Abstract
Purpose
The American College of Surgeons Oncology Group Z1071 trial reported a 12.6% false-negative rate (FNR) for sentinel lymph node (SLN) surgery after neoadjuvant chemotherapy (NAC) in cN1 disease. Patients were not selected for surgery based on response, but a secondary end point was to determine whether axillary ultrasound (AUS) after NAC after fine-needle aspiration cytology can identify abnormal nodes and guide patient selection for SLN surgery.
Patients and Methods
Patients with T0-4, N1-2, M0 breast cancer underwent AUS after neoadjuvant chemotherapy. AUS images were centrally reviewed and classified as normal or suspicious lymph nodes. AUS findings were tested for association with pathologic nodal status and SLN FNR. The impact of AUS results to select patients for SLN surgery to reduce the FNR was assessed.
Results
Postchemotherapy AUS images were reviewed for 611 patients. One hundred thirty (71.8%) of 181 AUS-suspicious patients were node positive at surgery compared with 243 (56.5%) of 430 AUS-normal patients (P < .001). Patients with AUS-suspicious nodes had a greater number of positive nodes and greater metastasis size (P < .001). The SLN FNR was not different based on AUS results; however, using a strategy where only patients with normal AUS undergo SLN surgery would potentially reduce the FNR in Z1071 patients with ≥ two SLNs removed from 12.6% to 9.8% when preoperative AUS results are considered as part of SLN surgery.
Conclusion
AUS is recommended after chemotherapy to guide axillary surgery. An FNR of 9.8% with the combination of AUS and SLN surgery would be acceptable for the adoption of SLN surgery for women with node-positive breast cancer treated with neoadjuvant chemotherapy.
INTRODUCTION
In early-stage breast cancer, axillary lymph node dissection (ALND) has been replaced by sentinel lymph node (SLN) surgery for nodal staging. SLN surgery accurately determines the status of the axilla in most patients and is associated with less morbidity than ALND for patients presenting with clinically node-negative disease.1–3 In the setting of neoadjuvant chemotherapy, SLN surgery is performed after completion of chemotherapy in patients who present with node-negative disease and, with recent data from several trials, is now being considered for use in patients with initially node-positive disease.4–9 The accuracy of SLN surgery after chemotherapy in patients who present with node-positive disease was recently evaluated in the American College of Surgeons Oncology Group (ACOSOG) Z1071 phase II study. The study identified a 12.6% false-negative rate (FNR) for SLN surgery after neoadjuvant chemotherapy in patients who presented with clinically node-positive disease and had two or more SLNs identified and removed.7 This was higher than the 10% cut point defined in the trial as an acceptable FNR, and therefore, improvements in technique or patient selection to decrease the FNR are important for the adoption of SLN surgery in this setting.
Axillary ultrasound (AUS) is frequently used to assess the axilla at the time of initial diagnosis of primary breast cancer to evaluate the axillary nodes for evidence of metastatic disease. When combined with percutaneous biopsy (fine-needle aspiration or core needle biopsy), it has a sensitivity of 25% to 95% and a specificity of 97% to 100%.10–21
AUS is not routinely used to assess axillary response after chemotherapy because ALND has been standard practice for surgical management of the axilla in patients who initially present with node-positive disease. However, with the selective use of neoadjuvant chemotherapy in appropriate patients, approximately 40% of patients convert from node-positive disease to node-negative disease, and with improvements in targeted therapy, pathologic nodal response rates to neoadjuvant therapy regimens have been reported to be as high as 70%. The use of AUS to assess response of nodal disease to chemotherapy may have a role in restaging the axilla and may be useful to guide surgical management of the axilla after completion of chemotherapy.
The prespecified secondary end points of the ACOSOG Z1071 trial were to determine how the post–neoadjuvant chemotherapy AUS appearance of the lymph nodes affects the FNR of SLN surgery and to determine how the AUS status after completion of neoadjuvant chemotherapy correlates with residual disease on final pathology. We hypothesized that patients with normal-appearing lymph nodes on AUS after chemotherapy are at lower risk of residual nodal disease and may be more suitable for SLN surgery. We also sought to determine whether AUS could improve patient selection for use of SLN surgery after chemotherapy in node-positive breast cancer and lower the FNR by selecting patients appropriate for this procedure.
