Abstract
Purpose
Given that the clinical course of oxaliplatin-induced neuropathy is not well defined, the current study was performed to better understand clinical parameters associated with its presentation.
Methods
Acute and chronic neuropathy was evaluated in patients receiving adjuvant FOLFOX (fluorouracil, leucovorin, and oxaliplatin) on study N08CB (North Central Cancer Treatment Group, Alliance). Acute neuropathy was assessed by having patients complete daily questionnaires for 6 days with each cycle of FOLFOX. Before each dose of FOLFOX and as long as 18 months after chemotherapy cessation, chronic neurotoxicity was assessed with use of the 20-item, European Organisation for Research and Treatment of Cancer quality-of-life questionnaire for patients with chemotherapy-induced peripheral neuropathy.
Results
Three hundred eight (89%) of the 346 patients had at least one symptom of acute neuropathy with the first cycle of FOLFOX; these symptoms included sensitivity to touching cold items (71%), sensitivity to swallowing cold items (71%), throat discomfort (63%), or muscle cramps (42%). Acute symptoms peaked at day 3 and improved, although they did not always resolve completely between treatments. These symptoms were about twice as severe in cycles 2 through 12 as they were in cycle 1. For chronic neurotoxicity, tingling was the most severe symptom, followed by numbness and then pain. During chemotherapy, symptoms in the hands were more prominent than they were in the feet; by 18 months, symptoms were more severe in the feet than they were in the hands. Patients with more severe acute neuropathy during the first cycle of therapy experienced more chronic sensory neurotoxicity (P < .0001).
Conclusion
Acute oxaliplatin-induced neuropathy symptoms do not always completely resolve between treatment cycles and are only half as severe on the first cycle as compared with subsequent cycles. There is a correlation between the severities of acute and chronic neuropathies.
INTRODUCTION
Oxaliplatin is associated with a commonly occurring acute neuropathy that is characterized by distal or perioral paresthesias or dysesthesias. These symptoms often occur during or immediately after oxaliplatin infusion and have been reported to be reversible within a few days. In addition, many patients develop a chronic sensory neurotoxicity that can substantially affect the quality of life.1–4
While general issues regarding oxaliplatin-induced neuropathy are well known, a better understanding of the clinical manifestations of this toxicity should help in the counseling of patients and in providing information regarding neuroprotective strategies. For example, how prominent are the four recognized components of acute neuropathy (ie, sensitivity to touching cold items, discomfort swallowing cold items, throat discomfort, and muscle cramps) and how do these symptoms correlate with each other? Are the symptoms of acute neuropathy always completely reversible? Is the grade of acute neuropathy in the first cycle the same as in subsequent cycles? Do the incidence and severity of the acute neuropathy experienced following the first dose of oxaliplatin predict the incidence and severity of acute neuropathy following subsequent doses?
In addition, a better understanding of chronic peripheral neurotoxicity is desired. How common and severe are long-term numbness, tingling, and pain symptoms, and how do they relate to each other? Are these symptoms, in the oxaliplatin treatment phase and after completion of therapy, more pronounced in upper or lower extremities? Is there any association between acute and chronic neuropathy, as has been speculated in some recent reports?5–7 This article addresses these questions.
METHODS
This study was conducted with use of patient data from North Central Cancer Treatment Group trial N08CB, a phase III placebo-controlled, double-blind study that tested the ability of intravenous (IV) calcium and magnesium (CaMg) to prevent oxaliplatin-induced neuropathy.8 In this trial, patients who had undergone curative-intent colon cancer resection were scheduled to receive 12 cycles of FOLFOX (fluorouracil, leucovorin, and oxaliplatin), involving 85 mg/m2 oxaliplatin every 2 weeks. Patients were randomized (approximately 115 patients per group) to three treatment groups: IV CaMg before and after FOLFOX, IV placebo before and after FOLFOX, or IV CaMg before and IV placebo after FOLFOX. A CONSORT diagram for the patients involved in this study is provided in the previously published Journal of Clinical Oncology article.8
Patients were ineligible if they had preexisting peripheral neuropathy, a family history of a genetic/familial neuropathy, or had received prior treatment with neurotoxic chemotherapy, such as oxaliplatin, cisplatin, a taxane, or a vinca alkaloid. The protocol was approved per US federal guidelines, and all patients provided written informed consent. Other details regarding trial methods have been previously published.8
To provide data regarding oxaliplatin-associated acute neuropathy, patients completed daily questionnaires before each cycle of FOLFOX and for 5 consecutive days after the initiation of each 2-week cycle of chemotherapy. Using a validated methodology,9–12 questions included a 0 (no problem) to 10 (major problem) numeric rating scale that addressed sensitivity to touching cold items, discomfort swallowing cold items, throat discomfort, and muscle cramps during the previous 24 hours. No information was collected regarding analgesic use to combat any of these symptoms.
