Abstract
Purpose
Limited resection has been increasingly used in older patients with stage IA lung cancer. However, the equivalency of limited resection versus lobectomy according to histology is unknown.
Methods
We identified patients older than 65 years with stage IA invasive adenocarcinoma or squamous cell carcinoma ≤ 2 cm who were treated with limited resection (wedge or segmentectomy) or lobectomy in the Surveillance, Epidemiology, and End Results–Medicare database. We estimated propensity scores that predicted the use of limited resection and compared survival of patients treated with limited resection versus lobectomy. Treatments were considered equivalent if the upper 95th percentile of the hazard ratio (HR) for limited resection was ≤ 1.25.
Results
Overall, 27% of 2,008 patients with adenocarcinoma and 32% of 1,139 patients with squamous cell carcinoma underwent limited resection. Survival analyses, adjusted for propensity score by using inverse probability weighting, showed that limited resection was not equivalent to lobectomy in patients with adenocarcinoma (HR, 1.21; upper 95% CI,1.34) or squamous cell carcinoma (HR, 1.21; upper 95% CI, 1.39). Although patients with adenocarcinomas treated with segmentectomy had equivalent survival rates to those treated with lobectomy (HR, 0.97; upper 95% CI, 1.07), outcomes of those treated with wedge resection (HR, 1.29; upper 95% CI, 1.42) did not. Among patients with squamous cell carcinoma, neither wedge resection (HR, 1.34; upper 95% CI, 1.53) nor segmentectomy (HR, 1.19; upper 95% CI, 1.36) were equivalent to lobectomy.
Conclusion
We found generally that limited resection is not equivalent to lobectomy in older patients with invasive non–small-cell lung cancer ≤ 2 cm in size, although segmentectomy may be equivalent in patients with adenocarcinoma.
INTRODUCTION
Approximately 10% to 15% of patients with non–small-cell lung cancer (NSCLC) are diagnosed with stage IA disease.1 However, the incidence of early-stage cancers will substantially increase after recently released US Preventive Services Task Force recommendations for lung cancer screening are fully implemented.2 Because surgical management of stage IA NSCLC leads to 5-year survival rates as high as 70%, decisions related to optimal surgical resection are critical toward a meaningful chance of cure.3
The National Comprehensive Cancer Network recommendation for the treatment of stage IA NSCLC is lobectomy with systematic lymph node sampling.4 Evidence supporting this practice derives from a single randomized controlled trial (RCT) of node-negative NSCLC ≤ 3 cm in size, which found a lower recurrence risk and a trend toward improved survival with lobectomy compared with limited resection (ie, wedge resection or segmentectomy).5 Since then, several observational studies have shown equivalent survival and lower rates of postoperative complications with limited resection for tumors ≤ 2 cm in size, particularly among older patients.6–10 Consequently, elective limited approaches are increasingly used for stage IA NSCLCs.
Lung cancer histology is an important predictor of survival, independent of tumor size.11 There is significant heterogeneity in lung cancer prognosis on the basis of histology, from relatively indolent tumors, such as adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), to more aggressive tumors, like invasive adenocarcinoma and squamous cell carcinoma.12 Patients with AIS and MIA have excellent survival rates after resection and, therefore, are likely to benefit from parenchymal sparing of limited resection without an increased risk of recurrence.13,14 However, it is unclear whether limited resection is equivalent to lobectomy for patients who have stage IA disease with invasive histology.
In this study, we used population-based data to assess the equivalency of limited resection versus lobectomy among older patients with stage IA invasive adenocarcinoma and squamous cell carcinoma ≤ 2 cm in size. We hypothesized that limited resection, compared with lobectomy, will not lead to equivalent survival among patients with more aggressive cell types.
METHODS
Study Population
Study patients were identified from the Surveillance, Epidemiology, and End Results (SEER) –Medicare database, a population-based tumor registry that includes clinical information on incident cancer cases within representative areas of the United States.15 The study cohort was limited to patients older than 65 years who were diagnosed between 1998 and 2010 with stage IA NSCLC ≤ 2 cm in size and treated with limited resection or lobectomy. Centrally located cancers, which generally are not amenable to limited resection, and patients treated with neoadjuvant radiotherapy, which could reflect cancers of higher stages, were excluded. Patients who resided in nursing homes or who were receiving hospice care were excluded because of a likely poor functional status. Finally, patients in health maintenance organizations or those without Part B (outpatient) Medicare coverage were excluded because of a lack of claims data. The Icahn School of Medicine at Mount Sinai Institutional Review Board considered the study exempt.
