Abstract
Cyclic electron flow (CEF) around PSI regulates acceptor-side limitations and has multiple functions in the green alga, Chlamydomonas reinhardtii. Here we draw on recent and historic literature and concentrate on its role in Photosystem I (PSI) photoprotection, outlining causes and consequences of damage to PSI and CEF’s role as an avoidance mechanism. We outline two functions of CEF in PSI photoprotection that are both linked to luminal acidification: firstly, its action on Photosystem II with non-photochemical quenching and photosynthetic control and secondly, its action in poising the stroma to overcome acceptor-side limitation by rebalancing NADPH and ATP ratios for carbon fixation.
Keywords: cyclic electron flow, PSI photoinhibition, non-photochemical quenching, oxygen photoreduction, luminal acidification, photosynthetic control, ATP, malate valve
In the early years of photosynthesis research, a cyclic photophosphorylation was described that required ferredoxin (Fd), did not evolve oxygen (O2) and resulted in the accumulation of ATP (Arnon et al., 1954). From this observation, experiments performed in a variety of organisms from cyanobacteria to higher plants using a combined pharmacological and in vitro approach created a robust model for what is now referred to as cyclic electron flow (CEF; thoroughly reviewed, in Bendall and Manasse, 1995). More recently, Arabidopsis thaliana lines altered in CEF have been identified and have enriched the ways we have to study these pathways (Joet et al., 2001; Munekage et al., 2002, 2004; DalCorso et al., 2008). Biochemical approaches have shown that the Proton-Gradient Regulator5 (PGR5) and PGR5-Like1 (PGRL1) proteins form an interaction that results in a ferredoxin-plastoquinone reductase (FQR) activity (Hertle et al., 2013). In the unicellular, green alga, Chlamydomonas reinhardtii, this pathway and the function of these proteins is conserved (Petroutsos et al., 2009; Tolleter et al., 2011; Johnson et al., 2014). In Chlamydomonas a second type of CEF is also in operation where the mediator at the level of the PQ pool is a type-2 NADPH dehydrogenase (Desplats et al., 2009), with the nda2 mutant shown to have a phenotype in CEF (Jans et al., 2008). Here, we focus on the PGR5 pathway and work done on the Chlamydomonas mutants pgr5 and pgrl1 mutants, that both demonstrate no PGR5/PGRL1-dependent CEF (Alric, 2014). Our focus is on Chlamydomonas but due to the conservation of this pathway we also make reference to work done in other photosynthetic organisms.
Cyclic electron flow is a generator of proton motive force that (i) can produce supplementary ATP to meet ATP:NADPH requirements for the Calvin Benson Bassham (CBB) cycle and the CO2 concentrating mechanism (CCM; reviewed by Alric, 2010), and (ii) triggers regulatory mechanisms, namely non-photochemical quenching (NPQ) and cytochrome b6f complex (cytb6f) “photosynthetic control” (Joliot and Johnson, 2011). Its rate is highest under conditions where the stromal poise is reduced, thus PGR5-CEF has been considered as a regulator of redox homeostasis for the photosynthetic chain (Nishikawa et al., 2012). Among the phenotypes observed in CEF-altered strains of both Arabidopsis and Chlamydomonas, Photosystem I (PSI) photoinhibition arose in conditions of high light or limiting CO2 (Munekage et al., 2002; Dang et al., 2014; Johnson et al., 2014) and fluctuating light (Suorsa et al., 2012) leading to the assignment of yet another role for PGR5-CEF. While Photosystem II (PSII) photoinhibition is frequently observed and has complex models that describe the mechanism (Murata et al., 2012), PSI photoinhibition remains poorly understood. In this work, we review the potential causes of photoinhibition that occur at the acceptor-side of PSI and the processes triggered by CEF that can contain it. For the sake of comprehensive reviewing of mechanisms involved in PSI photoprotection, other connected pathways are also introduced.
Acceptor-Side Limitation is the Cause of PSI Photoinhibition
PSI photoinhibition was first reported in isolated chloroplasts submitted to strong light (Jones and Kok, 1966). Satoh was able to differentiate two types of damage that corresponded to damage to the two photosystems using fragmented chloroplasts. Artificial donors were used to measure the capacity of PSI to transfer electrons to terminal acceptor, NADPH. These experiments showed that the addition of the PSII inhibitor, DCMU specifically but incompletely prevented photo-inactivation of PSI (Satoh, 1970a,b). Photo-inactivation of PSI was avoided by addition of excess Fd showing that PSI photoinhibition is an acceptor-side limited phenomenon. The observation that the same group of co-factors that could enhance CEF (including Fd) were also involved in the avoidance of photoinactivation of PSI led to the discussion of CEF as a photoprotectant for PSI (Satoh, 1970c). Further studies demonstrated the destruction of PSI-bound iron-sulfur centers (FX, FA/B) by oxidative species primarily superoxide anion radical () (Sonoike et al., 1995). Production of can occur within: the iron-sulfur centers of PSI, reduced Fd and stromal flavodehydrogenases (NADP+ ferredoxin dehydrogenase, glutathione reductase and monohydrate ascorbate reductase) in plant chloroplasts (discussed, in Asada, 2000). In permissive conditions, radicals are enzymatically neutralized into water, resulting in the net uptake of O2 reported by Mehler (1951), establishing a pseudo-cyclic pathway for electrons known as the water-water cycle. When radical production exceeds detoxifying capacity, irreversibly damages PSI primary acceptors (FX, FA/B) and prevents stable accumulation of P700+ in high light (Figure 1). This is because of fast charge recombination at the level of intermediary acceptors A0/1 (Setif and Brettel, 1990). The resultant decrease in the quantity of oxidizable P700 is thus a common measurement for probing the photoinhibition of PSI.