In addition, chronic peripheral neurotoxicity was measured at the initiation of each chemotherapy cycle with use of the 20-item, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with chemotherapy-induced peripheral neuropathy(EORTC QLQ-CIPN20), which has sensory, motor, and autonomic neurotoxicity subscales. When tested in patients with cancer who received a variety of chemotherapies, the EORTC QLQ-CIPN20 was shown to be reliable, valid, and responsive to change.13,14 The CIPN20 tool was also administered before each dose of chemotherapy, and at 1, 3, 6, 12, and 18 months after the last day of chemotherapy. The EORTC QLQ-CIPN20 subscales were computed according to the standard scoring algorithm and then transformed to a 0 to 100 scale, where high scores mean less symptom burden. In this analysis, descriptive statistics and plots were the primary tools to summarize the results and findings. Likelihood-based repeated measures models were used in an exploratory manner, as missing mechanisms remained unknown and thus may not have been appropriately accounted for. Therefore, the P values should be interpreted cautiously to give some quantitative sense, rather than reaching confirmative conclusions.
RESULTS
Between June 2010 and June 2012, 353 patients were accrued to the treatment study. Details of patient characteristics are included in a previously published article,8 which revealed that the IV CaMg did not prevent oxaliplatin-induced neuropathy. Consequently, the current article presents combined data from 346 patients in the total study population who had neuropathy data available. To confirm that presenting the total population was appropriate, an analysis was also performed in the placebo group alone and no appreciable difference was seen when comparing these data.
Oxaliplatin-Induced Acute Neuropathy
Of the 346 patients who completed questionnaires, 308 (89%) had at least one acute neuropathy symptom with the first cycle of FOLFOX. The degrees of the acute symptoms are shown in Table 1, along with the highest degree of symptoms during the 12 cycles of FOLFOX. Most patients reported moderate/severe symptoms for sensitivity to touching cold, discomfort swallowing cold liquids, and throat discomfort at some point during their therapy. Only 38 patients (11%) did not experience any acute symptom in cycle 1, and only three patients (1%) did not experience any acute symptom in all cycles of FOLFOX (only one of these three patients received 12 cycles of FOLFOX).
Table 1.
Severity of Acute Neuropathy Symptoms in Cycle 1 and in Cycles 1 Through 12 Combined
| Symptom | No. (%) of Patients With Symptom Score (N = 346) |
||
|---|---|---|---|
| Severity Score | |||
| None (0) | Mild (1-3) | Moderate/Severe (4-10) | |
| Sensitivity touching cold | |||
| Cycle 1 | 99 (29%) | 137 (40%) | 110 (32%) |
| Cycles 1 through 12* | 8 (2%) | 66 (19%) | 272 (79%) |
| Discomfort swallowing cold liquids | |||
| Cycle 1 | 100 (29%) | 145 (42%) | 101 (29%) |
| Cycles 1-12* | 12 (3%) | 86 (25%) | 248 (72%) |
| Throat discomfort | |||
| Cycle 1 | 129 (37%) | 143 (41%) | 74 (21%) |
| Cycles 1-12* | 20 (6%) | 123 (36%) | 203 (59%) |
| Muscle cramp | |||
| Cycle 1 | 200 (58%) | 104 (30%) | 42 (12%) |
| Cycles 1-12* | 66 (19%) | 140 (40%) | 140 (40%) |
| Worst of any symptom | |||
| Cycle 1 | 38 (11%) | 149 (43%) | 159 (46%) |
| Cycles 1-12* | 3 (1%) | 53 (15%) | 290 (84%) |
May be < 12 cycles if patient stopped treatment early.
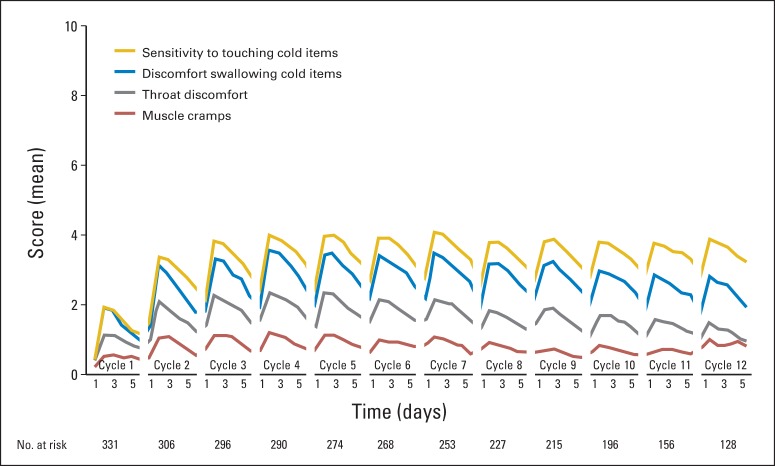
Acute neuropathy symptoms appeared within a day after the first dose of oxaliplatin, peaked in severity at day 3, and then improved (Fig 1). Sensitivity to touching cold items and discomfort swallowing cold items were rated as more severe problems than throat discomfort or muscle cramps. In some patients, these symptoms were still present at the beginning of the second cycle of chemotherapy two weeks later and were more severe in cycle 2 (P < .001, for comparison of symptom severity in cycle 1 v cycle 2). Similar to cycle 1, severity of symptoms peaked around day 3 after oxaliplatin treatment and then improved, but symptoms did not resolve before the next treatment cycle. Cycles 3 through 12 followed a pattern similar to cycle 2; the severity, on average, did not increase further.