We obtained patient sociodemographic information from SEER. By using Medicare data, we grouped patients into estimated income quartiles that were based on the median income in their census tract. Medicare claims allowed us to assess comorbidities by applying a modified version of the Charlson index.8,16 Claims that indicated home services were used as a proxy for poor performance status.17
Tumor histology was determined with SEER codes according to the International Classification of Diseases for Oncology.18 In 2011, a major revision to the classification of lung adenocarcinoma was proposed by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society, in which terminology was introduced to replace the bronchioloalveolar carcinoma (BAC) category with AIS.12 A new category of MIA was added, and tumors previously described as adenocarcinoma, mixed type, are now classified on the basis of the predominant histologic growth pattern. This new coding system has not yet been incorporated into SEER, leading to potential misclassification of AIS and MIA occurrences. For example, patient cases historically diagnosed as nonmucinous BAC could potentially be AIS, MIA, or invasive adenocarcinomas with lepidic components. To reduce the likelihood of misclassification, we excluded former BAC patient cases (SEER codes 8250 and 8252) from the analyses.19 However, current recommendations are to classify mucinous BAC (SEER codes 8251 and 8253) as invasive mucinous adenocarcinoma.20 Thus, we combined these BAC subtypes with invasive adenocarcinomas (SEER codes 8140, 8230, 8254 to 8255, 8260, 8310, 8333, 8470, 8480, 8481, 8490, and 8550) into a single group. Squamous cell carcinomas were identified using SEER codes 8052, 8070 to 8073, and 8083 to 8084. Other cell types were excluded given their low incidence.
By using SEER data on cancer location, size, extension, and lymph node status, we determined tumor stage according to the American Joint Commission on Cancer classification (seventh edition).21 Tests used for cancer diagnosis (eg, fine needle aspiration, bronchoscopy), preoperative evaluation (eg, ventilation/perfusion scan, cardiac/cardiopulmonary stress testing), and staging work-up (eg, positron emission tomography, mediastinoscopy) were ascertained using International Classification of Diseases, 9th revision (ICD-9), procedure codes and Current Procedural Terminology-4 codes from Medicare claims.
Resection type was determined with SEER and Medicare site-specific surgery variables. Patients were classified as undergoing limited resection (SEER codes 20 to 22 and ICD-9 procedure codes 32.29 and 32.3) or lobectomy (SEER codes 30, 31, and 33 and ICD-9 procedure code 32.4). We used Medicare claims to identify patients treated with video-assisted thoracic surgery (VATS; ICD-9 procedure code 34.21 and Current Procedural Terminology-4 code 32657). The use of postoperative radiotherapy was obtained from both SEER and Medicare data. Chemotherapy use was ascertained from Medicare claims by applying a validated algorithm.22
Survival was calculated from the date of diagnosis to the date of death; patients alive as of December 31, 2009, were censored at the date of last follow-up. Cause of death was established from SEER, which obtains the cause from state death certificate information. When we assessed lung-cancer specific survival, deaths as a result of other causes were censored at the date of death.
Statistical Analysis
Within each histologic group, we compared demographic and tumor characteristics of patients treated with limited resection or lobectomy using the t test or χ2 test, as appropriate. Because treatment allocation was not random, potential imbalances in patient and tumor characteristics between treatment groups may have existed. Therefore, we applied propensity score methods to minimize the effect of measured confounders. Logistic regression was used to calculate propensity scores that indicated the probability of undergoing limited resection on the basis of pretreatment information, including patient sociodemographic characteristics, comorbidities, and functional status; tumor characteristics; and the diagnostic, staging, and operative work-ups. Weights were calculated for each patient as the inverse of the estimated probability for undergoing the type of treatment received (limited resection v lobectomy). When the model was fitted, we evaluated whether baseline covariates were well balanced across groups after adjustment for propensity scores. All analyses were conducted with SAS statistical software (SAS Institute, Cary, NC).
The equivalence of limited resection versus lobectomy in terms of overall (primary outcome) and lung-cancer specific (secondary outcome) survival was assessed with the Confidence Interval Method.23 We used Cox proportional hazards regression to compare survival of patients with invasive adenocarcinoma and squamous cell carcinoma treated with limited resection versus lobectomy while adjusting for selection bias using inverse probability weighting.24 These models were also adjusted for the number of lymph nodes resected and the type of surgical approach (ie, VATS v open thoracotomy). On the basis of these models, we computed the one-sided upper-bound CI at a significance level of .05. Noninferiority was established at the level of α significance if the upper limit of a (1 − 2α) CI was below the prespecified equivalence margin.25 It is generally accepted in oncology studies that, if the hazard ratio (HR) associated with the new treatment versus the standard of care does not exceed 1.25 (ie, one-sided upper 95% CI is < 1.25), the two treatments can be considered equivalent.26 We used this criterion to assess if limited resection was equivalent to lobectomy.