FIGURE 1.
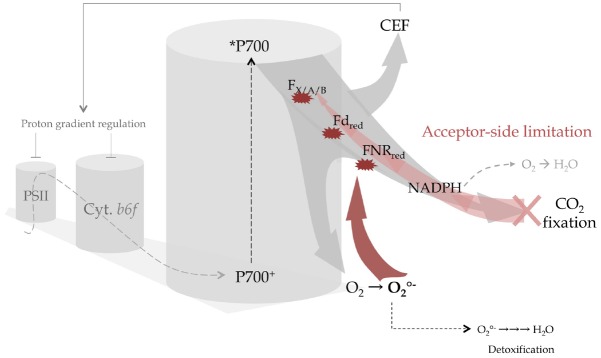
Acceptor-side limitation and excess electron flow promotes CEF or in its absence leads to the irreversible damage of PSI centers. The linear electron flow coming from PSII (gray dashed arrow) is the source of electrons for the PSI reaction center (P700+/P700) that transfers electrons from the chlorophyll excited state (P700*) and subsequently delivers to downstream acceptors within PSI (FX, FA, FB iron-sulfur centers) then to stromal electron carriers (ferredoxin, FNR, NADP+) (light gray arrow). When CO2 fixation decreases, acceptor-side limitation gradually leads to accumulation of NADPH and overreduction of stromal and PSI electron carriers (light red arrow). In this case electrons are redirected to O2 either at the level of NADPH without the production of reactive oxygen species (ROS; gray dashed arrow) or produce the very reactive superoxide anion radical () at the level of FX, FA, FB, Fd, and FNR with a rate exceeding the detoxification process. Thus will irreversibly destroys the centers (red arrows) resulting in an inability to oxidize *P700 and on a longer time scale the degradation of the entire PSI complex. Preventing this scenario, cyclic electron flow triggers downregulation of linear electron flow at the site of PSII and cytb6f by enhancing proton accumulation in the lumen.
Interestingly, no singlet oxygen (1O2) is produced in overexcited PSI (triplet excited state, 3P700) because P700 is sterically screened from O2 (Setif et al., 1981). Hence, P700+ and 3P700 are most probably efficient quenchers of excess excitation of plant PSI as observed for cyanobacterial PSI (Schlodder et al., 2005; Shubin et al., 2008). Contrarily, 1O2 is the main photo-damaging species produced in acceptor-side limited PSII (3P680) (Durrant et al., 1990). It is remarkable that O2 can be sensitized in PSII and not in PSI (Hideg and Vass, 1995), in other words that 1O2 production within PSII was not evolutionarily eliminated. Over time this may be why a signaling role has developed for 1O2 (Telfer, 2014) resulting in some selectivity in the degradation of PSII protein under photoinhibitory conditions. As compared to the “monolithic” architecture of PSI, the modular architecture of PSII allows for a unique degradation of damaged D1 and re-use of other subunits (extensively reviewed, in Caffarri et al., 2014) and may be another reason why PSII damage-and-repair cycle has been a target of selective pressure. On the contrary, PSI has no known molecular mechanism per se to set its turnover in tune with light intensity. The protection of PSI from photoinhibition would appear to require a set of distinctly different properties than that of PSII (Allahverdiyeva et al., 2015) which includes buffering acceptor side limitations in the stroma. Selective, irreversible photoinhibition of PSI in Chlamydomonas is observed to occur both in CEF-altered strains (Dang et al., 2014; Johnson et al., 2014; Kukuczka et al., 2014; Bergner et al., 2015) and in strains with severe acceptor side limitations such as those lacking RuBisCO (Johnson et al., 2010). Crpgr5 and Crpgrl1 strains demonstrate decreased amounts of oxidizable P700 and PSI protein measured by western hybridization after exposition to high light (Johnson et al., 2014; Kukuczka et al., 2014) and after transition from high (2%) to atmospheric concentrations of CO2 (Dang et al., 2014). In the following sections we present CEF’s role in triggering several mechanisms avoiding long-lasting limitations at the acceptor-side of PSI.
