Abstract
Helicobacter pylori (H. pylori) infection is generally acquired during early childhood; therefore, the immune response which usually takes place at this age may influence or even determine susceptibility to the infection contributing to the clinical outcomes in adulthood. Several cytokines including IL-6, IL-10, and TGF-β1 as well as Foxp3+ cell numbers have been shown to be higher; however, some other cytokines consisting of IL-1β, IL-17A, and IL-23 are lower in infected children than in infected adults. Immune response to H. pylori infection in children is predominant Treg instead of Th17 cell response. These results indicate that immune system responses probably play a role in persistent H. pylori infection. Childhood H. pylori infection is also associated with significantly lower levels of inflammation and ulceration compared with adults. This review, therefore, aimed to provide critical findings of the available literature about comparative immune system in children and adults with H. pylori infection.
1. Introduction
Helicobacter pylori (H. pylori) infections usually occur during childhood, continue throughout the life, and cause severe diseases such as gastritis, gastric ulcer, gastric carcinoma, and duodenum ulcer in adulthood. H. pylori is a well-known gastric pathogen infecting more than half of the world's people [1]. The outcomes of H. pylori infection seem to be dependent on some factors like gene regulation factors, genetic predisposition of the patient, receptor gene polymorphisms, particular cytokine, constituents, and environmental influences [2, 3]. Fortunately, most of the infected children do not develop any complications; however, the immunological events which usually develop in the children gastric mucosa are probably decisive in the immune response determining the final outcome the infection. The colonization of the stomach by this pathogen bacterium causes an inflammatory response and recruits neutrophils, lymphocytes, dendritic cells, and macrophages, to the gastric mucosa [4, 5]. There are complex mechanisms by which H. pylori may start and maintain the local immune response; however, cytokines produced by both adaptive immune and innate systems may lead to the development of gastric mucosa-associated lymphoid tissue lymphoma, gastric adenocarcinoma, and other ulcerative diseases; studies in H. pylori infection have revealed that childhood H. pylori infection is usually associated with significantly lower levels of gastric inflammation and ulceration in comparison to adults. Therefore, this review study was aimed to provide the critical findings of the available literature about comparative immune system in children and adults with H. pylori infection.
2. Bacterial Virulence Factors
Helicobacter pylori may express the virulence factors associated with inflammation as well as inflammatory symptoms in infected patients. The main pathogenicity factors of Helicobacter pylori include γ-glutamyl transpeptidase (GGT), cytotoxin-associated gene A (cagA) product, and virulence components vacuolating toxin (vacA), in addition to pathogen-associated molecular patterns (PAMPs) such as flagella and lipopolysaccharide (LPS) [6–9]. The cytotoxin-associated gene (cag) pathogenicity island (PAI) is one of these factors which has been extensively studied in regard to inflammation [10–12]. Colonization with the strains that possess cagA is more frequently associated with peptic ulceration gastric adenocarcinoma or other gastric mucosal complications than the cagA strains [13, 14]. It has been shown that cagA may play a role in production of IL-8 as well as activation of nuclear factor kappa-B (NF-κB) [15]. Furthermore, expression of cagA induces production of IL-8 and translocation of NF-κB nuclear in gastric epithelial cells [13, 16]. The vacA from H. pylori is capable of inducing intracellular vacuolation in gastric epithelial cells. Hence, it has been hypothesized that it may contribute in damage of gastric and duodenal mucosa which ultimately leads to ulcer formation, in vivo [14]. Moreover, the bacterial virulence factors vacA and cagA have important roles in pathogenesis of H. pylori infection. Others like blood group antigen-binding adhesion (BabA), outer inflammatory protein (oipA), sialic acid-binding adhesion (sabA), iceA (induced by contact with epithelium), and duodenal ulcer promoting gene (dupA) may promote colonization of the mucosa, too [17]. In regard to virulence factors cagA and vacA, these bacteria are very heterogeneous [18]. A lot of evidences have revealed that these genetic variations may have an important role in the outcome of infection [19, 20].