Fig 1.
Mean scores associated with the four acute neuropathy symptoms during treatment. Higher values indicate more severe symptoms.
The relationships between the different acute neuropathy symptoms were explored, illustrating moderately strong correlations between items assessing sensitivity to touching and to swallowing cold items (r = 0.69), sensitivity to touching cold items and throat discomfort (r = 0.61), and discomfort swallowing cold items and throat discomfort (r = 0.69). Muscle cramps, however, were not well correlated with the other three symptoms (all r < 0.25).
Thirty-eight patients (11%) did not experience any acute symptoms in cycle 1 (ie, scored 0 on each of the 5 days) Table 1. Patients without one of the acute symptoms in cycle 1 were less likely to experience the other acute neuropathic symptoms in cycle 1 and, when reported, these symptoms tended to be mild.
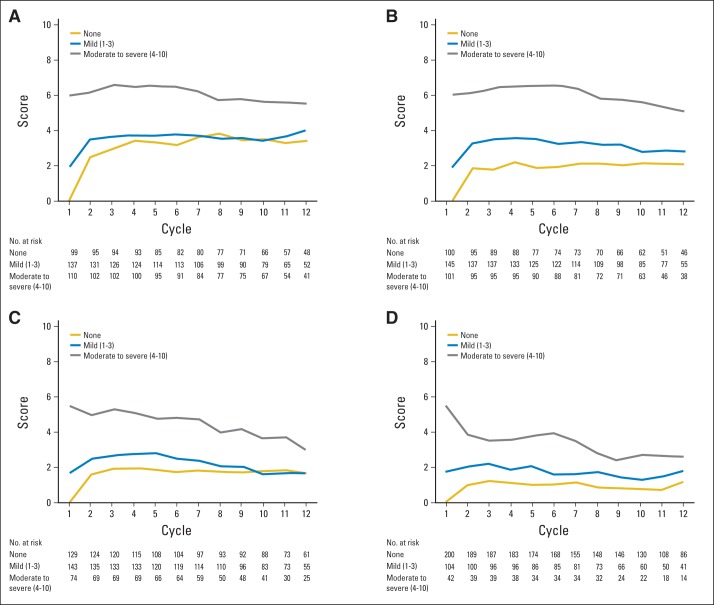
We also investigated whether the occurrence of oxaliplatin acute neuropathy symptoms in the first cycle predicted symptoms observed in subsequent cycles. Figure 2A. Patients who had moderate/severe sensitivity to touching cold items in the first cycle, on average, had similar sensitivity to touching cold items in subsequent cycles, whereas those with mild sensitivity to touching cold items in the first cycle had similar mild degrees of sensitivity to touching cold items in subsequent cycles. Those with no sensitivity to touching cold items in the first cycle, on average, developed mild sensitivity to touching cold items in subsequent cycles. Similar findings were observed for discomfort swallowing cold liquids (Fig 2B), throat discomfort (Fig 2C), and muscle cramps (Fig 2D). However, for the latter two symptoms, patients with moderate/severe symptoms in the first cycle, on average, had improved symptoms in subsequent cycles.
Fig 2.
Acute neuropathy symptom scores during the 12 cycles of oxaliplatin treatment, segregated by the worst mean maximum acute cycle 1 score (none v mild v moderate-severe) for (A) sensitivity to touching cold items, (B) discomfort swallowing cold liquids, (C) throat discomfort, and (D) muscle cramps. Higher values indicate more severe symptoms.
Individual patients who did not experience any acute symptoms in cycle 1 were further studied to better characterize those who developed symptoms in cycle 2 (Appendix Table A1, online only). Most patients (74%; 70 of 95 patients) who did not experience sensitivity to touching cold items in cycle 1 did develop sensitivity to touching cold items in cycle 2. This sensitivity was mild in 45% (43 of 95 patients) and, interestingly, became moderate/severe in 28% (27 of 95 patients), whereas 26% (25 of 95 patients) remained without any symptoms in cycle 2. Discomfort swallowing cold liquids and throat discomfort followed a similar pattern, with approximately 60% of patients developing mostly mild symptoms in cycle 2. Most patients who did not experience muscle cramps in cycle 1 did not experience them in the next cycle (65%; 122 of 189 patients).
Oxaliplatin-Induced Chronic Peripheral Neurotoxicity
Symptoms associated with chronic oxaliplatin neurotoxicity are depicted in Figure 3 as percent changes from baseline in the EORTC QLQ-CIPN20 subscale during the time that patients were receiving oxaliplatin. Sensory scores declined by 21 points, motor scores declined by 8 points, and autonomic scores dropped by 5 points. In the repeated measures model, during the 12-cycle treatment period, the sensory subscale score (mean score, 84) was observed to capture more severe symptoms than the autonomic subscale (mean score, 87) and the motor subscale (mean score, 90; P < .001) on a 0 to 100 quality-of-life scale in which higher values indicated fewer symptoms.
