs. 6.0 ± 2.9, P = 0.6 at discharge, respectively). The length of hospitalization proved to be similar in both sexes (15.3 ± 7.6 days in men vs. 16.3 ± 8.1days in women; P = 0.25).
Female population characteristics compared with male are displayed in Table 1.
CR programs have shown to improve secondary prevention in patients after cardiovascular procedures reducing mortality, re-hospitalization, interventional procedures and producing health behaviour changes (increased exercise, following diet prescription, smoking cessation).[2],[3] Despite the preponderance of evidence on the numerous benefits of CR and although women might be in greater need of the secondary prevention offered through CR, they are significantly less likely to access it than men.[14]–[16] These findings are also supported and summarized by two recent systematic reviews of Samayoa and Grace.[17],[18]
Although there are innate biological differences between genders [such as age of development of cardiovascular (CV) disease, CV risk factors, women's worse exercise performance than men] and, historically, women are less likely to be “physician referred” for cardiac rehabilitation,[19] the benefits of CR on exercise ability and on risk-factor modification are equally as good for women as for men of equivalent age,[20],[21] even if sex differences have been found for quality of life in some other studies.[22]
In a recent study, instead, Barth, et al.[23] have demonstrated that CR is less effective among women with regard to vital exhaustion and more effective with regard to social inhibition compared with men in a sample of low distressed patients. Similar findings are supported by Pasquali, et al.,[24] who have underlined that a significant improvement in 6-month physical functioning is present in CR patients, but tended to be greater in men versus women, in patients aged < 70 years versus ≥ 70 years, and in patients with coronary bypass graft versus patients with percutaneous intervention.[24]
However, there is no literature's evidence to suggest that women are less likely to benefit of CR than men also because this literature suffers from low sample sizes and lack of randomization and control groups; indeed women often present with lower physical fitness and such has a greater potential to benefit from CR.[18]
In our study, we confirm gender differences in referral to CR programs. In fact, the rate of women admitted for CR is just 29.4% and this percentage is similar to others previously observed.[7] In general, women are significantly older than men and more likely to have a preserved ventricular and renal function, whereas they present at admission a greater dependence in functional parameters of daily living (Barthel Index), a poorer performance at 6MWT and a higher percentage of anxiety and depression (HADS test). Although these baseline differences, both women and men improve significantly their discharge parameters of NYHA class, BNP, Barthel Index score and 6MWT after a CR program and no differences in the length of hospitalization emerged.
Our experience demonstrates that clinicians should evaluate older women (age > 70 years old) after cardiac surgical procedures for a CR treatment because of the efficacy of the therapy in improving clinical status. A low baseline physical performance and an alteration of mood (anxiety or depression) should not exclude women to CR treatment.
In conclusion, despite the high number of studies and reviews on CR's benefits in female population and strong recommendations of the literature, also our experience demonstrates that there still are sex differences in CR enrolment. This might be explained by the higher age of female population and the higher percentage of functional dependence and worse clinical performances at admission in women. However, as our study demonstrates, these wrong beliefs must be overcome as CR programs are useful to improve clinical performance and functional status also in a more compromised female population.