3. T Cell Subsets
T helper (Th) cells have been shown to differentiate into functional classes of two major CD4+ including Th1 cells (able to produce some cytokines such as IL-2 and IFN-γ) and Th2 cells (producing cytokines like IL-4, IL-5, and IL-10) [21, 22]. Th1 cells mediated cell immunity, which has an important role against intracellular parasites. However, Th2 generates humoral immunity as well as prevention of intestinal helminthes [23]. Other than Th1/Th2 paradigm, a unique subset of IL-17 producing Th17 cells has been discovered [24–26]. IL-23 has a crucial role in differentiation of Th17 cells. However, IL-4 and IL-12 promote, respectively, Th1 and Th2 cell differentiation [27]. It has been revealed that IL-17 possesses 6 family members (IL-17A–F), IL-17A (simply called IL-17) being the prototypic IL-17 family member [28, 29]. Furthermore, IL-17A exerts proinflammatory effects by stimulation of the production of chemokines such as IL-1, IL-6, cytokines, monocyte chemoattractant protein-1, and upregulation of cell adhesion molecules like vascular cell adhesion molecule-1 and intercellular adhesion molecule-1. The IL-17A plays a crucial role in induction of autoimmune diseases such as inflammatory bowel disease (IBD), experimental autoimmune encephalomyelitis (EAE), and rheumatoid arthritis (RA), as well as chronic inflammatory diseases [30–33]. Regulatory T (Treg) cells, by proliferation of antigen specific T cells and suppressing the activation, have important role in chronic inflammation. It should be noted that depletion or dysfunction of Treg cells is usually associated with inflammatory bowel disease, allergy, and autoimmune disease [34]. Treg cells comprise different subsets: Tr1 cells secreting interleukin IL-10, Th3 cells characterized by transforming growth factor (TGF-β1) secretion, and naturally occurring FOXP3-expressing CD4+CD25high Treg cells [35, 36]. The FOXP3+CD4+CD25high Treg cells are further divided into two subsets: thymus derived naturally occurring FOXP3+CD4+CD25high Treg cells and peripherally induced FOXP3+CD4+CD25high Treg cells [37].
4. Differences in Immunity of Children and Adults Infected and Uninfected with H. pylori
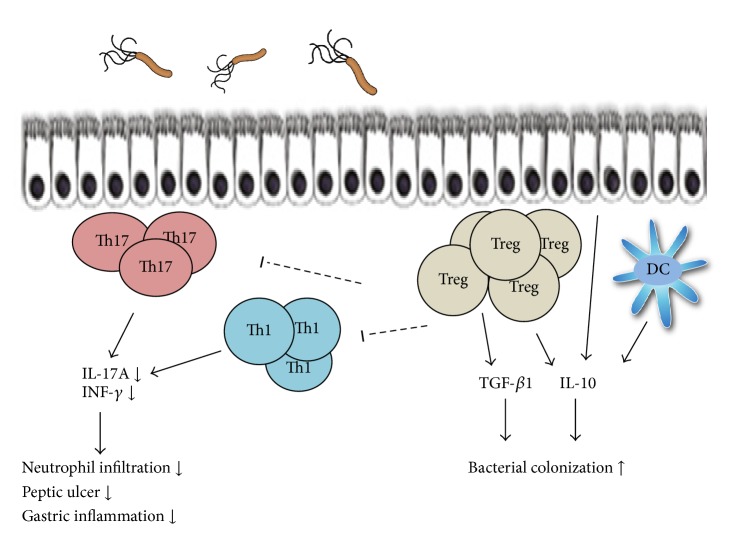
The human gastric mucosal biopsies revealed that people who were persistently infected with H. pylori, in comparison to uninfected ones, show an increased and higher level of infiltrated various types of leukocytes [38]. In these specimens, lymphocytes (T and B cells), monocytes, mast cells, neutrophils, macrophages, eosinophil, and dendritic cells are usually present [2, 4]. CD4+ T cells, B cells and dendritic cells may be organized in lymphoid follicles [39] indicating ongoing chronic immune responses and antigen presentation. In peripheral blood and gastric mucosa of infected humans, the H. pylori-specific CD4+ T cells are detectable which is not detectable in uninfected individuals [40]. The cytokines such as TNF-α, IFN-γ, IL-1, IL-6, IL-7, IL-8, IL-10, IL-17, IL-18, and IL-23 have usually increased levels in the stomach of H. pylori-infected patients in comparison to the uninfected subjects [2, 41, 42]. IL-4 is not usually detectable in the gastric mucosa of H. pylori-infected patients [43]. Hence, to show, in children, the mucosal regulation of H. pylori infection, which can provide a window to the early host response to bacteria, the mucosal cytokine response to the infection, the associated cellular infiltrate, and the characterized bacteria might be helpful. Studies on H. pylori-infected children and adults have shown that children possess reduced gastric inflammation in comparison