Fig 3.
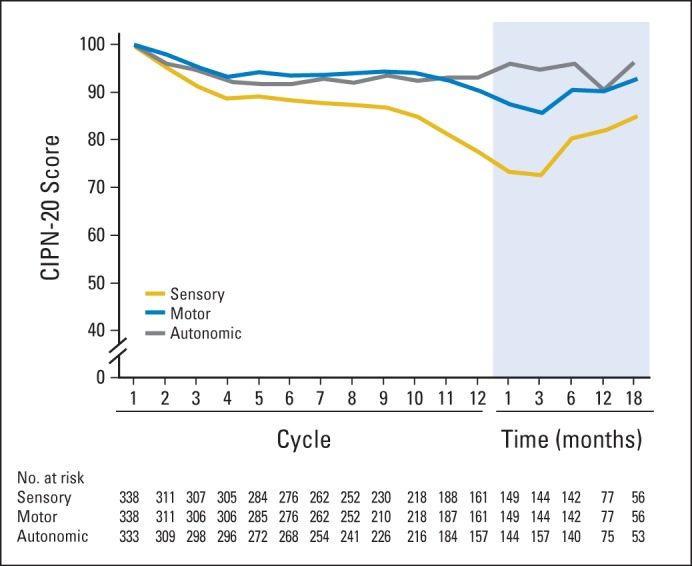
Mean sensory, motor, and autonomic subscores from the 20-item European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire for patients with chemotherapy-induced peripheral neuropathy (CIPN-20), during the treatment period and for 18 months of follow-up, in terms of percent of baseline score over time. Higher values indicate less severity of symptoms.
These sensory neuropathy changes were evaluated in more detail (Fig 4A) by examining data from the following individual EORTC QLQ-CIPN20 instrument questions: Do you have tingling fingers or hands? Do you have numbness in your fingers or hands? Do you have shooting or burning pain in your fingers or hands? Data from similar questions regarding toes and feet are also illustrated. With use of the original 1 to 4 scale of the CIPN questionnaire (corresponding to answers of none, mild, moderate, and severe), in which higher values indicated more symptoms, tingling (1.93) was the most highly rated symptom, followed by numbness (1.74) and pain (1.30; P < .001). During chemotherapy, symptoms in hands (1.72) were more prominent than in feet (1.59; P < .001). On average, most symptoms worsened for 3 months after treatment completion, but then improved.
Fig 4.
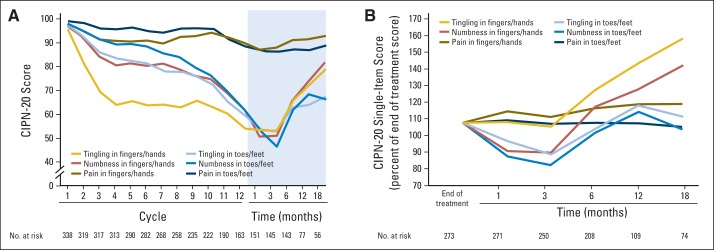
Mean tingling, numbness, and pain scores for toes/feet and fingers/hands from the 20-item European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire for patients with chemotherapy-induced peripheral neuropathy (CIPN-20) during 12 cycles of oxaliplatin therapy and after chemotherapy completion. Graphs showing (A) CIPN-20 subscale scores for the patients who completed all 12 cycles of treatment, and (B) the percent of end-of-treatment scores for these same items from the end of treatment through 18 months of follow-up for all patients, regardless of whether the patient stopped chemotherapy after all 12 planned cycles or earlier. Higher values indicate less severity of symptoms.