PATIENTS AND METHODS
The ACOSOG Z1071 trial enrolled women with histologically proven clinical stage T0-4, N1-2, M0, primary invasive breast cancer who had completed or were planning to undergo neoadjuvant chemotherapy.4 All patients had biopsy-proven node-positive breast cancer at presentation documented by fine-needle aspiration biopsy or core needle biopsy. The current analysis includes all patients who met protocol eligibility, completed neoadjuvant chemotherapy, underwent SLN surgery and axillary dissection, and had AUS images submitted for review. The institutional review boards of all participating institutions approved this study, and written informed consent was obtained from each patient before study entry.
Nodal Assessment on Ultrasound Imaging
After completion of neoadjuvant chemotherapy and within 4 weeks before surgery, all patients underwent an AUS to assess the morphologic appearance of the axillary lymph nodes. The AUS was submitted to the Quality Assurance Review Center at the University of Massachusetts (Worcester, MA) for central review.
Central Review of Nodal Assessment on Ultrasound Imaging
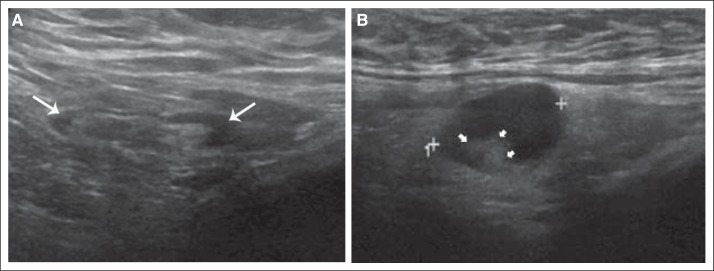
Hard copies or digital images from the post-treatment AUS examination were archived, and quality assurance for both exams was performed at the Quality Assurance Review Center. To standardize the evaluation of nodal status after neoadjuvant chemotherapy, retrospective remote central review of archived images from the post-treatment/preoperative AUS was conducted by the study radiologist (H.T.L.), who was blinded to the imaging reports, pathology, and surgical information. Classification of normal versus abnormal lymph node morphology was based on the cortex and hilum of the lymph node (Fig 1). A lymph node was considered normal if the cortex was hyperechoic and thin (< 3 mm thick) and the fatty hilum was visible. A lymph node was considered abnormal if the cortex was either focally or diffusely thickened (> 3 mm thick) and the fatty hilum was deformed or absent.
Fig 1.
(A) Illustration of normal lymph nodes on ultrasound. Ultrasound image of a morphologically normal lymph node with uniform thin hypoechoic cortex (white arrows) less than 3 mm in thickness. (B) Illustration of abnormal lymph nodes on ultrasound. Ultrasound image of a metastatic axillary lymph node with diffuse hypoechoic cortical thickness and deformity of the echogenic fatty hilum (small white arrowheads).
Statistical Analysis
Prespecified secondary objectives of the study to look at correlation of AUS findings with pathologic nodal status and SLN FNR were performed. Patient, tumor, and surgical variables were compared between different groups using a two-sample t test or Wilcoxon rank sum test, as appropriate, for continuous variables and a χ2 test or Fisher's exact test, as appropriate, for categorical variables. Although the correlation of AUS findings with study outcomes was prespecified, the study was not powered for these end points. Rather than performing a post hoc power analysis, we report relevant CIs for the estimates, which better convey whether the current analysis may have missed clinically important effects.
The FNR rate was computed as the number of patients with negative SLNs who had residual disease in the contents of the ALND divided by the total number of patients with residual disease. The analysis of the FNR for Z1071 was limited to patients with cN1 disease (metastases to movable ipsilateral level I or II axillary lymph nodes) and at least two SLNs identified on pathology and specified the use of 90% CIs; therefore, the same criteria were used in this study for consistency.
Additional analysis was performed to compare the FNRs in women after chemotherapy for the following two different strategies: using no additional selection criteria for SLN surgery (as patients were treated in the Z1071 trial) and using the criterion of normal lymph nodes on AUS to select patients for SLN surgery.
All tests were two-sided, and P < .05 was considered statistically significant. The analyses were done with SAS (version 9.3; SAS Institute, Cary, NC). Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. These analyses were based on data available on March 13, 2013.
