Abstract
Hydrogen peroxide (H2O2) is known to be generated in Photosystem II (PSII) via enzymatic and non-enzymatic pathways. Detection of H2O2 by different spectroscopic techniques has been explored, however its sensitive detection has always been a challenge in photosynthetic research. During the recent past, fluorescence probes such as Amplex Red (AR) has been used but is known to either lack specificity or limitation with respect to the minimum detection limit of H2O2. We have employed an electrochemical biosensor for real time monitoring of H2O2 generation at the level of sub-cellular organelles. The electrochemical biosensor comprises of counter electrode and working electrodes. The counter electrode is a platinum plate, while the working electrode is a mediator based catalytic amperometric biosensor device developed by the coating of a carbon electrode with osmium-horseradish peroxidase which acts as H2O2 detection sensor. In the current study, generation and kinetic behavior of H2O2 in PSII membranes have been studied under light illumination. Electrochemical detection of H2O2 using the catalytic amperometric biosensor device is claimed to serve as a promising technique for detection of H2O2 in photosynthetic cells and subcellular structures including PSII or thylakoid membranes. It can also provide a precise information on qualitative determination of H2O2 and thus can be widely used in photosynthetic research.
Keywords: photosystem II, superoxide anion radical, hydrogen peroxide, reactive oxygen species, amperometric biosensor, EPR-spin trapping
Introduction
Photosystem II (PSII) is a multi-subunit pigment-protein complex which is located in the thylakoid membrane of chloroplasts of cyanobacteria, algae and higher plants that comprises of more than 25 proteins and the concomitant cofactors (Ferreira et al., 2004; Loll et al., 2005; Guskov et al., 2009; Kawakami et al., 2011). In plants, photosynthesis has been considered as a source of reactive oxygen species (ROS) production which works in close association with regulated mechanism of antioxidant network under normal conditions. The ROS in plants are known to be involved in cell toxicity, defense and signaling, and have been recently overviewed (Krieger-Liszkay, 2005; Foyer and Shigeoka, 2011; Schmitt et al., 2014).
Chlorophyll pigments of the PSII antenna complex absorb light energy and use it for the oxidation of water molecules and reduction of plastoquinone. Light energy absorbed by chlorophyll pigments converted into the energy of separated charges and consequent water-plastoquinone oxidoreductase activity is involuntarily linked with the production of ROS (Pospíšil, 2009, 2012). In unison, released molecular oxygen serves as a forerunner of ROS, which at low concentration play an important role in cell regulation, whereas if formed in excess, is responsible for oxidation of biomolecules such as lipid, proteins, and nucleic acid (Halliwell and Gutteridge, 2007). In addition, direct oxidation of proteins and lipids by UV irradiation and toxic chemicals following subsequent chemical reactions are also known to be associated with formation of ROS (Halliwell and Gutteridge, 2007; Prasad and Pospíšil, 2012).
Production of ROS arises when excitation energy transfer to the PSII reaction center is inadequate or there is inhibition of electron transport chain in PSII. The redox couples in PSII covers a broad range of redox potential, it ranges from a very high negative value of redox couple, Pheo/Pheo− (Em = −610 mV) to a very high positive value of redox couple P680+/P680 (Em = +1250 mV) (Rappaport and Diner, 2008; Pospíšil, 2012). PSII is capable of either oxidizing water molecule or reducing molecular oxygen on the electron donor and on the electron acceptor side of the membrane, respectively (Pospíšil, 2012). There is leakage of electrons to molecular oxygen during the electron transport on the electron acceptor side of PSII (Pospíšil, 2009).
Formation of O results from non-enzymatic and enzymatic one-electron reductions of molecular oxygen. Pheophytin (Pheo•−) (Ananyev et al., 1994; Pospíšil et al., 2004), tightly bound plastosemiquinone (Q), (Cleland and Grace, 1999; Pospíšil et al., 2004), loosely bound plastosemiquinone (Q), (Zhang et al., 2003; Yadav et al., 2014), and free plastosemiquinone (PQ•−) (Mubarakshina and Ivanov, 2010) maintains non-enzymatic reduction of molecular oxygen to O. Heme iron of low-potential (LP) form of cyt b559 reduces molecular oxygen to O in the enzymatic reaction pathway (Pospíšil et al., 2006; Pospišil, 2011).
One electron reduction of O either via non-enzymatic or enzymatic reaction pathway results in the formation of H2O2. In spontaneous dismutation, O provides an additional electron to another O, and then with protonation brings about the formation of H2O2. In enzymatic dismutation, the ferrous heme iron of HP form of cyt b559 drives the catalysis of one-electron reduction of HO to H2O2 (Tiwari and Pospísil, 2009; Pospišil, 2011). Spontaneous dismutation is preferred where there is availability of protons e.g., at the membrane edge while PSII metal centers are chosen to catalyze the dismutation reaction in the interior of the membrane (Pospíšil, 2012). The one-electron reduction of O to H2O2 is catalyzed by superoxide dismutase (SOD) and is known to occur predominantly in the mitochondria, peroxisomes, and cytoplasm. At the physiological pH, the dismutation reaction is preferably catalyzed by SOD.
Several spectroscopic techniques (fluorescence and chemiluminescence) and chromatographic techniques (high performance liquid chromatography coupled with peroxyoxalate chemiluminescence detection) have been used in the past for the determination of H2O2 in living cells (Mills et al., 2007; Chen et al., 2009; Ahammad, 2013). Light induced production of H2O2 have been measured in PSII membranes by oxidation of thiobenzamide with lactoperoxidase. Thiobenzamide sulfoxide was quantified by its absorbance at 370 nm (Schröder and Åkerlund, 1990; Arató et al., 2004; Pospíšil et al., 2004). Production of H2O2 by chloroplasts have been measured by the AmplexRed fluorescence assays (Mubarakshina and Ivanov, 2010; Yadav and Pospíšil, 2012). Hydrogen peroxide (H2O2) detecting probes, 3,3 diaminobenzidine (DAB), Amplex Red (AR), Amplex Ultra Red (AUR), and a europium-tetracycline complex (Eu3Tc) have been compared by infiltrating into tobacco leaves and tested for sensitivity to light, toxicity, subcellular localization, and capacity to detect H2O2 in vivo (Šnyrychová et al., 2009). The induction of H2O2 generation at the leaf level after 3-acetyl-4-hydroxyl-5-isopropylpyrroline-2-dione (3-AIPTA) or bentazon treatment has been detected by performing histochemical analysis with 3, 3 DAB staining (Chen et al., 2012). Apart from several spectroscopic techniques (absorbance, fluorescence, chemiluminescence), cyclic voltammetry and histochemical technique, a reporter system based on HSP70A promoter-luciferase fusions have also been developed in past for the detection of H2O2 in vivo (Shao et al., 2007).