CEF Triggers Fast Quenching, Photosynthetic Control and PSII Photoinhibition Resulting in PSI Photoprotection
As already suggested (Sonoike, 2011), non-photochemical quenching (NPQ) of PSII avoids excessive electron flow to PSI via linear electron flow (LEF) to prevent photoinhibition. CEF limits electrons entering the thylakoid chain because it prompts both excitation-dependent quenching (qE) and indirectly PSII photoinhibition (qI), thus avoiding overflow to PSI. Acidification of the lumen triggers qE (Briantais et al., 1979) and occurs during CEF due to coupling of electron transfer and proton translocation in the cytb6f. Since both LEF and CEF pass through cytb6f, the exact contribution of CEF to the formation of a qE is hard to determine but an altered ability to develop qE is observed in Crpgr5 and Crpgrl1 strains (Tolleter et al., 2011; Dang et al., 2014; Johnson et al., 2014; Kukuczka et al., 2014) concomitantly with PSI photoinhibition (Dang et al., 2014; Johnson et al., 2014). This is also consistent both with the failure to acidify the lumen under short saturating illumination in Atpgr5 plants (Suorsa et al., 2012) and reduced growth of Crpgrl1 strains in fluctuating light (Dang et al., 2014). A recent report challenging the effects of rapid quenching of PSII in PSI photoprotection showed that an absence of qE (in Atnpq4 mutants lacking the PsbS protein that induces qE in higher plants, Li et al., 2000) does not have a dramatic effect on P700 oxidation kinetics at any light regime as opposed to Atpgr5 mutants where steady state oxidation of P700 is abolished (Tikkanen et al., 2015). Partial compensatory mechanisms may, however, act between CEF and qE as double mutant strain Crpgrl1npq4, (lacking both CEF and the LHCSR3 protein that acts as the activator for qE in Chlamydomonas, Peers et al., 2009), are particularly susceptible to PSI photoinhibition in comparison to the simple Crpgrl1 mutant (Kukuczka et al., 2014; Bergner et al., 2015). This may coincide with a PSI-photoprotective role recently proposed for LHCSR3 via its association with the PSI antenna system under “state 2 conditions” (Allorent et al., 2013; Bergner et al., 2015) but here LHCSR3-dependent quenching of LHCIs and/or PSI-bound LHCIIs has not been strictly established. The argument against would be that the quenching of PSI antenna is irrelevant given the harmlessness of *P700 and furthermore photo-oxidation events at PSI (measured on isolated complexes in vitro) have been shown to take place after photoinhibition is completed, chlorophyll oxidation being preceded by irreversible carotenoid oxidation (Santabarbara, 2006). For now, the literature would suggest that qE is a first level of photoprotection in photoinhibitory conditions and rapidly protects not only PSII but also PSI by reducing electron flow (Figure 2).
FIGURE 2.
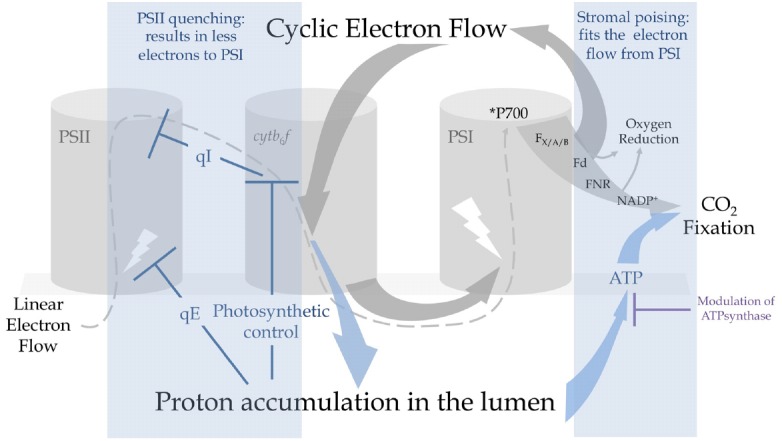
Cyclic electron flow promotes proton accumulation in the lumen and triggers regulatory mechanisms that can protect PSI from photoinhibition. Under constraining conditions, electrons are recycled from the acceptor-side of PSI by PGR5-CEF that results in a rapid acidification of the lumen. This promotes (i) the energy-dependent quenching of PSII antennas (qE) and (ii) photosynthetic control at the level of cytb6f that exerts reducing pressure on PSII to provoke a controlled photoinhibition (qI). These mechanisms result in a decrease of electron flow to PSI. Regulation of ATPsynthase conductivity by protons, electrochemical gradient partitioning and O2 photoreductive pathways produce ΔpH, producing ATP and contributing to the recycling of NADPH. Extra ATP produced by CEF is used by the Calvin-Benson-Bassham (CBB) cycle to assimilate CO2 and contributes to the regeneration of NADP+. Decreasing linear electron flow or increasing the sinks downstream of PSI avoids the over-reduction of ferredoxin (Fd) and PSI centers.