RESULTS
Patient Characteristics
A total of 756 patients with T0-4, N1-2, M0 breast cancer were enrolled onto ACOSOG Z1071 from 136 institutions. Of these, 687 patients met eligibility requirements for the main trial and underwent SLN and ALND surgery. Overall pathologic complete nodal response rate was 39.0%. The study group for the current analysis consists of 611 patients who underwent both SLN surgery and ALND and for whom an AUS examination after neoadjuvant chemotherapy was submitted for central review. Differences between the 611 patients who had postchemotherapy AUS data available and the 76 patients without AUS data available are listed in Table 1.
Table 1.
Patient and Treatment Characteristics by Post–Neoadjuvant Chemotherapy AUS Status
| Characteristic | AUS Result Available (n = 611) |
AUS Result Not Available (n = 76) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .28 | ||||
| Mean | 50.2 | 48.8 | |||
| SD | 11.0 | 9.8 | |||
| Median | 50 | 48 | |||
| Range | 23-93 | 26-70 | |||
| Race/ethnicity | .017 | ||||
| White | 486 | 79.3 | 69 | 90.8 | |
| Other | 127 | 20.7 | 7 | 9.2 | |
| Body mass index, kg/m2 | .52 | ||||
| Mean | 29.5 | 29.0 | |||
| SD | 6.5 | 6.3 | |||
| Median | 28.6 | 28.5 | |||
| Range | 15.5-64.1 | 18.5-44.2 | |||
| Performance score | .025 | ||||
| 0 | 501 | 82.0 | 53 | 69.7 | |
| 1 | 109 | 17.8 | 23 | 30.3 | |
| 2 | 1 | 0.2 | 0 | 0 | |
| Clinical T category at diagnosis | .71 | ||||
| T0/Tis | 7 | 1.1 | 0 | 0 | |
| T1 | 83 | 13.6 | 6 | 7.9 | |
| T2 | 334 | 54.7 | 45 | 59.2 | |
| T3 | 159 | 26.0 | 21 | 27.6 | |
| T4 | 28 | 4.6 | 4 | 5.3 | |
| Nodal category | .30 | ||||
| cN1 | 579 | 94.8 | 70 | 92.1 | |
| cN2 | 32 | 5.2 | 6 | 7.9 | |
| Approximated subtype | .16 | ||||
| HER2 positive | 179 | 29.3 | 27 | 35.5 | |
| Hormone receptor positive and HER2 negative | 276 | 45.2 | 37 | 48.7 | |
| Triple receptor negative | 156 | 25.5 | 12 | 15.8 | |
| Tumor histology | .91 | ||||
| IDC | 540 | 88.4 | 70 | 92.1 | |
| ILC | 34 | 5.6 | 3 | 4.0 | |
| Mixed | 10 | 1.6 | 1 | 1.3 | |
| Other | 27 | 4.4 | 2 | 2.6 | |
| Chemotherapy completed | .42 | ||||
| Yes | 563 | 92.1 | 68 | 89.5 | |
| No | 48 | 7.9 | 8 | 10.5 | |
| Physical examination of axilla after chemotherapy | .10 | ||||
| No palpable adenopathy | 512 | 83.8 | 58 | 76.3 | |
| Palpable lymph nodes | 74 | 12.1 | 11 | 14.5 | |
| Fixed or matted lymph nodes | 4 | 0.6 | 0 | 0 | |
| Not reported | 21 | 3.4 | 7 | 9.2 | |
| Type of breast surgery | .75 | ||||
| Partial mastectomy | 244 | 40.1 | 29 | 38.2 | |
| Total mastectomy | 365 | 59.9 | 47 | 61.8 | |
| Not available | 2 | 0 | |||
| No. of SLNs examined | .96 | ||||
| 0 | 44 | 7.2 | 6 | 7.9 | |
| 1 | 76 | 12.4 | 10 | 13.2 | |
| 2 | 148 | 24.2 | 17 | 22.4 | |
| 3 | 134 | 21.9 | 19 | 25.0 | |
| ≥ 4 | 209 | 34.2 | 24 | 31.6 | |
| Median | 3 | 3 | .66 | ||
| Range | 0-13 | 0-11 | |||
Abbreviations: AUS, axillary ultrasound; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; SD, standard deviation; SLN, sentinel lymph node.