Secondary analyses were conducted by stratifying the sample by age (< 70 v ≥ 70 years), by the type of limited resection (wedge resection v segmentectomy), and by including patients with stage IA tumors 3 cm or smaller in size.
On the basis of the number of deaths (primary outcome) among patients in the cohort, we estimated that the study had greater than 80% power to determine if the HR associated with limited resection did not exceed 1.25.
RESULTS
We identified 4,033 patients with histologically confirmed stage IA NSCLC 2 cm or less in size treated surgically between 1998 and 2009 from the SEER-Medicare registry. Of these, we excluded 604 patients with BAC and 282 patients with other cell types. The final study cohort consisted of 3,147 patients who had stage IA disease with invasive adenocarcinoma (64%) or squamous cell (36%) carcinomas. Overall, 29% of patients underwent limited resection (23%, wedge resection; 6%, segmentectomy). Baseline characteristics of study patients according to histology and type of resection are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of Patients With Stage IA (≤ 2 cm) Non–Small-Cell Lung Cancer, According to Histologic Type, Treated With Limited Resection Versus Lobectomy
| Characteristic | Invasive Adenocarcinoma |
Squamous Cell Carcinoma |
||||||
|---|---|---|---|---|---|---|---|---|
| Limited Resection (n = 546) | Lobectomy (n = 1,462) |
P |
Limited Resection (n = 362) | Lobectomy (n = 777) |
P |
|||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||||
| Mean ± SD age, years | 74.5 ± 5.3 | 73.4 ± 5.2 | < .001 | .97 | 74.7 ± 5.5 | 73.9 ± 4.9 | .04 | .94 |
| Female sex, No. (%) | 336 (62) | 853 (58) | .19 | .99 | 208 (58) | 375 (48) | .004 | .97 |
| Race/ethnicity, No. (%) | ||||||||
| White | 492 (90) | 1,302 (89) | .51 | .99 | 324 (90) | 699 (90) | .46 | .99 |
| Black | 26 (5) | 64 (5) | 24 (7) | 47 (6) | ||||
| Hispanic† | 13 (2) | 35 (2) | < 11 (< 28) | < 11 (< 28) | ||||
| Other† | 15 (3) | 61 (4) | < 11 (< 28) | > 11 (> 2) | ||||
| Married, No. (%) | 273 (50) | 865 (59) | < .001 | .96 | 185 (51) | 422 (54) | .31 | .97 |
| Median income quartile, No. (%) | ||||||||
| Lowest | 103 (19) | 277 (19) | .31 | .99 | 84 (23) | 187 (24) | .2 | .99 |
| Second | 121 (22) | 381 (26) | 87 (24) | 228 (30) | ||||
| Third | 137 (25) | 337 (23) | 105 (29) | 204 (26) | ||||
| Highest | 185 (34) | 467 (32) | 86 (24) | 158 (20) | ||||
| Comorbidity score, No. (%) | ||||||||
| ≤ 1 | 210 (39) | 761 (52) | < .001 | .96 | 86 (24) | 314 (40) | < .001 | .85 |
| > 1-2 | 172 (31) | 374 (26) | 130 (36) | 210 (27) | ||||
| > 2 | 164 (30) | 327 (22) | 146 (40) | 253 (33) | ||||
| Mean ± SD tumor size, mm | 14.6 ± 4.1 | 15.7 ± 3.7 | < .001 | .96 | 14.8 ± 3.9 | 15.5 ± 3.9 | .004 | .92 |
| Tumor site, No. (%) | ||||||||
| Upper lobe | 356 (66) | 921 (63) | < .001 | .99 | 253 (70) | 539 (69) | .43 | .98 |
| Middle lobe† | 13 (2) | 95 (7) | < 11 (< 28) | 32 (4) | ||||
| Lower lobe | 164 (30) | 430 (29) | 95 (26) | 194 (26) | ||||
| Other lobe† | 13 (2) | 16 (1) | < 11 (< 28) | 12 (1) | ||||
| Lymph nodes removed, No. (%)‡ | ||||||||
| ≤ 5 | 425 (78) | 589 (40) | < .001 | — | 314 (87) | 307 (40) | < .001 | — |
| > 5 | 73 (13) | 687 (47) | 31 (9) | 381 (49) | ||||
| Unknown | 48 (9) | 186 (13) | 17 (5) | 89 (11) | ||||
| VATS‡ | 189 (35) | 220 (15) | < .001 | — | 116 (32) | 92 (12) | < .001 | — |
Abbreviations: SD, standard deviation; VATS, video-assisted thoracic surgery.