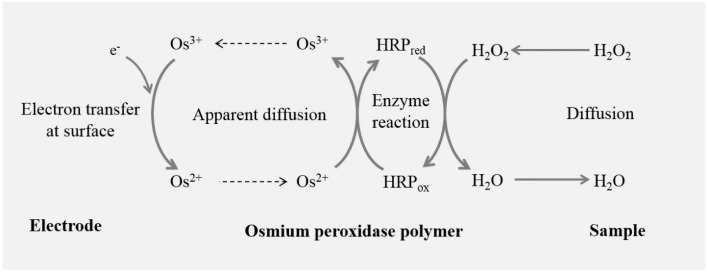
In the electrochemical method, mediator and non-mediator based electrode have been used in the past. Among the mediator based modified electrodes, HRP is the most commonly used material for the modification of the electrode in the last decade (Lin et al., 2000; Camacho et al., 2007; Radi et al., 2009; Ahammad, 2013). The sensitivity and selectivity of H2O2 biosensor depends on the material used and modifications involved (Lin et al., 2000; Nakabayashi and Yoshikawa, 2000; Yao et al., 2005; Wang and Zhang, 2006; Camacho et al., 2007; Radi et al., 2009; Inoue et al., 2010; Li et al., 2013). The mechanism of detection of H2O2 biosensor is described in Scheme 1. The enzyme HRP is converted to its oxidized form, which is than reduced at the surface of the carbon electrode by the transfer of the electron via the mediator. Different mediators have been used in the past including methylene blue (Lin et al., 2000; Kafi et al., 2009; Tiwari and Singh, 2011); quinones predominantly hydroquinones (Lei et al., 2004, 2005; Zhang et al., 2008; Yang et al., 2010); ferrocene (Wang et al., 2005; Senel et al., 2010), and ferrocene carboxylic acid (Tian et al., 2001; Tripathi et al., 2006; Liu et al., 2011; Luo et al., 2011). The detection limit in utmost cases were found to be in the concentration range of units of millimolar or micromolar (μM) while a limited reports showed detection limit in tens of nanomolar (nM) concentration (Zhang et al., 2008; Loew et al., 2009; Lu et al., 2010). Recently, Os-HRP was compared with native HRP based coated glassy carbon electrode and it was found to possess high sensitivity for hydroperoxide. The electrodes were tested for H2O2 and hydroperoxide in the concentration range of 0.01–1 μM (Loew et al., 2009).
Scheme 1.
Working principle of catalytic amperometric biosensor device: schematic illustration shows the working principle of Osmium-horseradish peroxidase (Os-HRP) modified carbon electrode depicting the oxidation-reduction cycle leading to generation of reduction current for H2O2.
Non-mediator based H2O2 biosensor has also been widely used in the past; however, it is known that the electron transfer between HRP and the electrode is difficult due to higher distance between the active site of HRP and the electrode. The voltammetric detection of H2O2 at carbon electrodes is challenging due to the slow electron transfer kinetics associated with the irreversible oxidation of peroxide. An anodic scan has been used as an electrochemical pretreatment and a rapid, sensitive and selective voltammetric method has been developed for the detection of physiological concentrations of H2O2 at uncoated carbon fiber microelectrodes (Sanford et al., 2011).
In this study, we provide an experimental evidence on the detection of H2O2 by using Osmium (Os) as a mediator which promotes shuttling of electrons between the electrode and the enzyme. Detection of H2O2 by using highly sensitive and selective Os-HRP modified electrode was tested in PSII membrane under light illumination. The current study introduces the use of catalytic amperometric biosensors in detection of low level of H2O2 production in PSII membrane.
Materials and methods
Material and chemical reagents
5-(ethoxycarbonyl)-5-methyl-1-pyrroline N-oxide (EMPO) spin trap was obtained from Alexis Biochemicals (Lausen, Switzerland). Capillary tube used for Electron paramagnetic resonance (EPR) measurements was purchased from Blaubrand intraMARK, Brand, Germany. All other chemicals of analytical grade were purchased either from Wako Pure Chemicals Industries, Ltd. (Osaka, Japan), Sigma-Aldrich chemie Gmbh (Munich, Germany), or Sigma-Aldrich Japan K.K (Tokyo, Japan).
Preparation of PSII membrane
Photosystem II (PSII) membranes were prepared from fresh spinach leaves using the method reported previously by Berthold et al. (1981) with modifications described by Ford and Evans (1983). All steps during the isolation procedure were done at 4°C in green light using green LED strip (Photon Systems Instruments (PSI), Drásov, Czech Republic) or under dark condition using different buffers (A and B). The composition of buffer A (pH 7.5) being 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 40 mM HEPES (pH 7.5), 5 mM Na-ascorbate, and 2 g/l bovine serum albumin (BSA) while buffer B was composed of 400 mM sucrose, 15 mM NaCl and 5 mM MgCl2, 40 mM MES (pH 6.5). Spinach leaves were washed twice with deionized water. Na-ascorbate and BSA were added immediately before crushing the spinach leaves. Dark adapted leaves (400 g) were homogenized with 500 ml of buffer A. This step was followed by filtering the homogenized mixture through 2 layers of nylon bolting cloth. Filtrate was transferred into ice-chilled centrifugation tubes and was centrifuged for 10 min at 9950 × g at 4°C. The supernatant was thrown out and pellet was mixed properly with paint brush. The pellet was then resuspended in 600 ml of buffer B and again centrifuged at 9950 × g for 10 min at 4°C. After the centrifugation, supernatant was discarded and pellet was again resuspended in buffer B, at this step concentration of chlorophyll was measured. This step was followed by treating the suspension with 5% Triton X-100 on ice bath with continuous stirring for 17 min, and then centrifugation at 7000 × g for 7 min. The pellet was discarded and supernatant was centrifuged again at 48,000 × g for 20 min at 4°C. Pellet was washed for 3 times with buffer B and at the final step, and chlorophyll concentration was measured. PSII membrane were diluted to final chlorophyll concentration (3–6 mg Chl ml−1) and were stored at −80°C until further use.
Chlorophyll concentration was determined in aqueous 80 % (v/v) acetone by absorbance at 646 and 663 nm according to the method described by Lichtenthaler (1987).
Light illumination
PSII membranes were exposed to continuous white light (1,000 μmol photons m−2s−1) for time period as required during the different experimental setups. The illumination was performed using halogen lamps with a light guide (KL1500 Electronic, Schott, Mainz, Germany and PL075, Hoya Candeo Optonics, Japan). The light intensity was measured by quantum radiometer LI-189 and LI-185B (LI-COR Inc., Lincoln, U.S.A.).
Electron paramagnetic resonance (EPR) spin-trapping spectroscopy
The detection of O was performed by EPR spin-trapping spectroscopy. Superoxide anion radical was detected by spin trapping using EMPO, 5-(ethoxycarbonyl)-5-methyl-1-pyrroline N-oxide. The experimental conditions are as follows: PSII membranes (150 μg Chl ml−1); EMPO, 25 mM; phosphate buffer, 40 mM (pH 6.5), DCMU, 20 μM and desferal, 50 μM. Spectra were recorded using EPR spectrometer Mini Scope MS400 (Magnettech GmbH, Berlin, Germany) with EPR conditions as follows: microwave power, 10 mW; modulation amplitude, 1 G; modulation frequency, 100 kHz; sweep width, 100 G; scan rate, 1.62 G s−1. Formation of light-induced EMPO-OOH adduct EPR spectra was measured in PSII membranes after illumination of PSII membranes in glass capillary tube with continuous white light (1000 μmol photons m−2 s−1).