Baseline Characteristics Associated With Postchemotherapy AUS Findings
Lymph nodes were classified as normal if the radiologist was unable to visualize any lymph nodes on AUS or indicated that the lymph nodes were normal in morphologic appearance. Lymph nodes with abnormal morphology on AUS were classified as suspicious. Of 611 patients with postchemotherapy AUS results, 430 (70.4%) had lymph nodes classified as normal on AUS, and 181 (29.6%) had suspicious lymph nodes. Table 2 lists patient, tumor, and treatment characteristics for patients with normal lymph nodes as assessed by postchemotherapy AUS and for patients with suspicious lymph nodes. Age, performance status, clinical N stage at presentation, and completion of all chemotherapy did not differ significantly between patients with normal lymph nodes and those with suspicious nodes as assessed by AUS. There was a greater proportion of nonwhite patients in the group with suspicious lymph nodes on AUS after chemotherapy compared with those with normal AUS findings.
Table 2.
Comparison of Patient and Tumor Characteristics Between Patients With Normal Postchemotherapy Lymph Nodes As Assessed by AUS and Patients With Suspicious Postchemotherapy Lymph Nodes
| Characteristic | Postchemotherapy AUS Normal (n = 430) |
Postchemotherapy AUS Suspicious (n = 181) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .29 | ||||
| Mean | 49.8 | 51.0 | |||
| SD | 10.8 | 11.4 | |||
| Median | 49 | 51 | |||
| Range | 23-83 | 23-93 | |||
| Race/ethnicity | .0017 | ||||
| White | 355 | 82.6 | 129 | 71.3 | |
| Other | 75 | 17.4 | 52 | 28.7 | |
| Body mass index, kg/m2 | .029 | ||||
| Mean | 29.1 | 30.5 | |||
| SD | 6.4 | 6.9 | |||
| Median | 28.4 | 29.3 | |||
| Range | 15.5-64.1 | 17.6-52.3 | |||
| Performance score | .47 | ||||
| 0 | 347 | 80.7 | 154 | 85.1 | |
| 1 | 82 | 19.1 | 27 | 14.9 | |
| 2 | 1 | 0.2 | 0 | 0 | |
| Clinical T stage at diagnosis | .016 | ||||
| T0/Tis | 5 | 1.2 | 2 | 1.1 | |
| T1 | 61 | 14.2 | 22 | 12.2 | |
| T2 | 233 | 54.2 | 101 | 55.8 | |
| T3 | 119 | 27.7 | 40 | 22.1 | |
| T4 | 12 | 2.8 | 16 | 8.8 | |
| Nodal category | .16 | ||||
| cN1 | 411 | 95.6 | 168 | 92.8 | |
| cN2 | 19 | 4.4 | 13 | 7.2 | |
| Chemotherapy completed | .12 | ||||
| No | 29 | 6.7 | 19 | 10.5 | |
| Yes | 401 | 93.3 | 162 | 89.5 | |
Abbreviations: AUS, axillary ultrasound; SD, standard deviation.
Association of Postchemotherapy AUS Findings With Pathologic Findings
The number of SLNs removed and the number of additional axillary lymph nodes removed did not differ significantly between patients with normal lymph nodes and those with suspicious nodes as assessed by AUS (Table 3). Postchemotherapy AUS status was associated with nodal pathologic findings. Of the 430 patients with normal nodes as assessed by AUS, 243 patients (56.5%; 95% CI, 51.6% to 61.2%) were node positive on final pathology. In comparison, 130 (71.8%; 95% CI, 64.7% to 78.3%) of 181 patients who had suspicious nodes identified by AUS were found to have residual node-positive disease (P < .001).
Table 3.