Adjusted for propensity scores.
Data from fewer than 11 patients were masked to maintain patient confidentiality.
Not preoperative characteristics (and no associated P values). These factors were included in the Cox model that compared survival in patients treated with limited resection versus lobectomy.
Among patients with invasive adenocarcinoma, those treated with limited resection were older (P < .001), were less likely to be married (P < .001), had more comorbidities (P < .001), and had smaller tumors (P < .001). Patients undergoing limited resection had fewer lymph nodes sampled (P < .001) and were more likely to undergo VATS (P < .001). There were no significant differences in the distribution of other baseline characteristics among patients with adenocarcinoma in both groups (P > .05 for all comparisons). Patients with squamous cell carcinoma who were treated with limited resection also were older (P = .04), were more likely to be women (P = .004), and had higher comorbidity scores (P < .001). Limited resection in this group also was associated with smaller tumors (P = .004), fewer lymph nodes sampled (P < .001), and VATS resections (P < .001). Other factors were not significantly different among treatment groups (P > .05 for all comparisons). Baseline characteristics were well matched across treatment groups after adjustment for propensity scores.
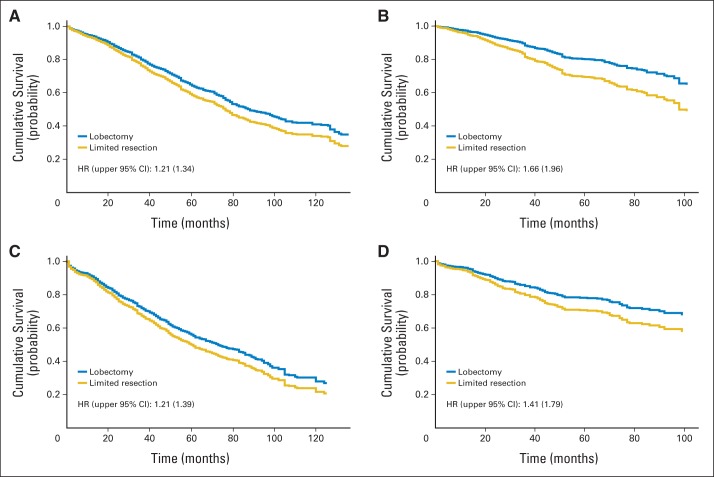
Analyses to adjust for propensity scores showed that limited resection was not equivalent to lobectomy in terms of overall survival (HR, 1.21; upper 95% CI, 1.34) or lung cancer–specific survival (HR, 1.66; upper 95% CI, 1.96) for patients with invasive adenocarcinoma. Patients with squamous cell carcinoma treated with limited resection also did not have equivalent overall survival (HR, 1.21; upper 95% CI, 1.39) or lung cancer–specific survival (HR, 1.41; upper 95% CI, 1.79) as those treated with lobectomy (Fig 1).
Fig 1.
Adjusted survival curves based on Cox models that compare treatment with limited resection versus lobectomy in patients with adenocarcinoma for (A) overall survival and (B) lung cancer–specific survival, and in patients with squamous cell carcinoma for (C) overall survival and (D) lung cancer–specific survival. HR, hazard ratio.