to the infected adults, in spite of similarity in H. pylori colonization level. Furthermore, inflammation in children has been shown to be less in comparison to that of adults, indicating a downregulation in immune response to infection in children [44, 45]. Moreover, the sequence analysis revealed that the bacteria isolated from the infected children and adults might have similar cagA and vacA gene profiles. The difference in bacterial strains and common virulence factors were not the cause of low level of inflammation in infected children in comparison to adults [44]. H. pylori-infected children in comparison to infected adults possess lower levels of protein and gastric IL-17-specific mRNA as well as fewer gastric Th17 cells, indicating more reduction in the mucosal Th17 response in infected children. Moreover, the gastric mucosa of the infected children has lower level of IFN-γ mRNA, confirming the findings indicated of reduced Th1 response in children with H. pylori infection [46, 47]. Recent study indicated that the gastric concentrations of cytokines representative of the innate and Th1 response were higher in the H. pylori-positive than in the H. pylori-negative children and adults. The gastric concentrations of IL-1α and TNF-α were significantly higher, while those of IL-2, IL-12p70, and IFN-γ were lower in the infected children than in the infected adults. In the infected children, the gastric concentration of IL-1α, IL-2, IL-12p70, and IFN-γ increased, whereas in adults the gastric concentrations of IFN-γ and IL-12p70 decreased with aging. Increased gastric concentration of Th1-associated cytokines correlated with increased degree of gastritis, that is, the background lesion for the development of the H. pylori-associated severe diseases [48]. Treg cells are described as the key regulator of the immune system in the maintenance of immunologic tolerance. Recently, the close relationship between H. pylori infection and immunosuppressive Treg cells has been reported in animal and human models [49]. Treg cells suppress H. pylori-induced Th1-mediated immune response to contribute to the bacteria's persistent colonization in the gastric mucosa and therefore may play a major role in inducing chronic gastritis. The TGF-β1 and IL-10 gastric levels and the gastric number of Treg Foxp3+ cells in H. pylori-positive groups are higher in children than in adults (Table 1) [44, 46, 49–51]. The consensus is that Treg cells and Th17 commitments might be mutually controlled. TGF-β is required for the differentiation of both Treg cells and Th17 by inducing key transcription factors, Foxp3 and RORγt/RORc, respectively [52–54]. But, in absence of IL-6, an exclusive Treg differentiation might occur as Foxp3 is capable of associating with and inhibiting the RORγt. In contrast, in presence of IL-6, this inhibition might be abrogated allowing Th17 differentiation [55]. In a paradoxical pattern, the gastric concentration of IL-6 is usually less in infected adults than in infected children. In this regard, it might be hypothesized that these results are due to the higher gastric levels of IL-23 in adults, compared with children, which might prevent the amplification/stabilization of the shifted Th17 cells. The other possibility might be the higher level of TGF-β in the gastric milieu of infected children. It should be noted that, at low levels, TGF-β synergizes with IL-6 to promote IL-23 receptor expression in favor of Th17 cell commitment. However, the high level of TGF-β represses IL-23 receptor expression favoring Foxp3+ Treg cell differentiation [49, 56]. A recently published study revealed