Comparison of Pathologic Variables Between Patients With Normal Postchemotherapy Lymph Nodes and Patients With Suspicious Postchemotherapy Lymph Nodes As Assessed by AUS
| Variable | Postchemotherapy AUS Normal (n = 430) |
Postchemotherapy AUS Suspicious (n = 181) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| No. of SLNs removed | .76 | ||||
| 0 | 34 | 7.9 | 10 | 5.5 | |
| 1 | 54 | 12.6 | 22 | 12.2 | |
| 2-6 | 316 | 73.5 | 137 | 75.7 | |
| ≥ 7 | 26 | 6.0 | 12 | 6.6 | |
| No. of SLNs positive on HE | .001 | ||||
| 0 | 206 | 52.0 | 63 | 36.8 | |
| 1 | 107 | 27.0 | 49 | 28.6 | |
| 2 | 49 | 12.4 | 30 | 17.5 | |
| ≥ 3 | 34 | 8.6 | 29 | 17.0 | |
| No. of additional ALNs removed | .70 | ||||
| 1-10 | 135 | 31.4 | 53 | 29.3 | |
| 11-20 | 212 | 49.3 | 98 | 54.1 | |
| 21-30 | 69 | 16.0 | 24 | 13.3 | |
| > 30 | 14 | 3.3 | 6 | 3.3 | |
| No. of additional ALNs positive on HE | .004 | ||||
| 0 | 269 | 62.6 | 85 | 47.0 | |
| 1-3 | 100 | 23.3 | 56 | 30.9 | |
| 4-10 | 50 | 11.6 | 32 | 17.7 | |
| > 10 | 11 | 2.6 | 8 | 4.4 | |
| Total No. of positive nodes (SLN+ALND) | .003 | ||||
| 0 | 171 | 43.2 | 47 | 27.5 | |
| 1-3 | 173 | 43.7 | 89 | 52.0 | |
| 4-10 | 42 | 10.6 | 28 | 16.4 | |
| > 10 | 10 | 2.5 | 7 | 4.1 | |
| Pathologic T stage (n = 3 missing) | .006 | ||||
| T0/Tis | 155 | 36.1 | 44 | 24.6 | |
| T1+ | 274 | 63.9 | 135 | 75.4 | |
| Pathologic N stage | < .001 | ||||
| N0 | 187 | 43.5 | 51 | 28.2 | |
| N1+ | 243 | 56.5 | 130 | 71.8 | |
| Axillary metastasis size (n = 22 missing) | < .001 | ||||
| N0 | 187 | 45.1 | 51 | 29.3 | |
| Micrometastasis | 38 | 9.2 | 6 | 3.4 | |
| Macrometastasis | 190 | 45.8 | 117 | 67.2 | |
| Axillary metastasis size, mm (n = 18 missing) | < .001 | ||||
| Median | 6.5 | 11.0 | |||
| Range | 0.1-35.0 | 0.5-80.0 | |||
Abbreviations: ALN, axillary lymph node; ALND, axillary lymph node dissection; AUS, axillary ultrasound; HE, hematoxylin and eosin; SLN, sentinel lymph node.
Patients with suspicious nodal status based on AUS were also more likely to have a greater number of positive SLNs than patients with normal AUS findings (34.5% v 21.0%, respectively, with two or more positive SLNs; P = .001). Patients with suspicious nodal status also had greater nodal disease burden than patients with AUS normal nodes, with a larger median SLN metastasis size (11.0 v 6.5 mm, respectively; P < .001). Patients with abnormal AUS findings were more likely to have additional positive axillary lymph nodes and a greater number of additional positive nodes (P = .004; Table 3). Patients with suspicious lymph nodes by AUS were also more likely to have residual invasive disease in the breast (pathologic T1 or greater) than T0/Tis disease compared with patients with normal AUS findings (75.4% v 63.9%, respectively; P = .006).
SLN FNRs by AUS Nodal Status
The FNR analysis of SLN surgery, which was the primary end point of the ACOSOG Z1071 study, was calculated from patients with clinical N1 disease who had two or more SLNs removed. Four hundred seventy (76.9%) of 611 patients with a postchemotherapy AUS met these criteria. Characteristics were compared between patients in this study (those with postchemotherapy AUS data) and patients in the original analysis of the SLN FNR, which included 527 patients.4 The differences between these groups (results not shown) were similar to the differences seen between patients with a postchemotherapy AUS and those without a postchemotherapy AUS for the overall patient group, as reported in Table 1.
In the 470 cN1 patients with a postchemotherapy AUS in whom at least two SLNs were removed, 286 had residual nodal disease with 36 false-negative events. This yields an FNR of 12.6% (90% CI, 9.5% to 16.3%), which is essentially identical to that reported as the trial's primary end point (12.6%; 90% CI, 9.8% to 16.0%). Among patients who had normal lymph node status by postchemotherapy AUS, 187 patients had residual disease with 28 false-negative events, for an FNR of 15.0% (90% CI, 10.9% to 20.0%). Among patients who had suspicious nodal status by AUS, 99 patients had residual disease with eight false-negative events, for an FNR of 8.1% (90% CI, 4.1% to 14.1%). Although the SLN surgery FNR was lower for patients with AUS-suspicious nodes, the difference in FNRs was not statistically significant (P = .09).