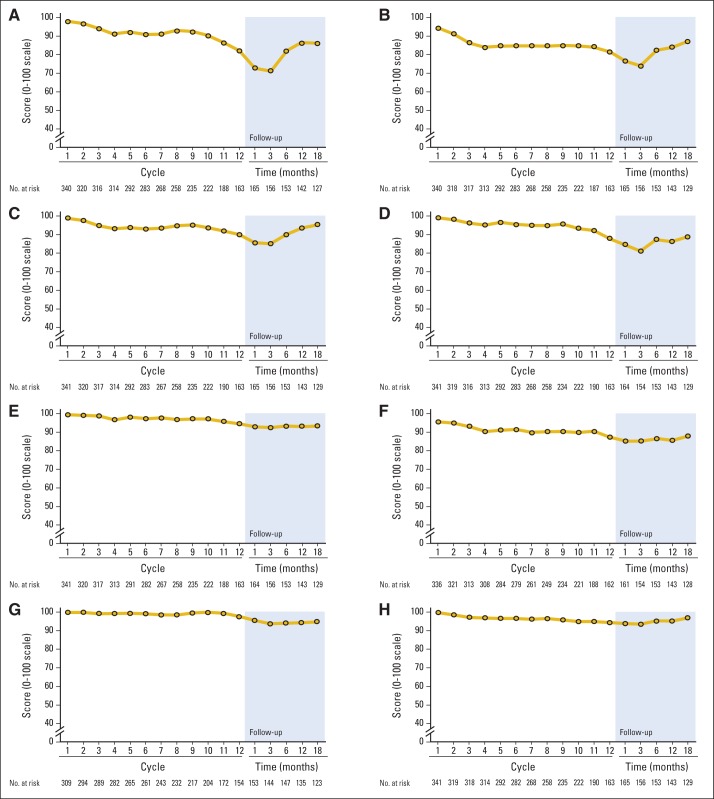
Figure 4B illustrates data from all patients who completed questionnaires after they stopped their chemotherapy, regardless of when chemotherapy was stopped (ie, not just those who completed all 12 planned cycles of FOLFOX, as shown in Fig 4A). Numbness and tingling in toes and/or feet, as well as numbness in fingers and/or hands worsened the most during the first 3 months after completion of therapy, while tingling in fingers and/or hands was more stable during this time. On average, all of the symptoms, except for pain, improved after 3 months, with finger and/or hand symptoms improving more than those in feet. By 18 months after the completion of chemotherapy, feet scores were more severe than hand scores were. Pain scores did not change much during the 18 months after treatment completion. Function was also evaluated with the CIPN20 questionnaire, revealing that patients had impairment in some measures of function, which was more severe in hands versus feet during treatment and follow-up (Appendix Fig A1, online only).
Appendix Table A2 (online only) provides details regarding the percent reduction from baseline in the CIPN20 sensory score at end of treatment, and at months 1, 3, 6, 12, and 18 from the time of chemotherapy completion. At 18 months, 50% of patients had a < 10% reduction in CIPN sensory scores from baseline, 31% had a 10% to 30% reduction, and 19% continued to experience severe CIPN symptoms, demonstrated by a > 30% reduction in CIPN sensory scores.
Appendix Figure A2 (online only) displays individual patient data for sensory neuropathy commencing with the completion of chemotherapy, illustrating that, although on average there is worsening of most symptoms in the first 3 months (Fig 4A and B), some individual patients do improve during this time. This was also seen with the autonomic and motor EORTC QLQ-CIPN20 subscales and with the individual symptoms of numbness, tingling, and pain, in both fingers and/or hands and toes and/or feet (data not shown).
Association Between Acute Neuropathy and Chronic Neurotoxicity
To determine whether there was any relationship between acute oxaliplatin neuropathy and the later appearance of chronic neurotoxicity, EORTC QLQ-CIPN20 sensory scores were compared between those patients who, with the first dose of oxaliplatin, had acute scores of 0 (none) versus 1 to 3 (mild) versus 4 to 10 (moderate/severe) for any of the four acute neuropathy symptoms. Figure 5 illustrates that those with more severe acute symptoms with cycle 1, for any of the four symptoms, tend to have more trouble with chronic neurotoxicity. The one patient who completed all 12 cycles of FOLFOX without experiencing any acute neuropathy symptoms did not develop any chronic neurotoxicity. Similar patterns are seen for each of the individual four acute neuropathy symptoms (P < .0001 for repeated measures analysis of the CIPN20 sensory subscale by maximum of any of the four acute symptoms during the first cycle).
Fig 5.
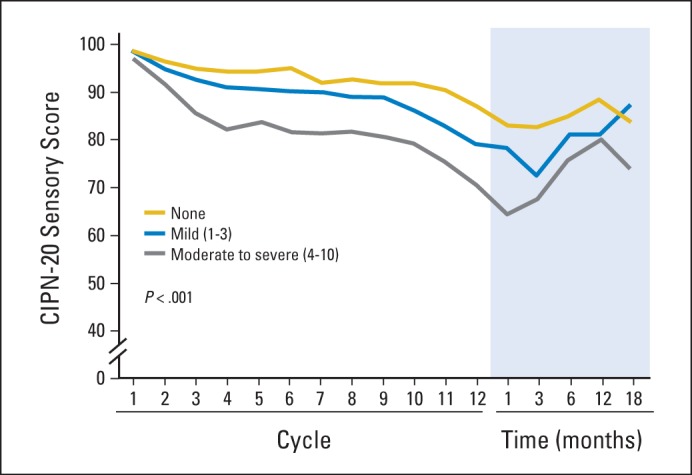
Total sensory neuropathy scores from the 20-item European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire for patients with chemotherapy-induced peripheral neuropathy (CIPN-20), segregated by the worst acute neuropathy scores (for any of the four acute neuropathy symptoms) during cycle 1. Higher values indicate less severity of symptoms.