Fabrication of Osmium-HRP (Os-HRP) modified carbon electrode
The electrochemical biosensor used in the study was prepared using carbon-electrode (BAS Inc, ALS Co., Ltd., Japan). Prior to each measurement, the carbon electrode was cleaned using PK-3 Electrode Polishing Kit (BAS Inc., ALS Co., Ltd., Japan) with the aim to remove redox reaction products accumulated on the electrode surface. This step was followed by spreading/dropping of 0.5 μL aliquot of Os-HRP polymer solution (Bioanalytical System, USA) on the carbon electrode (φ, 1 mm). The solution was allowed to form a circular thin film after overnight drying at 4°C under dark condition.
Cyclic voltammetry of Os-HRP modified carbon electrode
For the basic characterization of the Os-HRP modified carbon electrode, a portion of the electrode was soaked in 10 ml of 40 mM Na-phosphate buffer and cyclic voltammetry was performed. Cyclic voltammetry was conducted at a scan rate of 20 mV/s from 0.0 to +0.5 V at room temperature.
Hydrogen peroxide detection using catalytic amperometric biosensor
The electrochemical measurements were performed using a potentiostat (HA1010mM4S; Hokuto Denke Co. Ltd., Japan). For the assay to study H2O2 generated in PSII membrane, the PSII membranes at a concentration of 150 μg Chl ml−1 was introduced into a six well Repro plate (IFP, Research unit for the functional peptides, Yamagata, Japan). Ag/AgCl electrode was used as a reference electrode and Os-HRP modified carbon electrode was used as working electrode. Platinum plate in the dimension of 5*5*0.1 mm was used as a counter electrode. Kinetics on the formation of light-induced H2O2 was measured in PSII membranes after illumination under continuous white light (1000 μmol photons m−2 s−1) for a duration of 1 h. The sampling time was kept at 500 ms.
Results
Superoxide anion radical production in PSII membranes
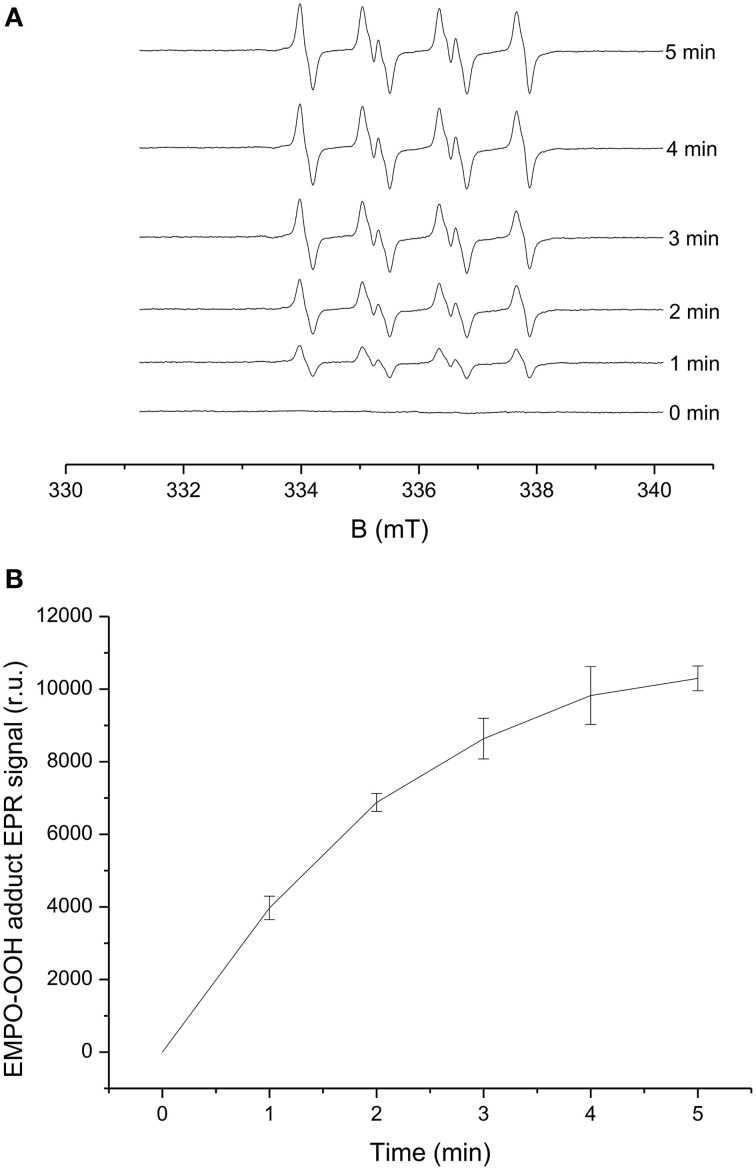
The light-induced formation of O in PSII membranes was measured using EPR spin-trapping spectroscopy. The spin-trapping was accomplished by the spin-trap compound 5-(ethoxycarbonyl)-5-methyl-1-pyrroline N-oxide (EMPO). The EMPO did not induce any EMPO-OOH adduct EPR signal in non-illuminated PSII membranes, whereas illumination with a continuous white light (1,000 μmol photons m−2 s−1) resulted in the formation of the EMPO-OOH adduct EPR signal (Figure 1A). Time dependence of EMPO-OOH adduct EPR signal shows that formation of O is enhanced linearly for a duration upto 5 min of light illumination (Figure 1B). These results suggest that illumination of PSII membranes with a continuous white light (1,000 μmol photons m−2 s−1) results in O production.
Figure 1.
Light-induced EMPO-OOH adduct EPR spectra measured in PSII membranes. EMPO-OOH adduct EPR spectra were measured under light illumination (1000 μmol photons m−2 s−1) of PSII membranes (150 μg Chl ml−1) in the presence of 25 mM EMPO, 100 μM desferal, and 40 mM phosphate buffer (pH 6.5). (A) Shows the spectra measured in the time range of 0–5 min of illumination and 1 (B) shows time profile of EMPO-OOH adduct EPR spectra. The intensity of EPR signal was evaluated by measuring the relative height of central peak of the first derivative of the EPR absorption spectrum. The data represent the mean value (±SD) where n = 3.