FNR When AUS Is Combined With SLN Surgery
Using a strategy whereby clinical response (normal AUS after chemotherapy) is used to select patients for SLN surgery, patients with normal nodes as assessed by postchemotherapy AUS could undergo SLN surgery. If any of the SLNs were positive, the patient would undergo an ALND, and if the SLNs were negative, then no further axillary surgery would be needed. Figure 2A shows the number of false-negative events and FNR seen in Z1071 patients when SLN surgery with an identification of at least two SLNs was performed on all patients regardless of AUS findings. Figure 2B shows the number of false-negative events and the FNR if the same patients enrolled onto Z1071 would have been selected for SLN surgery based on the AUS findings and undergone SLN surgery with resection of at least two SLNs. This selection process by AUS would result in the combination of AUS and SLN, resulting in an FNR of 9.8% (90% CI, 7.1% to 13.2%) compared with 12.6% when AUS is not used to select patients for SLN surgery.
Fig 2.
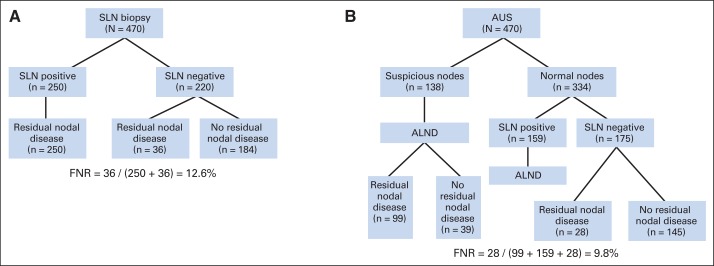
Comparison of false-negative rates (FNRs) when using (A) sentinel lymph node (SLN) surgery irrespective of axillary ultrasound (AUS) imaging findings or (B) using AUS imaging results for selective use of SLN surgery. The study participants from the American College of Surgeons Oncology Group Z1071 trial were used with the observed rates in our study. ALND, axillary lymph node dissection.
DISCUSSION
AUS for evaluation of axillary lymph node morphology has been routinely used for patients with newly diagnosed invasive breast cancer. The current study demonstrates the potential utility of AUS after completion of neoadjuvant chemotherapy before surgery to evaluate for residual nodal disease in patients who initially present with node-positive breast cancer. A prior single-institution study at The University of Texas MD Anderson Cancer Center showed that use of AUS for patient selection decreases the FNR of SLN surgery.22 To our knowledge, this is the first prospective multicenter study looking at the role of AUS after neoadjuvant chemotherapy in women presenting with node-positive breast cancer. We observed that abnormal lymph nodes on AUS after chemotherapy predict for a significantly increased likelihood of residual nodal positivity and also greater nodal disease burden. When AUS identified suspicious lymph nodes after completion of neoadjuvant chemotherapy, residual nodal disease was found in 71.8% of patients. Patients with normal lymph nodes on AUS examination have a significantly lower likelihood of having residual disease, and the overall nodal burden of disease is lower, with smaller metastases and fewer positive nodes (> four positive nodes in 13.1% of patients with normal lymph nodes on AUS v 20.5% of patients with abnormal lymph nodes).
In patients with positive SLNs at surgery after a normal AUS, 63% had no additional positive nodes in the ALND specimen. Therefore, in patients with normal AUS, SLN surgery could be used to identify patients who may be able to avoid ALND. This is being evaluated in the Alliance A11202 clinical trial, which is currently enrolling patients and comparing axillary radiation to axillary dissection for patients with positive SLNs after neoadjuvant chemotherapy.
Although the FNR is not significantly different between patients with normal and abnormal AUS findings, the AUS does correlate with the likelihood of residual nodal disease. Thus, AUS after neoadjuvant chemotherapy can be used to categorize patients based on likelihood of residual nodal disease. This information can then be used to decide whether to pursue SLN surgery. Thus, AUS may help triage patients when considering the use of SLN surgery for staging of axillary nodal response to neoadjuvant chemotherapy in women who present with node-positive disease. The strategy of combined AUS and SLN surgery results in a calculated FNR of 9.8%. When applying this strategy to the 470 patients in Z1071 with two SLNs resected and AUS images available, 28 patients with a negative SLN had disease remaining in the axilla (false-negative events; potential undertreatment with use of SLN only). In addition, 39 patients had no residual nodal disease and were subjected to ALND (false-positive events; potential overtreatment). The majority of patients (n = 403) would have appropriate axillary surgery, avoiding both overtreatment and undertreatment.