Aside from ATP production and qE, high pmf downregulates LEF (Rumberg et al., 1968) at the site of PQH2 oxidation: originally called “back pressure,” (Stiehl and Witt, 1969), and now known as “photosynthetic control.” Comparison of the CrΔrbcL mutant against the double mutant CrΔrbcL pgr5 or wildtype (WT) clearly shows the effects of photosynthetic control imposed by CEF witnessed by a strong increase in chlorophyll fluorescence in the single mutant indicative of a gradually decreasing electron flow, while the double mutant has fluorescence kinetics that resemble the WT (Johnson et al., 2014). The mutant lacking RuBisCO is an important genetic tool to observe the effects of an absence of CO2 fixation on CEF because it is difficult to modulate CO2 much below atmospheric concentrations in Chlamydomonas due to the efficiency of the carbon concentrating mechanism (CCM): thus this double mutant gives us a window on the mechanisms of CEF as if the WT were under strong CO2-limited conditions. As important as qE in fluctuating light, photosynthetic control is established when a rapid response to severe acceptor side limiting conditions is required to buffer a sudden burst of electron flow toward PSI (Suorsa et al., 2012). Moreover, while qE is not constitutive but inducible in Chlamydomonas, on the contrary to plants (Peers et al., 2009), photosynthetic control is likely to be crucial in the very first hours of exposition to drastic conditions. As a secondary consequence of photosynthetic control, reducing pressure increases on the QB site of PSII so that PSII centers remain in a closed state longer. Overexcited chlorophyll (3P680) activates O2 into 1O2 triggering photoinhibition of PSII (reviewed, in Sonoike, 2011; Murata et al., 2012). Highlighting the key role of pmf in control of linear electron transfer, PSI was shown more susceptible to photoinhibition than PSII in nigericin-infiltrated leaves where control at the level of cytb6f could not develop (Joliot and Johnson, 2011). Moreover, photoinhibition of PSI in Atpgr5 could be avoided by modulating PSII turnover with the addition of the protein translation inhibitor lincomycin (Tikkanen et al., 2014). Thus, PSII photoinhibition mediated by photosynthetic control is a secondary level of photoprotection in drastic photoinhibitory conditions that exceed qE dissipation capacity: it indirectly but effectively acts as a shunt to avoid sustained PSI acceptor-side limitations (Figure 2).
In Chlamydomonas, the pgr5 mutation combined with an absence of chloroplast ATPsynthase results in a less photosensitive phenotype than the ATPase mutant alone, where light sensitivity has been attributed to luminal over-acidification (Johnson et al., 2014). This observation shows that photosynthetic control relying on CEF actively contributes to decreasing the luminal pH and supports previous work (Rott et al., 2011). In higher plants, triggering of low luminal pH has also been correlated with changes in conductivity of the ATPsynthase to protons (Kanazawa and Kramer, 2002) and also to partitioning of the proton motive force between its osmotic (or concentration gradient, ΔpH) and electrical (ΔΨ) component (Avenson et al., 2005). ATPsynthase conductivity to protons is increased in Atpgr5 (Avenson et al., 2005; Wang et al., 2015) with similar observations seen in knocked-down PGR5 rice lines (Nishikawa et al., 2012). This may be ruled by the concentration of substrate for ATP production, i.e., ADP and phosphate (Pi): in spinach thylakoids, artificially decreasing Pi levels resulted in lower ATPase conductivity and a lower luminal pH, thus promoting qE (Takizawa et al., 2008). These observations show the metabolic interconnections between ATP, CEF and ATPsynthase. As already suggested (Shikanai, 2014), further studies should be done to explain the acceptor-side limitation occurring in strains affected in PGR5-CEF in the light of the scenario proposed by Kramer and coworkers for qE regulation.
O2 Reduction Works with CEF in Dissipating Excessive Electron Flow
A number of recent observations have shown the direct interaction of CEF with chloroplast metabolism because CEF regulates acceptor-side limitation in the absence of reactions for consumption of NADPH. Rubisco-less mutants but also CBB cycle mutants and those affected in starch metabolism show a strong increase in CEF or in CEF-dependent photosynthetic control that results in a repressed rate of LEF (Livingston et al., 2010; Johnson and Alric, 2012; Johnson et al., 2014; Krishnan et al., 2015). When the CBB cycle is an insufficient sink for reducing power, O2 photoreduction pathways may work in conjunction with CEF to protect PSI. On the other hand, under non-acceptor side limited conditions (steady state high light and/or high CO2) an absence of CEF in Crpgrl1 did not result in photoinhibition to PSI and this capacity to acclimate was shown to be due to a sustained dependence on O2 photoreduction pathways (Dang et al., 2014). While photorespiration is a minimal process in green algae due to the CCM, other important sinks exist for reducing equivalents downstream of PSI that terminate on O2. These include: (i) export of reducing power to the respiratory chain to stimulate oxidative phosphorylation in the mitochondria, (ii) ROS-producing (“Mehler”) reactions with a concomitant increase in detoxifying enzymes and (iii) ROS-independent (“Mehler-like”) NADPH:O2 oxidoreduction probably by flavodiiron proteins (FLV; Peltier et al., 2010). Mechanisms (ii) and (iii) dually generate proton gradient and thus ATP (Forti and Elli, 1995, 1996) and regenerate NADP+ thus avoiding PSI acceptor-side limitations, and over-expression of FLV proteins 1 and 3 in cyanobacteria have been observed to stabilize PSI under fluctuating light (Allahverdiyeva et al., 2013). Mechanism (i) mitochondrial cooperation, also generates ATP but the ATP is probably not reshuttled back into the chloroplast, its major role would be thus to regenerate oxidized NADP+ (Figure 2).