Effect of superoxide dismutase and catalase on O production in PSII membranes
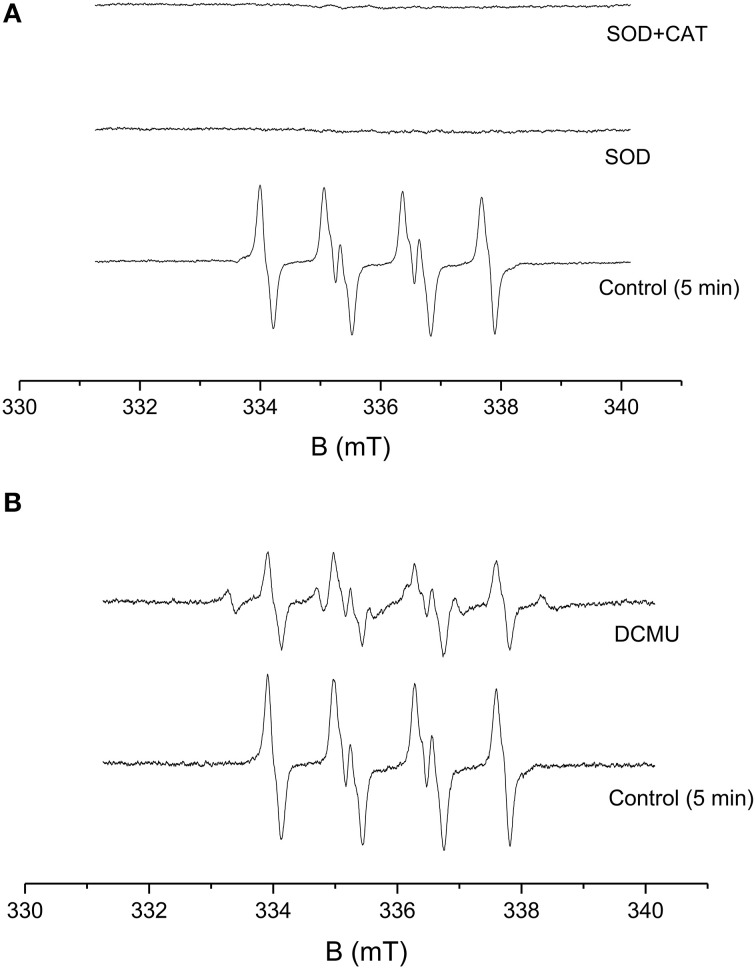
To study the contribution of O leading to H2O2 formation in PSII membranes under light illumination, the effect of SOD and catalase (CAT) were studied on O. Upon addition of exogenous SOD, which is known to catalyze the dismutation of O to H2O2 to PSII membranes prior to illumination, EMPO-OOH adduct EPR signal was found to diminish completely. The simultaneous addition of SOD and CAT was also found to suppress the EMPO-OOH EPR signal completely (Figure 2A). This observation indicates that O produced during light illumination is most likely involved in H2O2 formation in PSII membranes.
Figure 2.
Effect of SOD, CAT, and DCMU on EMPO-OOH adduct EPR spectra measured in PSII membranes. EMPO-OOH adduct EPR spectra were measured in PSII membranes in the presence of SOD and CAT under light illumination. The relative intensity of the light-induced EMPO-OOH adduct EPR signal measured in the presence of SOD (400 U/ml) and SOD+CAT (400 U/ml each) (A) and DCMU (20 μM) (B). The other experimental conditions were the same as described in Figure 1.
Effect of DCMU on O production in PSII membranes
The effect of herbicides, DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea] (Sigma Aldrich, Germany) was tested for its effect on EMPO-OOH adduct EPR signal in PSII membranes. The effect of DCMU which is known to block the electron transfer from QA to QB was studied on light-induced formation of O. Figure 2B shows that the addition of DCMU suppressed EMPO-OOH adduct EPR signal approximately by 50% (Figure 2B). These observations indicate that loosely bound plastosemiquinone bound at or after the QB site can contribute to the overall production of O via the reduction of molecular oxygen.
Characterization of Os-HRP modified carbon electrode
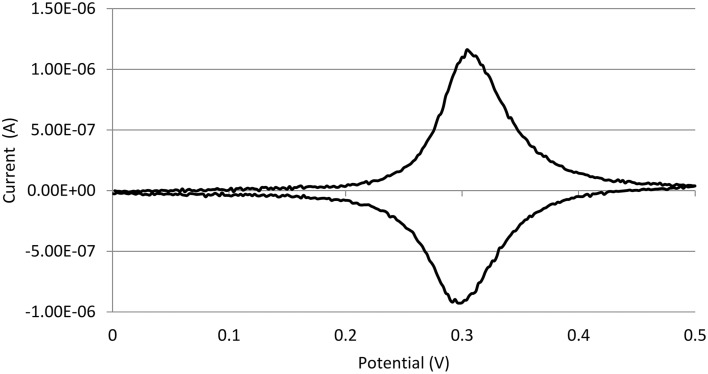
The characterization of the Os-HRP modified carbon electrode was performed using cyclic voltammetry (Figure 3). Cyclic voltammetry was conducted at a scan rate of 20 mV/s from 0.0 to + 0.5 V at room temperature. The oxidation and reduction current were obtained at 0.3 V vs. Ag/AgCl. Based on the data obtained, the surface concentration of Os-HRP on the carbon electrode was calculated to be 6.78 × 10−9 mol/cm2. The Calibration curve of Os-HRP modified carbon electrode was also measured using standard H2O2 solution (Supplementary Data).
Figure 3.
Characterization of Os-HRP modified carbon electrode. Characterization of the Os-HRP modified carbon electrode: cyclic voltammetry was performed for the basic characterization of the modified electrode. Cyclic voltammetry was conducted at a scan rate of 20 mV/s from 0.0 to +0.5 V at room temperature.
Hydrogen peroxide production in PSII membranes
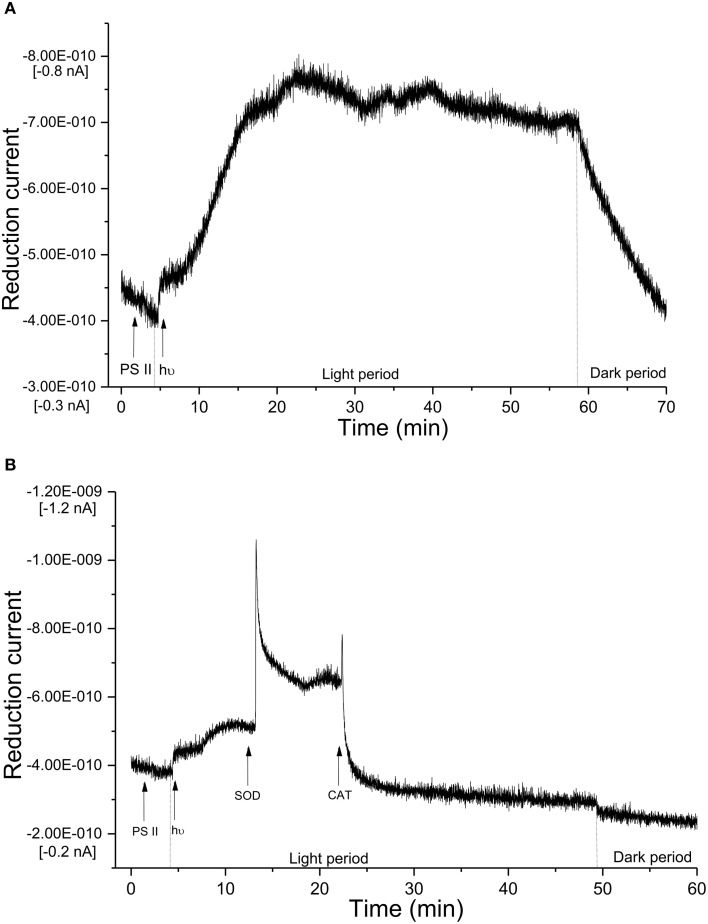
The light-induced formation of H2O2 in PSII membranes was measured using catalytic amperometric biosensor. The study of kinetics of H2O2 production was accomplished using Os-HRP modified carbon electrode. The reduction current generated in the presence of H2O2 was monitored. Under dark condition, no change in the reduction current was observed whereas illumination with a continuous white light (1,000 μ mol photons m−2 s−1) resulted in change in the reduction current. Figure 4A shows typical chronoamperometric responses, the reduction currents gradually increased after light illumination followed by a shoulder which continues for a duration of about 1 h which than rapidly drops at the switching off the light (dark period). The peak value of the reduction current was reached after about 15 min of light illumination with a maximum change in reduction currents of approximately 400 pA. The data presented shows continuous generation of H2O2 till the period of light illumination (Figure 4A). These results shows that illumination of PSII membranes with continuous white light results in H2O2 production.