Although 70.4% of patients had normal AUS findings after neoadjuvant chemotherapy, the pathologic complete nodal response rate was only 39.0%. This demonstrates that a normal-appearing lymph node on ultrasound does not preclude residual disease within the lymph node on final pathology, and surgical staging remains important to assess for residual nodal disease. Further refinements in axillary imaging technique are desired in this setting. Review of criteria for defining suspicious lymph nodes, use of clips to mark abnormal nodes, and standardization of AUS equipment and performance parameters may improve performance of AUS. This could help improve the sensitivity and specificity of AUS after neoadjuvant chemotherapy.
The ACOSOG Z1071 study reported an FNR of 12.6% for SLN surgery in women who presented with node-positive breast cancer and were treated with neoadjuvant chemotherapy. This FNR was higher than the predetermined acceptable FNR of 10%, and subsequently, the recommendation was that additional refinements in patient selection are needed to guide the use of SLN surgery in this setting. One of the limitations was that the Z1071 study included all comers for SLN surgery and did not select patients based on tumor response to chemotherapy. Herein, we show that the use of postchemotherapy, preoperative AUS can assist with patient selection to further reduce the number of false-negative events with the use of SLN surgery in this setting. AUS allows selection of patients with the greatest likelihood of nodal response to benefit from SLN surgery for axillary staging. These are additional data that can be used in the decision-making process when considering SLN surgery in this setting. Further advancement in the management of the axilla in patients with node-positive breast cancer is being evaluated in the A11202 trial, which is comparing axillary dissection to axillary-directed radiation for management of patients with SLN-positive disease after completion of neoadjuvant chemotherapy.
A couple limitations of this study are worth noting. The first is that we only had AUS findings for a subset of patients (611 of 687 patients; 88.9%). Although there was no discernable systematic bias with respect to who did or did not have an AUS, an undetectable bias cannot be dismissed. A second limitation is related to power. The FNR estimate when using a combination of AUS and SLN was 9.8% with a rather wide 90% CI (7.1% to 13.2%), reflecting limited precision as a result of the sample size. Because the original FNR from the trial when using SLN alone was 12.6%, which falls in the 90% CI for AUS plus SLN, we cannot rule out the possibility that AUS may not add useful information that would decrease the FNR from that of SLN alone.
In patients with node-positive disease at presentation who have a complete nodal response by AUS after neoadjuvant chemotherapy, SLN surgery can be used to stage the axilla for residual nodal disease. This allows selection of patients at the greatest likelihood of complete nodal response and provides them the opportunity to avoid axillary dissection if two or more SLNs are resected and found to be negative. An FNR of 9.8% with the AUS and SLN combination would be expected in this setting and could facilitate the adoption of SLN surgery for women with node-positive breast cancer treated with neoadjuvant chemotherapy.
Glossary Terms
- neoadjuvant therapy:
the administration of chemotherapy prior to surgery. Induction chemotherapy is generally designed to decrease the size of the tumor prior to resection and to increase the rate of complete (R0) resections.
- sentinel lymph node:
the lymph node that is anatomically located such that it is the first site of lymph drainage from the location of the primary tumor. It is suspected and assumed that if a malignancy is going to disseminate via the lymphatic system, metastases will first be evident in the sentinel lymph node. In this manner, this lymph node is said to stand guard or sentinel over the metastatic state of the tumor. For many cancers, the sentinel lymph node is biopsied as part of the staging process and presence of macro- or micrometastases in the sentinel lymph node is a negative prognostic factor.