Secondary analyses to stratify by age showed that limited resection was not equivalent to lobectomy in terms of overall survival (HR, 1.69; upper 95% CI, 2.14) and lung cancer–specific survival (HR, 1.58; upper 95% CI, 2.38) among patients with adenocarcinoma younger than 70 years of age. These results were consistent among patients age 70 years and older (HR, 1.16; upper 95% CI, 1.29 for overall survival; HR, 1.65; upper 95% CI, 1.99 for lung cancer–specific survival). Age-stratified analyses in patients with squamous cell carcinoma also showed a lack of equivalency in terms of overall and lung cancer–specific survival. Patients with adenocarcinoma who were treated with wedge resection did not have equivalent overall survival (HR, 1.29; upper 95% CI, 1.42) or lung cancer–specific survival (HR, 1.78; upper 95% CI, 2.09) compared with those who underwent lobectomy. However, overall survival (HR, 0.97; upper 95% CI, 1.07) and lung cancer–specific survival (HR, 0.89; upper 95% CI, 1.07) of patients with adenocarcinoma treated with segmentectomy versus lobectomy was equivalent. Conversely, neither wedge resection (HR, 1.34; upper 95% CI, 1.53 for overall survival; HR, 1.85; upper 95% CI, 2.32 for lung cancer–specific survival) nor segmentectomy (HR, 1.19; upper 95% CI, 1.36 for overall survival; HR, 1.17; upper 95% CI, 1.48 for lung cancer–specific survival) was equivalent to lobectomy among patients with squamous cell carcinoma. Finally, patients with tumors 3 cm or smaller in size who had adenocarcinoma (HR, 1.31; upper 95% CI, 1.41 for overall survival; HR, 1.90; upper 95% CI, 2.13 for lung cancer–specific survival) or squamous cell carcinoma (HR, 1.16; upper 95% CI, 1.27 for overall survival; HR, 1.62; upper 95% CI, 1.88 for lung cancer–specific survival) did not have equivalent outcomes when treated with limited resection versus lobectomy (Table 2).
Table 2.
Adjusted Association of Limited Resection With Overall and Lung Cancer–Specific Survival Among Patients With Invasive Adenocarcinoma and Squamous Cell Carcinoma
| Model | Histologic Type |
|||||
|---|---|---|---|---|---|---|
| Invasive Adenocarcinoma |
Squamous Cell Carcinoma |
|||||
| No. of Patients | Overall Survival, HR (upper 95% CI) | Lung Cancer–Specific Survival HR, (upper 95% CI) | No. of Patients | Overall Survival, HR (upper 95% CI) | Lung Cancer–Specific Survival, HR (upper 95% CI) | |
| Full cohort with tumors ≤ 2 cm | 2,008 | 1.21 (1.34) | 1.66 (1.96) | 1,139 | 1.21 (1.39) | 1.41 (1.79) |
| By age | ||||||
| Limited to patients age ≥ 70 years | 1,490 | 1.16 (1.29) | 1.65 (1.99) | 892 | 1.14 (1.33) | 1.33 (1.74) |
| Limited to patients age < 70 years | 518 | 1.69 (2.14) | 1.58 (2.38) | 247 | 1.46 (2.04) | 2.04 (3.43) |
| By treatment | ||||||
| Limited to patients treated with wedge resection v lobectomy | 1,897 | 1.29 (1.42) | 1.78 (2.09) | 1,060 | 1.34 (1.53) | 1.85 (2.32) |
| Limited to patients treated with segmentectomy v lobectomy | 1,596 | 0.97 (1.07) | 0.89 (1.07) | 854 | 1.19 (1.36) | 1.17 (1.48) |
| Full cohort with tumors ≤ 3 cm | 3,384 | 1.31 (1.41) | 1.90 (2.13) | 2,085 | 1.16 (1.27) | 1.62 (1.88) |
Abbreviation: HR, hazard ratio.
DISCUSSION
Limited resection increasingly is being adopted for the treatment of older patients with small NSCLC. In this population-based study of older patients, we evaluated the survival outcomes of limited resection versus lobectomy for those with invasive adenocarcinoma and squamous cell carcinoma. We found that limited surgical approaches, particularly wedge resection, are not equivalent to lobectomy in this population. Conversely, segmentectomy led to equivalent outcomes only for patients with invasive adenocarcinoma. Our results highlight the importance of tumor histology as a determinant of long-term outcomes for patients with early-stage NSCLC.
In the two decades since the Lung Cancer Study Group trial, the surgical management of early-stage NSCLC has evolved as a result of improved staging, preoperative evaluation, and adoption of VATS.27 These changes have led to increased skepticism about extrapolating the Lung Cancer Study Group conclusions to patients currently receiving treatment. Several more recent observational studies that compare limited resection with lobectomy have demonstrated equivalent long-term outcomes in older patients.6–10 Elderly patients undergoing lobectomy are more likely to experience peri- and postoperative complications, and, at the same time, they are at a lower risk of cancer recurrence because of competing risks of death as a result of comorbidities.28–31 However, the impact of histologic type on survival was not assessed in these studies. The observed survival equivalence with limited resection versus lobectomy may be driven by outcomes of less aggressive tumors, such as AIS and MIA, which are over-represented among NSCLCs 2 cm or less in size. Thus, it is unclear whether patients with more aggressive cancers are also good candidates for limited resection. Our findings should help decide the best treatment for older patients by balancing the potential short- and long-term risks of limited resection versus lobectomy.