Figure 4.
Real-time monitoring of reduction current for hydrogen peroxide in PSII membrane. (A) Kinetics of the production of H2O2 was measured using Os-HRP modified carbon electrode during light illumination in PSII membranes. The light illumination was started at 5 min from the start of the measurement and the reduction current was measured for a duration of 1 h. (B) Effect of SOD and CAT on reduction current was measured in the presence of SOD (400 U/ml) and SOD + CAT (400 U/ml each).
Effect of SOD and CAT on H2O2 production in PSII membranes
To monitor the functionality of the sensor, the effect of SOD was measured under light-illumination. Reduction current for H2O2was measured for first 5 min of light illumination where a linear increase was observed similar to control (Figure 4A). Upon addition of exogenous SOD to PSII membranes during illumination, a fast increase in reduction current was observed bringing a considerable change of about 600 pA. The fast increase was followed by a rapid drop however; the reduction current was still higher as compared to reduction current recorded before the addition of SOD. The addition of CAT completely suppressed the reduction current by about 100% (Figure 4B). These results show that under illumination of PSII membranes with a continuous white light, H2O2 production in PSII membrane is contributed via dismutation of O.
Discussion
In the current study, we used spectroscopic and amperometric techniques to measure the production of ROS in spinach PSII membrane. Our prime objective was to study the effect of high light stress in PSII membrane reflected by ROS production primarily the generation of H2O2 as the stress response formed via the dismutation of O. Superoxide anion radical which is known to be formed by one-electron reduction of molecular oxygen was measured under light illumination (Figure 1). EPR spin-trapping data obtained using the urea-type herbicide (DCMU) supports the evidence on the involvement of loosely bound plastosemiquinone in O production (Figure 2B). Suppression of EMPO-OOH adduct EPR signal is in agreement with our previously published results where contribution of loosely bound plastosemiquinone at or after the QB site (DCMU-sensitive site) might contribute to the overall O production (Yadav et al., 2014). The EMPO-OOH adduct EPR signal in the presence of DCMU indicates that molecular oxygen is reduced prior to the QB site which is known to occur due to reduction of molecular oxygen by Pheo•− or Q which serves as electron donors to molecular oxygen due to their low redox potentials (Pospíšil, 2012). The observation that the EMPO-OOH adduct was completely suppressed in PSII membranes illuminated in presence of SOD indicates that O formed during the light illumination dismutates to H2O2 prior to its interaction with spin trap (Figure 2A). It can be concluded here that the H2O2 formed in PSII membrane under light illumination is contributed by the dismutation of O.
Amperometric methods for the direct detection of H2O2 has been used since last decades in animal cells (Mouithys-Mickalad et al., 2001; Inoue et al., 2010) however, very limited evidences exist on its application in plant cells (Cleland and Grace, 1999). Amperometric measurements have been implemented at the level of protoplast to study the photosynthetic activity under the effect of benzoquinone (Yasukawa et al., 1998, 1999). Redox response of benzoquinone, p-hydroquinone and oxygen was measured by placing a microelectrode close to an algal protoplast to localize concentration of these species (Yasukawa et al., 1999). Direct detection of H2O2using electrochemical methods; however, had never been reported previously in photosynthetic organelles. H2O2 detection in subchloroplast oxygen-evolving PSII particles and isolated reaction center complexes of PSII has been studied using luminol-peroxidase chemiluminescence and pulse photoactivation (Zastrizhnaya et al., 1997). Production of H2O2 was detected in PSII membranes using AR fluorescent assay (Yadav and Pospíšil, 2012). Levels of H2O2 including O and singlet oxygen has been determined by using both histochemical and fluorescent probes in leaves and thylakoids (Zulfugarov et al., 2014), however, there exist arguments over the selectivity of the molecular probes used.
Two different approaches of the detection of H2O2via electrochemical methods are being used in animal cells. The detection of H2O2 is either via a non-mediator or a mediator based biosensor device (Lin et al., 2000; Nakabayashi and Yoshikawa, 2000; Yao et al., 2005; Wang and Zhang, 2006; Camacho et al., 2007; Jia, 2008; Radi et al., 2009; Ahammad, 2013). In non-mediator based biosensor, the transfer of electron occurs between the electrode and the enzyme (Ahammad, 2013). The preparation process being very simple, the non-mediator based biosensors have been extensively used in the past. In the mediator based biosensor device, the mediator plays a key role to shuttle electron between the electrode and the enzyme (Scheme 1). Most commonly, methylene blue, ferrocene and carboxylic acid are used as mediators (Lin et al., 2000; Tian et al., 2001; Li et al., 2004; Tripathi et al., 2006; Senel et al., 2010). The mediator in the case of HRP is pointed to be important because the shuttling of electron between electrode and HRP is large as the active site of HRP is located deep in the protein sheath (Ahammad, 2013). In our current device, Os acts as a mediator where Os2+ is reduced to Os3+ in the process (Scheme 1). Different modified electrode has been introduced in the past, the detection limit in most case were in the range of μM concentration with a limited contribution where mediator-based HRP biosensor reached a lower detection limit in the range of tens-hundreds nm concentration (Chen et al., 2006; Zhang et al., 2008; Lu et al., 2010).
Using Os-HRP modified carbon electrode, we have observed a change in the reduction current during illumination of PSII membranes reflecting the production of H2O2 (Figure 4A). A change of ~400 pA reflects generation of H2O2 which was then found to be stable in the period of 20–60 min of light illumination. The reduction current was found to drop immediately under dark condition. Based on the considerable changes monitored in reduction current, the biosensor device is claimed to be sensitive for its application in photosynthetic samples.
ROS generated in the cell or organelles are eliminated by antioxidant enzymes such as SOD, glutathione peroxidase, CAT or by low molecular antioxidants such as vitamins, glutathione etc. (Halliwell and Gutteridge, 2007). When SOD which dismutates O to H2O2 was added to the illuminated PSII membrane, the reduction current for H2O2 was observed to gradually enhance leading to the conclusion that the H2O2 generated in the PSII membrane is formed via the formation of O. The addition of SOD (Figure 4B) during the light illumination drastically enhanced the reduction current by 600 pA followed by a sudden drop indicate the fast conversion of O into H2O2 available in the PSII pool. This is in agreement with observed result with EPR signal of the EMPO-OOH adduct observed under the effect of SOD (Figure 2A). The subsequent addition of CAT which converts H2O2 to H2O and molecular oxygen brings back the reduction current close to the value observed under dark condition.