Radmer and Kok (1976) first observed the potential for O2 to replace CO2 fixation during a light-to-dark transition or in the presence of CBB cycle inhibitors. The role of such an acceptor side activity within the chloroplast such as Mehler (O2 reduction) or hydrogenase (H+ reduction) would enable Chlamydomonas cells to reoxidise the electron transport chain in the light, convincingly shown after anaerobic incubation (Forti et al., 2005; Ghysels et al., 2013). In the Crpgr5 ΔrbcL, lacking both CO2 fixation and CEF, O2 photoreduction rates can completely compensate for CO2 fixation resulting in WT O2 evolution levels (Johnson et al., 2014). Similarly, in a detached leaf assay addition of antimycin A provokes both production of H2O2 and a strong sustained malate dehydrogenase activity resulting in high rates of mitochondrial O2 uptake (Fridlyand et al., 1998). While very removed from the steady-state metabolic flow observed in WT strains under standard conditions, these experimental observations provide us with the maximal rates for the different pathways, and suggest possible compensatory reactions. It would appear that CEF down regulates ATP-independent O2 reducing pathways and up regulates ATP-dependent CO2 reduction by CBB cycle. Therefore, CEF can be seen as limiting ROS production under acceptor side limitations. Furthermore, it has been suggested that an interplay between CEF and O2 photoreduction acts as a buffer to poise electron flow toward carbon fixation (Backhausen et al., 2000). The action of H2O2 as an activator of NDH CEF in Arabidopsis provides further evidence that O2 photoreduction pathways and CEF are working in tandem (Strand et al., 2015). The model that emerges is that regulation of temporary excesses of reductant at the acceptor side of PSI is controlled by an interplay between CEF, the Mehler reaction, FLV proteins and the malate valve with another level of control exerted by redox regulators such as thioredoxins (Scheibe and Dietz, 2012). These pathways likely form a set of communicating reactions that can rebalance NADPH/NADP+ ratios and avoid PSI photoinhibition.
Concluding Remarks
While the study of mutants reveals to us the limitations of a system, the complete photosynthetic apparatus is perfectly able to acclimate to both light and changing redox conditions with CEF and its protective role over PSI placed centrally as a regulator of this flexibility. Further understanding of PSI photoinhibition, proposed to be a major determinant in crop productivity (Tikkanen et al., 2014), may allow the rational modification of photosynthesis to improve the efficiency of plant crops and the production of renewable algal biomass.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Agence Nationale de la Recherche (ChloroPaths: ANR-14-CE05-0041-01) and the CEA Tech Department of Commissariat des Energies Atomiques et Energies Alternatives (CEA) for FC grant. We thank Jean Alric for constructive discussions.