DISCUSSION
Oxaliplatin-Induced Acute Neuropathy
The current study elucidates five characteristics of oxaliplatin-associated acute neuropathy that have not previously been recognized in the literature. First, in contrast to the claims that the acute symptoms associated with oxaliplatin are reversible,6,15 the current study demonstrates that some patients continued to report residual symptoms at the initiation of subsequent doses of oxaliplatin, two weeks after each prior dose, illustrating that these neuropathy symptoms are not always completely reversible between cycles. Accordingly, this further supports murine data that illustrate that oxaliplatin causes long-term cold-induced neurotoxicity.16 Second, this study demonstrates that the acute neuropathy symptoms tend to be more severe with cycle 2 than with cycle 1, and then have similar patterns for cycles 3 to 12. Third, these data show that the severity of acute symptoms that are experienced in cycle 1 predicts the severity of symptoms that will be experienced in the remaining cycles. Fourth, study data illustrate that most patients who do not experience any of the acute symptoms with cycle 1 will eventually develop symptoms with the remaining cycles, the majority of which will be mild. Fifth, the study illustrates moderate correlations between sensitivity to touching cold items, discomfort swallowing cold items, and throat discomfort, with a much lower correlation with muscle cramps, thus pointing to a potentially different mechanism of action for acute neuropathy and muscle cramps. Finally, study findings suggest that the majority of patients will report moderate/severe acute symptoms during their treatment with FOLFOX, supporting the clinical relevance of this syndrome and the need for potential interventions to alleviate it.
Oxaliplatin-Induced Chronic Neurotoxicity
The data from the current trial confirm the data from other studies, supporting the theory that chronic neurotoxicity is predominantly sensory and worsens over the course of the 12 cycles of FOLFOX treatment, in relation to the cumulative dose of oxaliplatin that is administered7,17,18; these data also confirm other reports that suggest that tingling and numbness are higher-rated symptoms than pain is.19,20 While one might argue that a more prominently-rated tingling score might actually be less bothersome to patients than a lower-rated pain score, anecdotal evidence supports that many patients do claim that tingling is a more bothersome symptom than pain is.
The current study's findings expand on the description that, during chemotherapy, sensory symptoms are more severe in the fingers and/or hands than they are in the toes and/or feet, and that this anatomic severity distribution is reversed in the months following completion of therapy. This phenomenon has been described before in the literature, but not in as much detail as in the current study. The results from a small study of 33 patients receiving oxaliplatin indicated that, during therapy, symptoms were more common in hands as opposed to feet.19 Additional data supporting this phenomenon come from the National Surgical Adjuvant Breast and Bowel Project Protocol C-07, which compared the efficacy of adjuvant bolus fluorouracil and leucovorin (FU/LV) versus fluorouracil and leucovorin plus oxaliplatin.17 Patients receiving oxaliplatin experienced more neurotoxic symptoms in their hands, as opposed to feet, during therapy, whereas, after completing therapy, symptoms were more severe in their feet. An update on these data, at a median follow-up of 7 years, noted that patients who received oxaliplatin reported numbness and/or tingling in both hands and feet; however, foot symptoms were more prevalent.1 In addition, a recent study assessed neuropathy symptoms and quality of life 2 to 11 years after diagnosis in survivors of colorectal cancer, 162 of whom had received oxaliplatin. This study also utilized the EORTC QLQ-CIPN20 instrument and reported that those treated with oxaliplatin more often reported tingling, numbness, and pain in toes and/or feet as compared with those not treated with oxaliplatin; however, there was no statistically significant difference in these same symptoms in patients' hands when these patients were compared with patients who either did or did not receive oxaliplatin.2
The current study's illustration that neuropathy symptoms continue to worsen for 3 months after completing the chemotherapy has often been referred to as “coasting”; this phenomenon is well described with cisplatin, and it has been proposed that this might be related to accumulation of the drug in dorsal root ganglia and its effects on mitochondria.21–24 Coasting has also been reported with oxaliplatin1,15,25; however, the details of the time frame and the clinical course of the individual sensory symptoms have not been previously reported. The worsening neuropathy slope in the current study (Fig 4A) appears to be similar to that seen in the time before therapy discontinuation, suggesting that there was a steady progression of neurotoxicity until 3 months after stopping oxaliplatin, at which time the nerves begin to recover.
Oxaliplatin Acute Neuropathy Predicts Chronic Neurotoxicity
The current study's finding that the degree of acute neuropathy appears to predict the development of chronic neurotoxicity supports the results of previous studies.5–7,26 However, the current study demonstrates that the relationship between acute neuropathy and more chronic neuropathy lasts throughout 18 months, while the previous reports only followed patients until 1 month after the completion of chemotherapy. In addition, the previous studies assessed acute neuropathy halfway through chemotherapy, while the current study demonstrates a relationship between acute symptoms in cycle 1 and chronic neurotoxicity.
Thus, the current report adds to the literature by providing a number of new findings regarding the clinical manifestations of oxaliplatin-induced neuropathy that had not previously been reported. In addition, it confirms multiple aspects of the clinical manifestations of oxaliplatin-induced neuropathy that have been suggested by other studies. This more in-depth understanding of the clinical course and features of oxaliplatin neuropathy will allow clinicians to better counsel patients; accordingly, it may guide further research to investigate the pathophysiology of this clinical syndrome with the goal of identifying possible preventative and/or therapeutic interventions. It also provides data by which this oxaliplatin neurotoxicity syndrome can be compared with neuropathy syndromes from other known neurotoxic agents, such as paclitaxel. Ongoing work will compare and contrast the neuropathy manifestations of oxaliplatin and paclitaxel,27,28 both of which have been evaluated with use of similar instruments.