Electrochemical detection of H2O2 is suggested here to be of great importance in addition to other methods that has been used in the recent past. In the detailed study performed by Šnyrychová and co-workers, different H2O2 detecting probes were tested. Probes such as amplex red and amplex ultra-red were found to be sensitive to light and thus are not appropriate for the study on the generation of H2O2 in plant sample where effect of light stress is frequently studied. Based on the results, the authors also suggested that these probes should be used with caution to avoid artifacts (Šnyrychová et al., 2009). In addition to this, the toxicity caused by the exogenous addition of probes cannot be completely excluded. The method of electrochemical measurements is also preferred because of the simplicity, low-cost, high-sensitivity and selectivity (Ahammad, 2013). The simplicity and low-cost of electrochemical measurements is because of the fact that the electrodes can be used for endless time with easy fabrication with designated mediator and enzyme and overnight incubation at 4°C under dark condition or as per standards. Based on the redox potential of the redox couple, the modified electrode are specific for different species. Os-HRP modified electrode is specific for H2O2 and thus can be widely used in photosynthetic research where detection of H2O2 has always been a challenge. The sensitivity of the modified electrode depends on the size and the material used. In our current report, Os-HRP modified carbon electrode has been claimed to be sensitive and highly selective among other H2O2 detection techniques available till date. This fact opens the possibility for using Os-HRP modified electrode for H2O2 detection in photosystem I (PSI) where H2O2 is formed by the transfer of electron from reduced ferredoxin to molecular oxygen via ferredoxin-thioredoxin reductase (Gechev et al., 2006). Plasma membrane NADPH oxidase complex are considered as the major producer of ROS including O and H2O2 in cells (Sagi and Fluhr, 2001, 2006; Halliwell and Gutteridge, 2007). In addition to this, among the enzymatic source of O and H2O2, cell wall bound peroxidases, aminooxidases, flavin containing oxidases, oxalate, and plasma membrane oxidases are known to be involved (Bolwell et al., 2002; Mori and Schroeder, 2004; Svedruzic et al., 2005). In the case of apoplastic oxidative burst, ROS are produced by cell wall bound oxidases, peroxidases and polyamine oxidases (Minibayeva et al., 1998, 2009). Thus, the current electrode also opens the possibility for measuring generation of H2O2 from different and localized structures of plants and animals cells.
Author contributions
AP and SK contributed to the conception and design of the work. AP, AK, MS, HK, TS performed the measurements. AP analyzed, interpreted the data and drafted the manuscript. AK, PP participated in drafting the manuscript. SK, MK, and MT revised it critically for important content. All authors approved the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, Japan. PP would like to thank Ministry of Education, Youth and Sports of the Czech Republic grants no. LO1204 (National Program of Sustainability I), no.CZ.1.07/2.3.00/30.0041 (Support for Building Excellent Research Teams and Intersectoral Mobility at Palacký University) and by Czech Science Foundation grant no. GP13-29294S. We thank Ketaki Vasant Phadke (Department of Biophysics, Palacký University) for cross-checking the manuscript for errors.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00862
References
- Ahammad A. J. S. (2013). Hydrogen peroxide biosensors based on horseradish peroxidase and hemoglobin. J. Biosens. Bioelectron. S9:001 10.4172/2155-6210.S9-001 [DOI] [Google Scholar]
- Ananyev G., Renger G., Wacker U., Klimov V. (1994). The photoproduction of superoxide radicals and the superoxide-dismutase activity of photosystem II. The possible involvement of cytochrome b559. Photosynth. Res. 41, 327–338. 10.1007/BF00019410 [DOI] [PubMed] [Google Scholar]
- Arató A., Bondarava N., Krieger-Liszkay A. (2004). Production of reactive oxygen species in chloride- and calcium-depleted photosystem II and their involvement in photoinhibition. Biochim. Biophys. Acta 1608, 171–180. 10.1016/j.bbabio.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Berthold D. A., Babcock G. T., Yocum C. F. (1981). A highly resolved oxygen evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 134, 231–234. 10.1016/0014-5793(81)80608-4 [DOI] [Google Scholar]
- Bolwell G. P., Bindschedler L. V., Blee K. A., Butt V. S., Davies D. R., Gardner S. L., et al. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp. Bot. 53, 1367–1376. 10.1093/jexbot/53.372.1367 [DOI] [PubMed] [Google Scholar]
- Camacho C., Matías J. C., Chico B., Cao R., Gómez L., Simpson B. K., et al. (2007). Amperometric biosensor for hydrogen peroxide, using supramolecularly immobilized horseradish peroxidase on the β-cyclodextrin-coated gold electrode. Electroanalysis 19, 2538–2542. 10.1002/elan.200703993 [DOI] [Google Scholar]
- Chen S., Yin C., Strasser R. J., Govindjee Yang, C., Qiang S. (2012). Reactive oxygen species from chloroplasts contribute to 3-acetyl-5- isopropyltetramic acid-induced leaf necrosis of Arabidopsis thaliana. Plant Physiol. Biochem. 52, 38–51. 10.1016/j.plaphy.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Chen S., Yuan R., Chai Y., Xu L., Wang N., Li X., et al. (2006). Amperometric hydrogen peroxide biosensor based on the immobilization of Horseradish Peroxidase (HRP) on the layer-by-layer assembly films of gold colloidal nanoparticles and toluidine blue. Electroanalysis 18, 471–477. 10.1002/elan.200503424 [DOI] [Google Scholar]
- Chen W., Li B., Xu C., Wang L. (2009). Chemiluminescence flow biosensor for hydrogen peroxide using DNAzyme immobilized on eggshell membrane as a thermally stable biocatalyst. Biosens. Bioelectron. 24, 2534–2540. 10.1016/j.bios.2009.01.010 [DOI] [PubMed] [Google Scholar]
- Cleland R. E., Grace S. C. (1999). Voltammetric detection of superoxide production by photosystem II. FEBS Lett. 457, 348–352. 10.1016/S0014-5793(99)01067-4 [DOI] [PubMed] [Google Scholar]
- Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S. (2004). Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838. 10.1126/science.1093087 [DOI] [PubMed] [Google Scholar]
- Ford R. C., Evans M. C. W. (1983). Isolation of a photosystem II from higher plants with highly enriched oxygen evolution activity. FEBS Lett. 160, 159–164. 10.1016/0014-5793(83)80957-0 [DOI] [Google Scholar]
- Foyer C. H., Shigeoka S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. 10.1104/pp.110.166181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev T. S., Van Breusegem F., Stone J. M., Denev I., Laloi C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28, 1091–1101. 10.1002/bies.20493 [DOI] [PubMed] [Google Scholar]