References
- Allahverdiyeva Y., Mustila H., Ermakova M., Bersanini L., Richaud P., Ajlani G., et al. (2013). Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. U.S.A. 110, 4111–4116. 10.1073/pnas.1221194110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y., Suorsa M., Tikkanen M., Aro E. M. (2015). Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 66, 2427–2436. 10.1093/jxb/eru463 [DOI] [PubMed] [Google Scholar]
- Allorent G., Tokutsu R., Roach T., Peers G., Cardol P., Girard-Bascou J., et al. (2013). A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25, 545–557. 10.1105/tpc.112.108274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alric J. (2010). Cyclic electron flow around photosystem I in unicellular green algae. Photosynth. Res. 106, 47–56. 10.1007/s11120-010-9566-4 [DOI] [PubMed] [Google Scholar]
- Alric J. (2014). Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii: (II) involvement of the PGR5-PGRL1 pathway under anaerobic conditions. Biochim. Biophys. Acta 1837, 825–834. 10.1016/j.bbabio.2014.01.024 [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Allen M. B., Whatley F. R. (1954). Photosynthesis by isolated chloroplasts. Nature 174, 394–396. 10.1038/174394a0 [DOI] [PubMed] [Google Scholar]
- Asada K. (2000). The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1419–1431. 10.1098/rstb.2000.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson T. J., Cruz J. A., Kanazawa A., Kramer D. M. (2005). Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 102, 9709–9713. 10.1073/pnas.0503952102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhausen J. E., Kitzmann C., Horton P., Scheibe R. (2000). Electron acceptors in isolated intact spinach chloroplasts act hierarchically to prevent over-reduction and competition for electrons. Photosynth. Res. 64, 1–13. 10.1023/A:1026523809147 [DOI] [PubMed] [Google Scholar]
- Bendall D. S., Manasse R. S. (1995). Cyclic Photophosphorylation and Electron Transport. Biochim. Biophys. Acta 1229, 23–38. 10.1016/0005-2728(94)00195-B [DOI] [Google Scholar]
- Bergner S. V., Scholz M., Trompelt K., Barth J., Gabelein P., Steinbeck J., et al. (2015). STATE TRANSITION7-dependent phosphorylation is modulated by changing environmental conditions, and its absence triggers remodeling of photosynthetic protein complexes. Plant Physiol. 168, 615–634. 10.1104/pp.15.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. (1979). A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim. Biophys. Acta 548, 128–138. 10.1016/0005-2728(79)90193-2 [DOI] [PubMed] [Google Scholar]
- Caffarri S., Tibiletti T., Jennings R. C., Santabarbara S. (2014). A comparison between plant photosystem I and photosystem II architecture and functioning. Curr. Peptide Sci. 15, 296–331. 10.2174/1389203715666140327102218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G., Pesaresi P., Masiero S., Aseeva E., Schunemann D., Finazzi G., et al. (2008). A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132, 273–285. 10.1016/j.cell.2007.12.028 [DOI] [PubMed] [Google Scholar]
- Dang K. V., Plet J., Tolleter D., Jokel M., Cuine S., Carrier P, et al. (2014). Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 26, 3036–3050. 10.1105/tpc.114.126375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats C., Mus F., Cuine S., Billon E., Cournac L., Peltier G. (2009). Characterization of Nda2, a plastoquinone-reducing type II NAD(P)H dehydrogenase in Chlamydomonas chloroplasts. J. Biol. Chem. 284, 4148–4157. 10.1074/jbc.M804546200 [DOI] [PubMed] [Google Scholar]
- Durrant J. R., Giorgi L. B., Barber J., Klug D. R., Porter G. (1990). Characterization of triplet states in isolated photosystem II reaction centers—oxygen quenshing as a mechanism for photodamage. Biochim. Biophysica Acta 42, 9205–9213. [Google Scholar]
- Forti G., Caldiroli G. (2005). State transitions in Chlamydomonas reinhardtii. The role of the Mehler reaction in state 2-to-state 1 transition. Plant Physiol. 137, 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti G., Elli G. (1995). The function of ascorbic acid in photosynthetic phosphorylation. Plant Physiol. 109, 1207–1211. 10.1007/978-94-009-0173-5_431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti G., Elli G. (1996). Stimulation of photophosphorylation by ascorbate as a function of light intensity. Plant Physiol. 112, 1509–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlyand L. E., Backhausen J. E., Scheibe R. (1998). Flux control of the malate valve in leaf cells. Arch. Biochem. Biophys. 349, 290–298. 10.1006/abbi.1997.0482 [DOI] [PubMed] [Google Scholar]
- Ghysels B., Godaux D., Matagne R. F., Cardol P., Franck F. (2013). Function of the chloroplast hydrogenase in the microalga Chlamydomonas: the role of hydrogenase and state transitions during photosynthetic activation in anaerobiosis. PLoS ONE 8:e64161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle A. P., Blunder T., Wunder T., Pesaresi P., Pribil M., Armbruster U., et al. (2013). PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol. Cell. 49, 511–523. 10.1016/j.molcel.2012.11.030 [DOI] [PubMed] [Google Scholar]