As our understanding of NSCLC has advanced, a new classification system of adenocarcinoma has been introduced that highlights the prognostic differences between histologic subtypes that have potential therapeutic implications.12 AIS and MIA, tumors that exhibit pure lepidic growth or have minimal invasion, respectively, have nearly 100% 5-year survival rates after resection and, therefore, are more amenable to limited resection.14 However, invasive adenocarcinomas are characterized by stromal invasion and can be additionally classified into a predominant histologic type. Lepidic, acinar, and papillary predominant subtypes of stage IA adenocarcinomas are associated with 5-year survival rates after resection between 83% and 90%, whereas solid and micropapillary histologies have 5-year survival rates of 67% to 76%.32 Our subset analysis shows that segmentectomy is equivalent to lobectomy for these tumors. However, only 15% to 20% of patients with stage IA disease who are undergoing limited resection are usually candidates for segmentectomy because of tumor characteristics that do not allow for adequate surgical margins. Squamous cell carcinomas are more likely to be locally aggressive and to invade adjacent structures. When matched by stage, these tumors have slightly better survival rates but similar recurrence risks as adenocarcinomas.20,33 However, patients with squamous cell carcinoma who were treated with either wedge resection or segmentectomy did not have equivalent outcomes to those treated with lobectomy, which suggests that they should receive lobectomy.
The diagnosis of histologic subtype can only be definitively determined from pathologic examination of postsurgical specimens. Our findings, nevertheless, are important for several reasons. First, a strong correlation exists between radiologic features on computed tomography with the histologic spectrum of tumor aggressiveness.34–38 Ground-glass opacities in sub–solid nodules correlate with lepidic histologic pattern, whereas the solid component is strongly predictive of invasive growth. Several recent studies also have shown the accuracy of the ratio of the percentage of solid component over the total tumor volume (consolidation:tumor) as a predictor of invasiveness.39–44 This preoperative information may be used, in combination with our findings, to guide decisions regarding type of surgical approach. Second, our findings suggest that tumors with squamous cell histology, when identified preoperatively via small biopsy samples, should be considered for lobectomy. Third, ongoing studies are evaluating potential biomarkers to predict adenocarcinoma invasiveness in small biopsy samples.45,46 Finally, postoperative pathologic identification of aggressive tumors might create an opportunity for completion lobectomy among younger patients or elderly patients undergoing elective sublobar resections. Furthermore, future studies should evaluate the potential role of adjuvant therapy or the use of stereotactic body radiation treatment for patients who, despite having aggressive histologic types, are not candidates for full lobectomy.
This study has some strengths and limitations. The SEER-Medicare registry provides data from a large number of older individuals from multiple geographic areas. Thus, our findings have strong external validity. Moreover, availability of long-term follow-up data provided sufficient power to assess the equivalence of limited resection versus lobectomy according to histologic type. Although we focused on the most frequently affected age group, a particular strength in our study, our results cannot be extrapolated to younger patients with longer life expectancies and, thus, increased risks of recurrence after treatment with limited resection.
The main study limitation is the lack of random treatment allocation. Patient- and provider-level processes to determine treatment allocation may have generated systematic differences in the treatment groups. To mitigate this bias, we restricted the study population to patients who were potential candidates for either approach and used propensity score methods to successfully balance groups for all measured confounders. We cannot exclude hidden biases, though, and these findings do not provide the same level of evidence as an RCT; however, the few RCTs that evaluate the role of limited resection for patients with small NSCLCs have not been completed because of low enrollment rates.47 Moreover, these studies (except a Japanese study focused on low-aggressive cancers) were not stratified by histology.48 Thus, in the absence of information from prospective trials, our findings provide useful information about the management of older patients with invasive early-stage NSCLC. Another limitation is the lack of updated histologic data in SEER to reflect the latest classification of adenocarcinoma. Although we excluded occurrences previously classified as BAC, our invasive adenocarcinoma group may still consist of somewhat heterogeneous tumors with varied prognoses. However, the potential inclusion of MIAs as invasive adenocarcinomas cannot explain our findings of worse outcomes with limited resection in this group. Finally, the pattern of disease relapse (not available in SEER) would help to better understand the benefits of lobectomy versus limited resection.