- Guskov A., Kern J., Gabdulkhakov A., Broser M., Zouni A., Saenger W. (2009). Cynobacterial photosystem II at 2.9 Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 16, 334–342. 10.1038/nsmb.1559 [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. C. (2007). Free Radicals in Biology and Medicine, 4th Edn. New York, NY: Oxford University Press. [Google Scholar]
- Inoue K. Y., Ino K., Shiku H., Kasai S., Yasukawa T., Mizutani F., et al. (2010). Electrochemical monitoring of hydrogen peroxide released from leucocytes on horseradish peroxidase redox polymer coated electrode chip. Biosens. Bioelectron. 25, 1723–1728. 10.1016/j.bios.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Jia J. (2008). Hydrogen peroxide biosensor based on horseradish peroxidase– Au nanoparticles at a viologen grafted glassy carbon electrode. Microchim. Acta 163, 237–241. 10.1007/s00604-008-0002-9 [DOI] [Google Scholar]
- Kafi A. K., Wu G., Chen A. (2009). A novel hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase onto Au-modified titanium dioxide nanotube arrays. Biosens. Bioelectron. 24, 566–571. 10.1016/j.bios.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Kawakami K., Umena Y., Kamiya N., Shen J.-R. (2011). Structure of the catalytic, inorganic core of oxygen-evolving photosystem II at 1.9 Å resolution. J. Photochem. Photobiol. B 104, 9–18. 10.1016/j.jphotobiol.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. (2005). Singlet oxygen production in photosynthesis. J. Exp. Bot. 56, 337–346. 10.1093/jxb/erh237 [DOI] [PubMed] [Google Scholar]
- Lei C. X., Long L. P., Cao Z. L. (2005). An H2O2 biosensor based on immobilization of horseradish peroxidase labeled nano-Au in silica sol-gel/alginate composite film. Anal. Lett. 38, 1721–1734. 10.1080/00032710500207762 [DOI] [Google Scholar]
- Lei C. X., Wang H., Shen G. L., Yu R. Q. (2004). Immobilization of enzymes on the nano-au film modified glassy carbon electrode for the determination of hydrogen peroxide and glucose. Electroanalysis 16, 736–740. 10.1002/elan.200302877 [DOI] [Google Scholar]
- Li C. X., Deng K. Q., Shen G. L., Yu R. Q. (2004). Amperometric hydrogen peroxide biosensor based on horseradish peroxidase-labeled nano-Au colloids immobilized on poly(2,6-pyridinedicarboxylic acid) layer by cysteamine. Anal. Sci. 20, 1277–1281. 10.2116/analsci.20.1277 [DOI] [PubMed] [Google Scholar]
- Li Z. H., Guedri H., Viguier B., Sun S. G., Marty J. L. (2013). Optimization of hydrogen peroxide detection for a methyl mercaptan biosensor. Sensors 13, 5028–5039. 10.3390/s130405028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H. K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382. 10.1016/0076-6879(87)48036-1 [DOI] [Google Scholar]
- Lin X. Q., Chen J., Chen Z. H. (2000). Amperometric biosensor for hydrogen peroxide based on immobilization of horseradish peroxidase on methylene blue modified graphite electrode. Electroanalysis 12, 306–310. 10.1002/(sici)1521-4109(20000301) [DOI] [Google Scholar]
- Liu X., Luo L., Ding Y., Xu Y., Li F. (2011). Hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase on γ-Al2O3 nanoparticles/chitosan film-modified electrode. J. Solid State Electrochem. 15, 447–453. 10.1007/s10008-010-1120-y [DOI] [Google Scholar]
- Loew N., Wollenberger U., Scheller F. W., Katterle M. (2009). Direct electrochemistry and spectroelectrochemistry of osmium substituted horseradish peroxidase. Bioelectrochemistry 76, 28–33. 10.1016/j.bioelechem.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Loll B., Kern J., Saenger W., Zouni A., Biesiadka J. (2005). Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438, 1040–1044. 10.1038/nature04224 [DOI] [PubMed] [Google Scholar]
- Lu L., Zhang L., Zhang X., Wu Z., Huan S., Shen G., et al. (2010). A MgO nanoparticles composite matrix-based electrochemical biosensor for hydrogen peroxide with high sensitivity. Electroanalysis 22, 471–477. 10.1002/elan.200900429 [DOI] [Google Scholar]
- Luo L., Zhu L., Xu Y., Shen L., Wang X., Ding Y., et al. (2011). Hydrogen peroxide biosensor based on horseradish peroxidase immobilized on chitosan-wrapped NiFe2O4 nanoparticles. Microchim. Acta 174, 55–61. 10.1007/s00604-011-0591-6 [DOI] [Google Scholar]
- Mills A., Tommons C., Bailey R. T., Tedford M. C., Crilly P. J. (2007). Reversible, fluorescence-based optical sensor for hydrogen peroxide. Analyst 132, 566–571. 10.1039/b618506a [DOI] [PubMed] [Google Scholar]
- Minibayeva F., Kolesnikov O., Chasov A., Beckett R. P., Lüthje S., Vylegzhanina N., et al. (2009). Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species. Plant Cell. Environ. 32, 497–508. 10.1111/j.1365-3040.2009.01944.x [DOI] [PubMed] [Google Scholar]
- Minibayeva F., Kolesnikov O. P., Gordon L. K. (1998). Contribution of a plasma membrane redox system to the superoxide production by wheat root cells Protoplasma 205, 101–106. 10.1007/BF01279299 [DOI] [PubMed] [Google Scholar]
- Mori I. C., Schroeder J. I. (2004). Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol. 135, 702–708. 10.1104/pp.104.042069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouithys-Mickalad A., Deby-Dupont G., Nys M., Lamy M., Deby C. (2001). Oxidative processes in human promonocytic cells (THP-1) after differentiation into macrophages by incubation with Chlamydia pneumoniae extracts. Biochem. Biophys. Res. Commun. 287, 781–788. 10.1006/bbrc.2001.5643 [DOI] [PubMed] [Google Scholar]
- Mubarakshina M. M., Ivanov B. N. (2010). The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol. Plant. 140, 103–110. 10.1111/j.1399-3054.2010.01391.x [DOI] [PubMed] [Google Scholar]
- Nakabayashi Y., Yoshikawa H. (2000). Amperometric biosensors for sensing of hydrogen peroxide based on electron transfer between horseradish peroxidase and ferrocene as a mediator. Anal. Sci. 16, 609–613. 10.2116/analsci.16.609 [DOI] [Google Scholar]
- Pospíšil P. (2009). Production of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1787, 1151–1160. 10.1016/j.bbabio.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Pospíšil P. (2011). Enzymatic function of cytochrome b559 in photosystem II. J Photochem. Photobiol. B 104, 341–347. 10.1016/j.jphotobiol.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Pospíšil P. (2012). Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1817, 218–231. 10.1016/j.bbabio.2011.05.017 [DOI] [PubMed] [Google Scholar]
- Pospíšil P., Arató A., Krieger-Liszkay A., Rutherford A. W. (2004). Hydroxyl radical generation by Photosystem II. Biochemistry 43, 6783–6792. 10.1021/bi036219i [DOI] [PubMed] [Google Scholar]
- Pospíšil P., Šnyrychová I., Kruk J., Strzałka K., Nauš J. (2006). Evidence that cytochrome b559 is involved in superoxide production in Photosystem II: effect of synthetic short-chain plastoquinones in a cytochrome b559 tobacco mutant. Biochem. J. 397, 321–327. 10.1042/BJ20060068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A., Pospíšil P. (2012). Ultra-weak photon emission induced by visible light and ultraviolet A radiation via photoactivated skin chromophores: in-vivo charge coupled device imaging. J. Biomed. Opt. 17:085004. 10.1117/1.JBO.17.8.085004 [DOI] [PubMed] [Google Scholar]