Appendix
Table A1.
Frequency of Acute Neuropathy Symptoms During Cycle 2 for Patients Who Reported No Problems for That Symptom During Cycle 1
| Symptom | Symptom Severity Score |
|||
|---|---|---|---|---|
| Cycle 1* |
Cycle 2 |
|||
| None (0) | None (0) | Mild (1-3) | Moderate/Severe (4-10) | |
| Sensitivity to touching cold items, % (No. of pts) | 100% (95) | 26% (25) | 45% (43) | 28% (27) |
| Discomfort swallowing cold liquids, % (No. of pts) | 100% (95) | 36% (34) | 41% (41) | 21% (20) |
| Throat discomfort, % (No. of pts) | 100% (124) | 40% (50) | 44% (55) | 15% (19) |
| Muscle cramps, % (No. of pts) | 100% (189) | 65% (122) | 25% (48) | 10% (19) |
No. of patients refers to patients who provided data for both cycles 1 and 2.
Table A2.
Percent Reduction from Baseline in CIPN-20 Sensory Score
| Percent Reduction | Time Since Completion of Chemotherapy |
|||||
|---|---|---|---|---|---|---|
| End of Treatment (N = 335) | Month 1 (N = 311) | Month 3 (N = 293) | Month 6 (N = 242) | Month 12 (N = 132) | Month 18 (N = 86) | |
| < 10%, No. of pts (%) | 124 (37%) | 96 (31%) | 83 (28%) | 99 (41%) | 58 (44%) | 43 (50%) |
| 10% to 30%, No. of pts (%) | 141 (42%) | 110 (35%) | 107 (37%) | 88 (36%) | 52 (39%) | 27 (31%) |
| > 30%, No. of pts (%) | 70 (21%) | 105 (34%) | 103 (35%) | 55 (23%) | 22 (17%) | 16 (19%) |
Abbreviation: CIPN-20, 20-item European Organisation for Research and Treatment of CancerQuality-of-Life Questionnaire for patients with chemotherapy-induced peripheral neuropathy.
Fig A1.
Mean functional subscores from the 20-item European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire for patients with chemotherapy-induced peripheral neuropathy during 12 cycles of oxaliplatin therapy and follow-up. Graphs show results for (A) “Difficulty manipulating small objects with your fingers,” (B) “Difficulty opening a jar or bottle because of weakness in your hands,” (C) “Problems holding a pen,” (D) “Problems standing or walking because of difficulty feeling ground,” (E) Difficulty walking because your feet dropped downward,” (F) “Difficulty climbing stairs or getting up out of a chair because of weakness in your legs,” (G) “Difficulty using car pedals,” and (H) “Difficulty distinguishing between hot and cold water.”
Fig A2.
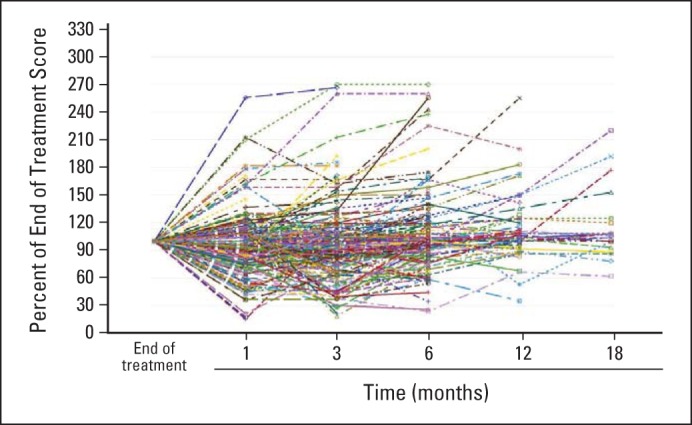
Changes in chemotherapy-induced peripheral neuropathy sensory scores for individual patients in the time following the last dose of chemotherapy. Higher values indicate lower severity of symptoms.
Footnotes
Supported in part by National Cancer Institute Grants No. CA31946 to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, MD, Chair) and CA33601 to the Alliance Statistics and Data Center (Daniel J. Sargent, PhD), and by Public Health Service Grants No. CA-25224 and CA-37404.