Footnotes
Supported by National Cancer Institute Grant No. U10 CA76001 to the American College of Surgeons Oncology Group. Also supported, in part, by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology (Grant No. CA31946; Monica M. Bertagnolli, MD, Chair) and to the Alliance Statistics and Data Center (Grant No. CA33601; Daniel J. Sargent, PhD).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00881361.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Judy C. Boughey, Karla V. Ballman, Kelly K. Hunt, Huong T. Le-Petross
Provision of study materials or patients: Judy C. Boughey, Kelly K. Hunt, Linda M. McCall, Elizabeth A. Mittendorf, Gretchen M. Ahrendt, Lee G. Wilke, Huong T. Le-Petross
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Judy C. Boughey
No relationship to disclose
Karla V. Ballman
No relationship to disclose
Kelly K. Hunt
No relationship to disclose
Linda M. McCall
No relationship to disclose
Elizabeth A. Mittendorf
Research Funding: Galena Biopharma (Inst), Antigen Express (Inst)
Gretchen M. Ahrendt
No relationship to disclose
Lee G. Wilke
No relationship to disclose
Huong T. Le-Petross
No relationship to disclose
REFERENCES
- 1.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 2.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 4.Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558–566. doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 5.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 6.Fontein DB, van de Water W, Mieog JS, et al. Timing of the sentinel lymph node biopsy in breast cancer patients receiving neoadjuvant therapy: Recommendations for clinical guidance. Eur J Surg Oncol. 2013;39:417–424. doi: 10.1016/j.ejso.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 9.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy following neoadjuvant chemotherapy in biopsy proven node positive breast cancer: The SN FNAC study. J Clin Oncol. 2013;(suppl 15s):31. doi: 10.1200/JCO.2014.55.7827. abstr 1018. [DOI] [PubMed] [Google Scholar]
- 10.Bonnema J, van Geel AN, van Ooijen B, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: New diagnostic method. World J Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Ortega MJ, Benito MA, Vahamonde EF, et al. Pretreatment axillary ultrasonography and core biopsy in patients with suspected breast cancer: Diagnostic accuracy and impact on management. Eur J Radiol. 2011;79:64–72. doi: 10.1016/j.ejrad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Hinson JL, McGrath P, Moore A, et al. The critical role of axillary ultrasound and aspiration biopsy in the management of breast cancer patients with clinically negative axilla. Ann Surg Oncol. 2008;15:250–255. doi: 10.1245/s10434-007-9524-3. [DOI] [PubMed] [Google Scholar]
- 13.Sahoo S, Sanders MA, Roland L, et al. A strategic approach to the evaluation of axillary lymph nodes in breast cancer patients: Analysis of 168 patients at a single institution. Am J Surg. 2007;194:524–526. doi: 10.1016/j.amjsurg.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. AJR Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Haisfield-Wolfe ME, Lange J, et al. The role of ultrasound-guided fine-needle aspiration of axillary nodes in the staging of breast cancer. Ann Surg Oncol. 2008;15:462–471. doi: 10.1245/s10434-007-9623-1. [DOI] [PubMed] [Google Scholar]
- 16.Koelliker SL, Chung MA, Mainiero MB, et al. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer—Correlation with primary tumor size. Radiology. 2008;246:81–89. doi: 10.1148/radiol.2463061463. [DOI] [PubMed] [Google Scholar]
- 17.Ciatto S, Brancato B, Risso G, et al. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res Treat. 2007;103:85–91. doi: 10.1007/s10549-006-9355-0. [DOI] [PubMed] [Google Scholar]
- 18.Gilissen F, Oostenbroek R, Storm R, et al. Prevention of futile sentinel node procedures in breast cancer: Ultrasonography of the axilla and fine-needle aspiration cytology are obligatory. Eur J Surg Oncol. 2008;34:497–500. doi: 10.1016/j.ejso.2007.07.198. [DOI] [PubMed] [Google Scholar]
- 19.Altomare V, Guerriero G, Carino R, et al. Axillary lymph node echo-guided fine-needle aspiration cytology enables breast cancer patients to avoid a sentinel lymph node biopsy: Preliminary experience and a review of the literature. Surg Today. 2007;37:735–739. doi: 10.1007/s00595-006-3366-7. [DOI] [PubMed] [Google Scholar]
- 20.Alkuwari E, Auger M. Accuracy of fine-needle aspiration cytology of axillary lymph nodes in breast cancer patients: A study of 115 cases with cytologic-histologic correlation. Cancer. 2008;114:89–93. doi: 10.1002/cncr.23344. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 22.Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19:3177–3184. doi: 10.1245/s10434-012-2484-2. [DOI] [PubMed] [Google Scholar]