- Radi A. E., Muñoz-Berbel X., Cortina-Puig M., Marty J. L. (2009). A third generation hydrogen peroxide biosensor based on horseradish peroxidase covalently immobilized on electrografted organic film on screen-printed carbon electrode. Electroanalysis 21, 1624–1629. 10.1002/elan.200904587 [DOI] [Google Scholar]
- Rappaport F., Diner B. A. (2008). Primary photochemistry and energetics leading to the oxidation of the (Mn)4Ca cluster and to the evolution of molecular oxygen in Photosystem II. Coord. Chem. Rev. 252, 259–272. 10.1016/j.ccr.2007.07.016 [DOI] [Google Scholar]
- Sagi M., Fluhr R. (2001). Superoxide production by plant homologues of the Gp91phox NADPH oxidase. modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 126, 1281–1290. 10.1104/pp.126.3.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M., Fluhr R. (2006). Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 141, 336–340. 10.1104/pp.106.078089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford A. L., Morton S. W., Whitehouse K. L., Oara H. M., Lugo-Morales L. Z., Roberts J. G., et al. (2011). Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Anal Chem. 82, 5205–5210. 10.1021/ac100536s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F. J., Renger G., Friedrich T., Kreslavksi V. D., Zharmukhadmedov S. K., Los D. A., et al. (2014). Reactive oxygen species: re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta 1837, 385–848. 10.1016/j.bbabio.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Schröder W. P., Åkerlund H.-E. (1990). Hydrogen peroxide production in photosystem II preparations, in Current Research in Photosynthesis, Vol. 1, ed Baltscheffsky M. (Dordrecht: Kluwer Academic Publisher; ), 901–904. [Google Scholar]
- Senel M. C., Cevik E., Abasıyanık M. F. (2010). Amperometric hydrogen peroxide biosensor based on covalent immobilization of horseradish peroxidase on ferrocene containing polymeric mediator. Sens. Actuators. B Chem. 145, 444–450. 10.1016/j.snb.2009.12.055 [DOI] [Google Scholar]
- Shao N., Krieger-Liszkay A., Schroda M., Beck Christoph F. (2007). A reporter system for the individual detection of hydrogen peroxide and singlet oxygen: its use for the assay of reactive oxygen species produced in vivo. Plant J. 50, 475–487. 10.1111/j.1365-313X.2007.03065.x [DOI] [PubMed] [Google Scholar]
- Šnyrychová I., Ayaydin F., Hideg É. (2009). Detecting hydrogen peroxide in leaves in vivo—a comparison of methods. Physiol. Plant. 135, 1–18. 10.1111/j.1399-3054.2008.01176.x [DOI] [PubMed] [Google Scholar]
- Svedruzic D., Jónsson S., Toyota C. G., Reinhardt L. A., Ricagno S, Lindqvist, Y., et al. (2005). The enzymes of oxalate metabolism: unexpected structures and mechanisms. Arch. Biochem. Biophys. 433, 176–192. 10.1016/j.abb.2004.08.032 [DOI] [PubMed] [Google Scholar]
- Tian F., Xu B., Zhu L., Zhu G. (2001). Hydrogen peroxide biosensor with enzyme entrapped within electrodeposited polypyrrole based on mediated sol–gel derived composite carbon electrode. Anal. Chim. Acta 443, 9–16. 10.1016/S0003-2670(01)01187-4 [DOI] [Google Scholar]
- Tiwari A., Pospísil P. (2009). Superoxide oxidase and reductase activity of cytochrome b559 in photosystem II. Biochim. Biophys. Acta 1787, 985–994. 10.1016/j.bbabio.2009.03.017 [DOI] [PubMed] [Google Scholar]
- Tiwari I., Singh M. (2011). Preparation and characterization of methylene blue-SDS-multiwalled carbon nanotubes nanocomposite for the detection of hydrogen peroxide. Microchim. Acta 174, 223–230. 10.1007/s00604-011-0620-5 [DOI] [Google Scholar]
- Tripathi V. S., Kandimalla V. B., Ju H. (2006). Amperometric biosensor for hydrogen peroxide based on ferrocene-bovine serum albumin and multiwall carbon nanotube modified ormosil composite. Biosens. Bioelectron. 21, 1529–1535. 10.1016/j.bios.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Wang G. H., Zhang L. M. (2006). Using novel polysaccharide-silica hybrid material to construct an amperometric biosensor for hydrogen peroxide. J. Phys. Chem. B 110, 24864–24868. 10.1021/jp0657078 [DOI] [PubMed] [Google Scholar]
- Wang H. S., Pan Q. X., Wang G. X. (2005). A biosensor based on immobilization of horseradish peroxidase in chitosan matrix cross-linked with glyoxal for amperometric determination of hydrogen peroxide. Sensors 5, 266–276. 10.3390/s5040266 [DOI] [Google Scholar]
- Yadav D. K., Pospíšil P. (2012). Role of chloride ion in hydroxyl radical production in photosystem II under heat stress: electron paramagnetic resonance spin-trapping study. J. Bioenerg. Biomembr. 44, 365–372. 10.1007/s10863-012-9433-4 [DOI] [PubMed] [Google Scholar]
- Yadav D. K., Prasad A., Kruk J., Pospíšil P. (2014). Evidence for the involvement of loosely bound plastosemiquinones in superoxide anion radical production in photosystem II. PLoS ONE 9:e115466. 10.1371/journal.pone.0115466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Zong X., Ye Z., Zhao B., Wang Q., Wang P. (2010). The application of complex multiple forklike ZnO nanostructures to rapid and ultrahigh sensitive hydrogen peroxide biosensors. Biomaterials 31, 7534–7541. 10.1016/j.biomaterials.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Yao H., Li N., Wei Y. L., Zhu J. J. (2005). A H2O2 biosensor based on immobilization of horseradishperoxidase in a gelatine network matrix. Sensors 5, 277–283. 10.3390/s5040277 [DOI] [Google Scholar]
- Yasukawa T., Uchida I., Matsue T. (1998). Permeation of redox species through a cell membrane of a single, living algal protoplast studied by microamperometry. Biochim. Biophys. Acta 1369, 152–158. 10.1016/S0005-2736(97)00220-4 [DOI] [PubMed] [Google Scholar]
- Yasukawa T., Uchida I., Matsue T. (1999). Microamperometric measurements of photosynthetic activity in a single algal protoplast. Biophys. J. 76, 1129–1135. 10.1016/S0006-3495(99)77277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrizhnaya O. M., Khorobrykh A. A., Khristin M. S., Klimov V. V. (1997). Photoinduced production of hydrogen peroxide at the acceptor side of photosystem II. Biochemistry 62, 357–361. [Google Scholar]
- Zhang H. L., Lai G. S., Han D. Y., Yu A. M. (2008). An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres. Anal. Bioanal. Chem. 390, 971–977. 10.1007/s00216-007-1748-3 [DOI] [PubMed] [Google Scholar]
- Zhang S., Weng J., Pan J., Tu T., Yao S., Xu C. (2003). Study on the photo-generation of superoxide radicals in Photosystem II with EPR spin trapping techniques. Photosynth. Res. 75, 41–48. 10.1023/A:1022439009587 [DOI] [PubMed] [Google Scholar]
- Zulfugarov I. S., Tovuu A., Eu Y. J., Dogsom B., Poudyal R. S., Nath K., et al. (2014). Production of superoxide from Photosystem II in a rice (Oryza sativa L.) mutant lacking PsbS. BMC Plant Biol. 14:242. 10.1186/s12870-014-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.