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group/Alliance and Mayo Clinic. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00316914.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Axel Grothey, Charles L. Loprinzi
Collection and assembly of data: Axel Grothey, Charles L. Loprinzi
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinical Course of Oxaliplatin-Induced Neuropathy: Results From the Randomized Phase III Trial N08CB (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Deirdre R. Pachman
No relationship to disclose
Rui Qin
Stock or Other Ownership: UnitedHealth Group (I)
Drew K. Seisler
No relationship to disclose
Ellen M.L. Smith
No relationship to disclose
Andreas S. Beutler
No relationship to disclose
Lauren E. Ta
No relationship to disclose
Jacqueline M. Lafky
No relationship to disclose
Nina D. Wagner-Johnston
Honoraria: Gilead Sciences
Consulting or Advisory Role: Gilead Sciences
Kathryn J. Ruddy
No relationship to disclose
Shaker Dakhil
No relationship to disclose
Nathan P. Staff
No relationship to disclose
Axel Grothey
Consulting or Advisory Role: Genentech/Roche (Inst), Bayer (Inst), Sanofi (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Amgen (Inst)
Research Funding: Genentech/Roche (Inst), Bayer (Inst), Pfizer (Inst), Eisai (Inst), Sanofi (Inst), Eli Lilly (Inst), Boston Biomedical (Inst)
Travel, Accommodations, Expenses: Genentech/Roche, Bayer, Bristol-Myers Squibb, Boston Biomedical, Amgen
Charles L. Loprinzi
Consulting or Advisory Role: Helsinn Therapeutics
Research Funding: Pfizer (Inst)
REFERENCES
- 1.Kidwell KM, Yothers G, Ganz PA, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer. 2012;118:5614–5622. doi: 10.1002/cncr.27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 3.Pietrangeli A, Leandri M, Terzoli E, et al. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol. 2006;56:13–16. doi: 10.1159/000094376. [DOI] [PubMed] [Google Scholar]
- 4.Tofthagen C, Donovan KA, Morgan MA, et al. Oxaliplatin-induced peripheral neuropathy's effects on health-related quality of life of colorectal cancer survivors. Supp Care Cancer. 2013;21:3307–3313. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer. 2013;119:438–444. doi: 10.1002/cncr.27732. [DOI] [PubMed] [Google Scholar]
- 6.Park SB, Goldstein D, Lin CS, et al. Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol. 2009;27:1243–1249. doi: 10.1200/JCO.2008.19.3425. [DOI] [PubMed] [Google Scholar]
- 7.Velasco R, Bruna J, Briani C, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014;85:392–398. doi: 10.1136/jnnp-2013-305334. [DOI] [PubMed] [Google Scholar]
- 8.Loprinzi CL, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity, (N08CB/Alliance) J Clin Oncol. 2014;32:997–1005. doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 10.Hauser K, Walsh D. Visual analogue scales and assessment of quality of life in cancer. J Support Oncol. 2008;6:277–282. [PubMed] [Google Scholar]
- 11.Kindler CH, Harms C, Amsler F, et al. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients' anesthetic concerns. Anesth Analg. 2000;90:706–712. doi: 10.1097/00000539-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 12.Locke DE, Decker PA, Sloan JA, et al. Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage. 2007;34:628–638. doi: 10.1016/j.jpainsymman.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavoie Smith EM, Barton DL, Qin R, et al. Assessing patient-reported peripheral neuropathy: The reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res. 2013;22:2787–2799. doi: 10.1007/s11136-013-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Lehky TJ, Leonard GD, Wilson RH, et al. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- 16.Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Molecular Pain. 2009;5:9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 18.Leonard GD, Wright MA, Quinn MG, et al. Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer. 2005;5:116. doi: 10.1186/1471-2407-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tofthagen C, McAllister RD, McMillan SC. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin J Oncol Nurs. 2011;15:182–188. doi: 10.1188/11.CJON.182-188. [DOI] [PubMed] [Google Scholar]
- 20.Wolf SL, Barton DL, Qin R, et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Supp Care Cancer. 2012;20:625–632. doi: 10.1007/s00520-011-1141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunberg SM, Sonka S, Stevenson LL, et al. Progressive paresthesias after cessation of therapy with very high-dose cisplatin. Cancer Chemother Pharmacol. 1989;25:62–64. doi: 10.1007/BF00694340. [DOI] [PubMed] [Google Scholar]
- 22.LoMonaco M, Milone M, Batocchi AP, et al. Cisplatin neuropathy: Clinical course and neurophysiological findings. J Neurol. 1992;239:199–204. doi: 10.1007/BF00839140. [DOI] [PubMed] [Google Scholar]
- 23.Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41:661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Kong K, Mozaffar T, et al. Delayed oxaliplatin-associated neurotoxicity following adjuvant chemotherapy for stage III colon cancer. Anticancer Drugs. 2006;17:103–105. doi: 10.1097/01.cad.0000185185.64980.70. [DOI] [PubMed] [Google Scholar]
- 26.Alejandro LM, Behrendt CE, Chen K, et al. Predicting acute and persistent neuropathy associated with oxaliplatin. Am J Clin Oncol. 2013;36:331–337. doi: 10.1097/COC.0b013e318246b50d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loprinzi CL, Reeves BN, Dakhil SR, et al. Natural history of paclitaxel-associated acute pain syndrome: Prospective cohort study NCCTG N08C1. J Clin Oncol. 2011;29:1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves BN, Dakhil SR, Sloan JA, et al. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer. 2012;118:5171–5178. doi: 10.1002/cncr.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]