- Hideg E., Vass I. (1995). Singlet oxygen is not produced in photosystem-I under photoinhibitory conditions. Photochem. Photobiol. 62, 949–952. 10.1111/j.1751-1097.1995.tb09162.x [DOI] [Google Scholar]
- Jans F., Mignolet E., Houyoux P. A., Cardol P., Ghysels B., Cuine S., et al. (2008). A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc. Natl. Acad. Sci. U.S.A. 105, 20546–20551. 10.1073/pnas.0806896105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joet T., Cournac L., Horvath E. M., Medgyesy P., Peltier G. (2001). Increased sensitivity of photosynthesis to antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol. 125, 1919–1929. 10.1104/pp.125.4.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X., Alric J. (2012). Interaction between starch breakdown, acetate assimilation, and photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. J. Biol. Chem. 287, 26445–26452. 10.1074/jbc.M112.370205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X., Steinbeck J., Dent R. M., Takahashi H., Richaud P., Ozawa S., et al. (2014). Proton gradient regulation 5-mediated cyclic electron flow under ATP- or redox-limited conditions: a study of DeltaATpase pgr5 and DeltarbcL pgr5 mutants in the green alga Chlamydomonas reinhardtii. Plant Physiol. 165, 438–452. 10.1104/pp.113.233593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X., Wostrikoff K., Finazzi G., Kuras R., Schwarz C., Bujaldon S., et al. (2010). MRL1, a conserved Pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in Chlamydomonas and Arabidopsis. Plant Cell 22, 234–248. 10.1105/tpc.109.066266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P., Johnson G. N. (2011). Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. U.S.A. 108, 13317–13322. 10.1073/pnas.1110189108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. (1966). Photoinhibition of chloroplast reactions. II. Multiple Effects. Plant Physiol. 41, 1044–1049. 10.1104/pp.41.6.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa A., Kramer D. M. (2002). In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc. Nat. Acad. Sci. U.S.A. 99, 12789–12794. 10.1073/pnas.182427499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Kumaraswamy G. K., Vinyard D. J., Gu H., Ananyev G., Posewitz M. C., et al. (2015). Metabolic and photosynthetic consequences of blocking starch biosynthesis in the green alga Chlamydomonas reinhardtii sta6 mutant. Plant J. 81, 947–960. 10.1111/tpj.12783 [DOI] [PubMed] [Google Scholar]
- Kukuczka B., Magneschi L., Petroutsos D., Steinbeck J., Bald T., Powikrowska M., et al. (2014). Proton gradient regulation5-like1-mediated cyclic electron flow is crucial for acclimation to anoxia and complementary to nonphotochemical quenching in stress adaptation. Plant Physiol. 165, 1604–1617. 10.1104/pp.114.240648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. P., Bjorkman O., Shih C., Grossman A. R., Rosenquist M., Jansson S., et al. (2000). A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395. 10.1038/35000131 [DOI] [PubMed] [Google Scholar]
- Livingston A. K., Kanazawa A., Cruz J. A., Kramer D. M. (2010). Regulation of cyclic electron flow in C(3) plants: differential effects of limiting photosynthesis at ribulose-1,5-bisphosphate carboxylase/oxygenase and glyceraldehyde-3-phosphate dehydrogenase. Plant Cell Environ. 33, 1779–1788. 10.1111/j.1365-3040.2010.02183.x [DOI] [PubMed] [Google Scholar]
- Mehler A. H. (1951). Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch. Biochem. Biophys. 34, 339–351. 10.1016/0003-9861(51)90012-4 [DOI] [PubMed] [Google Scholar]
- Munekage Y., Hashimoto M., Miyake C., Tomizawa K., Endo T., Tasaka M., et al. (2004). Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429, 579–582. 10.1038/nature02598 [DOI] [PubMed] [Google Scholar]
- Munekage Y., Hojo M., Meurer J., Endo T., Tasaka M., Shikanai T. (2002). PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110, 361–371. 10.1016/S0092-8674(02)00867-X [DOI] [PubMed] [Google Scholar]
- Murata N., Allakhverdiev S. I., Nishiyama Y. (2012). The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, alpha-tocopherol, non-photochemical quenching, and electron transport. Biochim. Biophys. Acta 1817, 1127–1133. 10.1016/j.bbabio.2012.02.020 [DOI] [PubMed] [Google Scholar]
- Nishikawa Y., Yamamoto H., Okegawa Y., Wada S., Sato N., Taira Y., et al. (2012). PGR5-dependent cyclic electron transport around PSI contributes to the redox homeostasis in chloroplasts rather than CO(2) fixation and biomass production in rice. Plant Cell Physiol. 53, 2117–2126. 10.1093/pcp/pcs153 [DOI] [PubMed] [Google Scholar]
- Peers G., Truong T. B., Ostendorf E., Busch A., Elrad D., Grossman A. R., et al. (2009). An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462, 518–521. 10.1038/nature08587 [DOI] [PubMed] [Google Scholar]
- Peltier G., Tolleter D., Billon E., Cournac L. (2010). Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth. Res. 106, 19–31. 10.1007/s11120-010-9575-3 [DOI] [PubMed] [Google Scholar]