In summary, our study showed that limited resection is not equivalent to lobectomy when used to treat older patients with invasive adenocarcinoma or squamous cell carcinoma. These patients may be considered for completion lobectomy or, potentially, for adjuvant treatments, if they are proven effective in this setting. This information is important, given the increasing uptake of lung cancer screening and the expected growth in the number of early-stage NSCLCs, the majority (> 60%) of which are expected to be of invasive histology on the basis of the results of the National Lung Cancer Screening Trial.49 Understanding the role of limited resection versus lobectomy in the treatment of these patients will be critical to successful implementation of screening.
Acknowledgment
We thank the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services and the Office of Strategic Planning, Health Care Finance Administration; Information Management Services; and the Surveillance, Epidemiology, and End Results (SEER) program tumor registries for their efforts in the creation of the SEER-Medicare database.
Footnotes
See accompanying article on page 3488
Supported by the Agency for Healthcare Research and Quality (Grant No. 5R01 HS019670).
All authors fulfilled the authorship credit requirements outlined by the International Committee of Medical Journal Editors recommendations. They have approved the final submitted version and agree to be accountable for the interpretation and reporting of the presented data.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Rajwanth R. Veluswamy, Nicole Ezer, Marcelo Bonomi, Alfred I. Neugut, Scott Swanson, Charles A. Powell, Mary B. Beasley, Juan P. Wisnivesky
Collection and assembly of data: Rajwanth R. Veluswamy, Grace Mhango, Emily Goodman, Juan P. Wisnivesky
Data analysis and interpretation: Rajwanth R. Veluswamy, Nicole Ezer, Grace Mhango, Alfred I. Neugut, Charles A. Powell, Mary B. Beasley, Juan P. Wisnivesky
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rajwanth R. Veluswamy
No relationship to disclose
Nicole Ezer
No relationship to disclose
Grace Mhango
No relationship to disclose
Emily Goodman
No relationship to disclose
Marcelo Bonomi
No relationship to disclose
Alfred I. Neugut
Consulting or Advisory Role: Pfizer, Otsuka, Teva Neuroscience, EHE International, United BioSource
Scott Swanson
Consulting or Advisory Role: Ethicon, Covidien
Charles A. Powell
No relationship to disclose
Mary B. Beasley
Consulting or Advisory Role: Genentech
Travel, Accommodations, Expenses: Genentech
Juan P. Wisnivesky
Honoraria: IMS Health, Merck, Bristol-Myers Squibb, Quintiles
Consulting or Advisory Role: EHE International
Research Funding: Sanofi
REFERENCES
- 1.Spiro SG, Porter JC. Lung cancer: Where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med. 2002;166:1166–1196. doi: 10.1164/rccm.200202-070SO. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 3.Strand T-E, Rostad H, Møller B, et al. Survival after resection for primary lung cancer: A population based study of 3211 resected patients. Thorax. 2006;61:710–715. doi: 10.1136/thx.2005.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Non–small-cell lung cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 5.Thomas P, Rubinstein L. Cancer recurrence after resection: T1 N0 non–small-cell lung cancer—Lung Cancer Study Group. Ann Thorac Surg. 1990;49:242–246. doi: 10.1016/0003-4975(90)90145-v. discussion 246-247. [DOI] [PubMed] [Google Scholar]
- 6.Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg. 2003;125:924–928. doi: 10.1067/mtc.2003.156. [DOI] [PubMed] [Google Scholar]
- 7.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non–small-cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 9.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–233. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy versus limited resection for stage I lung cancer: A meta-analysis. Br J Cancer. 2005;92:1033–1037. doi: 10.1038/sj.bjc.6602414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ost D, Goldberg J, Rolnitzky L, et al. Survival after surgery in stage IA and IB non–small-cell lung cancer. Am J Respir Crit Care Med. 2008;177:516–523. doi: 10.1164/rccm.200706-815OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 14.Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Wisnivesky JP, Smith CB, Packer S, et al. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II-IIIA lung cancer: Observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz A PC, Jack A. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 19.National Cancer Institute. Surveillance, Epidemiology, and End Results. http://www.seer.cancer.gov.