References
- 1.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 2.Alter DA, Oh PI, Chong A. Relationship between cardiac rehabilitation and survival after acute cardiac hospitalization within a universal health care system. Eur J Cardiovasc Prev Rehabil. 2009;16:102–113. doi: 10.1097/HJR.0b013e328325d662. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RJ, Miller NH, Lamendola C, et al. National Survey on gender differences in CR. J Cardiopulm Rehabil. 1996;16:402–422. doi: 10.1097/00008483-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Karoff M, Held K, Bjarnason-Wehrens B. Cardiac rehabilitation in Germany. Eur J Cardiovasc Prev Rehabil. 2007;14:18–27. doi: 10.1097/HJR.0b013e3280128bde. [DOI] [PubMed] [Google Scholar]
- 6.Held K, Ritter P. Age distribution in cardiac rehabilitation. Herzmedizin. 2003;20:160–163. [Google Scholar]
- 7.De Feo S, Tramarin R, Ambrosetti M, et al. Gender differences in cardiac rehabilitation programs from the Italian survey on cardiac rehabilitation (ISYDE-2008) Int J Cardiol. 2012;160:133–139. doi: 10.1016/j.ijcard.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. Mini Mental State. A practical method for grading the congestive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 10.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–83;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 11.Sahn DJ, Allen HD, George W, et al. The utility of contrast echocardiographic techniques in the care of critically ill infants with cardiac and pulmonary disease. Circulation. 1977;56:959–968. doi: 10.1161/01.cir.56.6.959. [DOI] [PubMed] [Google Scholar]
- 12.ATS Statement: Guidelines for the six-minute walk test. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Granger CV, Dewis LS, Peters NC, et al. Stroke rehabilitation: analysis of repeated Barthel index measures. Arch Phys Med Rehabil. 1979;60:14–17. [PubMed] [Google Scholar]
- 14.Benz Scott LA, Ben-Or K, Allen JK. Why are women missing from outpatient cardiac rehabilitation programs? A review of multilevel factors affecting referral, enrolment, and completion. J Womens Health. 2002;11:773–791. doi: 10.1089/15409990260430927. [DOI] [PubMed] [Google Scholar]
- 15.Bittner V, Sanderson BK. Women in cardiac rehabilitation. J Am Med Womens Assoc. 2003;58:227–235. [PubMed] [Google Scholar]
- 16.McCarthy MM, Vaughan Dickson V, Chyun D. Barriers to cardiac rehabilitation in women with cardiovascular disease: an integrative review. J Cardiovasc Nurs. 2011;26:E1–E10. doi: 10.1097/JCN.0b013e3181f877e9. [DOI] [PubMed] [Google Scholar]
- 17.Samayoa L, Grace SL, Gravely S, et al. Sex differences in cardiac rehabilitation enrolment: a meta-analysis. Can J Cardiol. 2014;30:793–800. doi: 10.1016/j.cjca.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Grace SL, Racco C, Chessex C, et al. A narrative review on women and cardiac rehabilitation: program adherence and preferences for alternative models of care. Maturitas. 2010;67:203–208. doi: 10.1016/j.maturitas.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Caulin-Glaser T, Blum M, Schmeizl R, et al. Gender differences in referral to cardiac rehabilitation programs after revascularization. J Cardiopulm Rehabil. 2001;21:24–30. doi: 10.1097/00008483-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Balady GJ, Jette D, Scheer J, et al. Changes in exercise capacity following cardiac rehabilitation in patients stratified according to age and gender. Results of the Massachusetts Association of Cardiovascular and Pulmonary Rehabilitation Multicenter Database. J Cardiopulm Rehabil. 1996;16:38–46. doi: 10.1097/00008483-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training on exercise capacity, coronary risk factors, behavioural characteristics, and quality of life in women. Am J Cardiol. 1995;75:340–343. doi: 10.1016/s0002-9149(99)80550-5. [DOI] [PubMed] [Google Scholar]
- 22.Deshotels A, Planchock N, Dech Z, et al. Gender differences in perceptions of quality of life in cardiac rehabilitation patients. J Womens Health (Larchmt) 1995;15:143–148. doi: 10.1097/00008483-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Barth J, Volz A, Schmid JP, et al. Gender differences in cardiac rehabilitation outcomes: do women benefit equally in psychological health? J Womens Health (Larchmt) 2009;18:2033–2039. doi: 10.1089/jwh.2008.1058. [DOI] [PubMed] [Google Scholar]
- 24.Pasquali SK, Alexander KP, Coombs LP, et al. Effect of cardiac rehabilitation on functional outcomes after coronary revascularization. Am Heart J. 2003;145:445–451. doi: 10.1067/mhj.2003.172. [DOI] [PubMed] [Google Scholar]