- Petroutsos D., Terauchi A. M., Busch A., Hirschmann I., Merchant S. S., Finazzi G., et al. (2009). PGRL1 participates in iron-induced remodeling of the photosynthetic apparatus and in energy metabolism in Chlamydomonas reinhardtii. J. Biol. Chem. 284, 32770–32781. 10.1074/jbc.M109.050468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. (1976). Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 58, 336–340. 10.1104/pp.58.3.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott M., Martins N. F., Thiele W., Lein W., Bock R., Kramer D. M., et al. (2011). ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23, 304–321. 10.1105/tpc.110.079111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumberg B., Reinwald E., Schroder H., Siggel U. (1968). Correlation between electron flow, proton translocation and phosphorylation in chloroplasts. Naturwissenschaften 55, 77–79. 10.1007/BF00599483 [DOI] [PubMed] [Google Scholar]
- Santabarbara S. (2006). Limited sensitivity of pigment photo-oxidation in isolated thylakoids to singlet excited state quenching in photosystem II antenna. Arch. Biochem. Biophys. 455, 77–88. 10.1016/j.abb.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Satoh K. (1970a). Mechanism of photoinactivation in photosynthetic systems.1. dark reaction in photoinactivation. Plant Cell Physiol. 11, 15–27. [Google Scholar]
- Satoh K. (1970b). Mechanism of photoinactivation in photosynthetic systems.2. occurrence and properties of 2 different types of photoinactivation. Plant Cell Physiol. 11, 29–38. [Google Scholar]
- Satoh K. (1970c). Mechanism of photoinactivation in photosynthetic systems.3. site and mode of photoinactivation in photosystem.1. Plant Cell Physiol. 11, 187–197. [Google Scholar]
- Scheibe R., Dietz K. J. (2012). Reduction-oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant Cell Environ. 35, 202–216. 10.1111/j.1365-3040.2011.02319.x [DOI] [PubMed] [Google Scholar]
- Schlodder E., Cetin M., Byrdin M., Terekhova I. V., Karapetyan N. V. (2005). P700+- and 3P700-induced quenching of the fluorescence at 760 nm in trimeric Photosystem I complexes from the cyanobacterium Arthrospira platensis. Biochim. Biophys. Acta 1706, 53–67. 10.1016/j.bbabio.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Setif P., Brettel K. (1990). Photosystem-I photochemistry under highly reducing conditions—study of the P700 triplet-state formation from the secondary radical pair (P700+-A1-). Biochim. Biophys. Acta 1020, 232–238. 10.1016/0005-2728(90)90152-T [DOI] [Google Scholar]
- Setif P., Hervo G., Mathis H. (1981). Flash-induced absorption chnages in photosystem I. Radical Pair or Triplet Formation? Biochim. Biophys. Acta 638, 257–267. 10.1016/0005-2728(81)90235-8 [DOI] [Google Scholar]
- Shikanai T. (2014). Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr. Opin. Biotechnol. 26, 25–30. 10.1016/j.copbio.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Shubin V. V., Terekhova I. N., Kirillov B. A., Karapetyan N. V. (2008). Quantum yield of P700+ photodestruction in isolated photosystem I complexes of the cyanobacterium Arthrospira platensis. Photochem. Photobiol. Sci. 7, 956–962. 10.1039/b719122g [DOI] [PubMed] [Google Scholar]
- Sonoike K. (2011). Photoinhibition of photosystem I. Physiol. Plant. 142, 56–64. 10.1111/j.1399-3054.2010.01437.x [DOI] [PubMed] [Google Scholar]
- Sonoike K., Terashima I., Iwaki M., Itoh S. (1995). Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 362, 235–238. 10.1016/0014-5793(95)00254-7 [DOI] [PubMed] [Google Scholar]
- Stiehl H. H., Witt H. T. (1969). Quantitative treatment of the function of plastoquinone in phostosynthesis. Z. Naturforsch. B 24, 1588–1598. 10.1515/znb-1969-1219 [DOI] [PubMed] [Google Scholar]
- Strand D. D., Livingston A. K., Satoh-Cruz M., Froehlich J. E., Maurino V. G., Kramer D. M. (2015). Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc. Natl. Acad. Sci. U.S.A. 112, 5539–5544. 10.1073/pnas.1418223112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M., Jarvi S., Grieco M., Nurmi M., Pietrzykowska M., Rantala M., et al. (2012). PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948. 10.1105/tpc.112.097162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K., Kanazawa A., Kramer D. M. (2008). Depletion of stromal P(i) induces high ‘energy-dependent’ antenna exciton quenching (q(E)) by decreasing proton conductivity at CF(O)-CF(1) ATP synthase. Plant Cell Environ. 31, 235–243. 10.1111/j.1365-3040.2007.01753.x [DOI] [PubMed] [Google Scholar]
- Telfer A. (2014). Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of beta-carotene. Plant Cell Physiol. 55, 1216–1223. 10.1093/pcp/pcu040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M., Mekala N. R., Aro E. M. (2014). Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta 1837, 210–215. 10.1016/j.bbabio.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Rantala S., Aro E. M. (2015). Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front. Plant Sci. 6:521. 10.3389/fpls.2015.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleter D., Ghysels B., Alric J., Petroutsos D., Tolstygina I., Krawietz D., et al. (2011). Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23, 2619–2630. 10.1105/tpc.111.086876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yamamoto H., Shikanai T. (2015). Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochim. Biophys. Acta 1847, 931–938. 10.1016/j.bbabio.2014.11.013 [DOI] [PubMed] [Google Scholar]