- 20.Rezaei MK, Nolan NJ, Schwartz AM. Surgical pathology of lung cancer. Semin Respir Crit Care Med. 2013;34:770–786. doi: 10.1055/s-0033-1358558. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual (7th ed) New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 22.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(suppl 8):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 23.Rogers JL, Howard KI, Vessey JT. Using significance tests to evaluate equivalence between two experimental groups. Psychol Bull. 1993;113:553–565. doi: 10.1037/0033-2909.113.3.553. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, van Dyk DA. Causal inference with general treatment regimes: Generalizing the propensity score. J Am Stat Assoc. 2004;99:854–866. [Google Scholar]
- 25.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–196. doi: 10.1007/s11606-010-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothmann M, Li N, Chen G, et al. Design and analysis of non-inferiority mortality trials in oncology. Stat Med. 2003;22:239–264. doi: 10.1002/sim.1400. [DOI] [PubMed] [Google Scholar]
- 27.Patel AN, Santos RS, De Hoyos A, et al. Clinical trials of peripheral stage I (T1N0M0) non–small-cell lung cancer. Semin Thorac Cardiovasc Surg. 2003;15:421–430. doi: 10.1053/j.semtcvs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non–small-cell lung cancer. Chest. 2012;142:1620–1635. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 29.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 30.Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: Initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–1019. doi: 10.1016/j.athoracsur.2005.06.066. discussion 1019-1020. [DOI] [PubMed] [Google Scholar]
- 31.Sigel K, Bonomi M, Packer S, et al. Effect of age on survival of clinical stage I non–small-cell lung cancer. Ann Surg Oncol. 2009;16:1912–1917. doi: 10.1245/s10434-009-0475-8. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 33.Chansky K, Sculier JP, Crowley JJ, et al. The International Association for the Study of Lung Cancer staging project: Prognostic factors and pathologic TNM stage in surgically managed non–small-cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 34.Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. 2001;33:17–25. doi: 10.1016/s0169-5002(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 35.Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: Correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220:803–809. doi: 10.1148/radiol.2203001701. [DOI] [PubMed] [Google Scholar]
- 36.Kondo T, Yamada K, Noda K, et al. Radiologic-prognostic correlation in patients with small pulmonary adenocarcinomas. Lung Cancer. 2002;36:49–57. doi: 10.1016/s0169-5002(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 37.Kuriyama K, Seto M, Kasugai T, et al. Ground-glass opacity on thin-section CT: Value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol. 1999;173:465–469. doi: 10.2214/ajr.173.2.10430155. [DOI] [PubMed] [Google Scholar]
- 38.Hashizume T, Yamada K, Okamoto N, et al. Prognostic significance of thin-section CT scan findings in small-sized lung adenocarcinoma. Chest. 2008;133:441–447. doi: 10.1378/chest.07-1533. [DOI] [PubMed] [Google Scholar]
- 39.Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg. 2002;124:278–284. doi: 10.1067/mtc.2002.122298. [DOI] [PubMed] [Google Scholar]
- 40.Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer. 2003;42:303–310. doi: 10.1016/j.lungcan.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Okada M, Nishio W, Sakamoto T, et al. Correlation between computed tomographic findings, bronchioloalveolar carcinoma component, and biologic behavior of small-sized lung adenocarcinomas. J Thorac Cardiovasc Surg. 2004;127:857–861. doi: 10.1016/j.jtcvs.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 42.Asamura H. Minimally invasive approach to early, peripheral adenocarcinoma with ground-glass opacity appearance. Ann Thorac Surg. 2008;85:S701–S704. doi: 10.1016/j.athoracsur.2007.10.104. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201) J Thorac Oncol. 2011;6:751–756. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: Prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–1639. doi: 10.1016/s0003-4975(02)03895-x. [DOI] [PubMed] [Google Scholar]
- 45.Nana-Sinkam SP, Powell CA. Molecular biology of lung cancer: Diagnosis and management of lung cancer, 3rd ed—American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e30S–e39S. doi: 10.1378/chest.12-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heymann JJ, Bulman WA, Maxfield RA, et al. Molecular testing guidelines for lung adenocarcinoma: Utility of cell blocks and concordance between fine-needle aspiration cytology and histology samples. Cytojournal. 2014;11:12. doi: 10.4103/1742-6413.132989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ClinicalTrials.gov. Comparison of different types of surgery in treating patients with stage IA non-small cell lung cancer. http://clinicaltrials.gov/ct2/show/NCT00499330?term=calgb+140503&rank=1.
- 48.Rusch VW, Tsuchiya R, Tsuboi M, et al. Surgery for bronchioloalveolar carcinoma and “very early” adenocarcinoma: An evolving standard of care? J Thorac Oncol. 2006;1:S27–S31. [PubMed] [Google Scholar]
- 49.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]