that IL-6 overproductions by IL-6 transgenic mice do not affect the function and development of natural Treg [57]. In this regard, the predominant Treg differentiation in children infected with H. pylori might account for more susceptibility of children to the H. pylori infection as well as to the bacterium persistence. Study in mouse stomach showed that H. pylori-induced dendritic cells skew the Th17/Treg balance toward a Treg-biased response that suppresses Th17 immunity through a cagA and vacA independent, TGF-β and IL-10-dependent mechanism [58, 59]. In support of these findings, recent study showed that H. pylori was capable of stimulating human gastric dendritic cells to produce IL-10, potentially supplementing Treg suppression of inflammation in the gastric mucosa [60, 61] (Figure 1). From these findings we might conclude that H. pylori-induced gastritis in adult is the consequence of both Th1 and Th17 immune-mediated inflammatory pathway involvement and that both pathways might be downregulated in the gastric mucosa of infected children.
Table 1.
Comparative immune response and clinical outcome in children and adults infected with H. pylori.
| Children | Adults | |
|---|---|---|
| Th1 | ↓ | ↑ |
| Th17 | ↓ | ↑ |
| Treg | ↑ | ↓ |
| TGF-β1 | ↑ | ↓ |
| IL-10 | ↑ | ↓ |
| Gastric inflammation | ↓ | ↑ |
| Neutrophil infiltration | ↓ | ↑ |
| Peptic ulcer | ↓ | ↑ |
| Virulence factors | Similar | Similar |
Figure 1.
Diagram of how the Treg cell response may influence inflammation, bacterial colonization density, and occurrence of H. pylori-mediated disease.
5. Conclusion
In conclusion, H. pylori infection in children is associated with high Treg response, as well as low Th1 and Th17 response [44, 46]. But, H. pylori-specific Th17/Th1 detection in chronically infected patients may reveal that the initial response is progressively lost [43, 62], indicating that, with progression of time, the mucosal immune system probably identifies H. pylori, as a pathogen. Hence, Th1, Th17, and Treg results may imply gastric mucosal response to H. pylori. More data from immune-mediated mechanism(s) of mucosal inflammation is required to provide strategies against this challenging pathogen, particularly for children who are living in countries with high rate of gastric cancer and/or H. pylori infection.
Acknowledgments
This study was financially supported by Research Deputy of Shahrekord University of Medical Sciences. The authors are grateful to the staffs of Cellular and Molecular Research Center, Shahrekord University of Medical Sciences, and the authorities of the Endoscopy Unit of Shahrekord Hajar Hospital for their valuable help.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Alireza Razavi and Nader Bagheri contributed equally in preparation of this paper.
References
- 1.Correa P., Piazuelo M. B. Natural history of Helicobacter pylori infection. Digestive and Liver Disease. 2008;40(7):490–496. doi: 10.1016/j.dld.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagheri N., Taghikhani A., Rahimian G., et al. Association between virulence factors of helicobacter pylori and gastric mucosal interleukin-18 mRNA expression in dyspeptic patients. Microbial Pathogenesis. 2013;65:7–13. doi: 10.1016/j.micpath.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Salimzadeh L., Bagheri N., Zamanzad B., et al. Frequency of virulence factors in Helicobacter pylori-infected patients with gastritis. Microbial Pathogenesis. 2015;80:67–72. doi: 10.1016/j.micpath.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T., Kato K., Ohara S., et al. Localization of antigen-presenting cells in Helicobacter pylori-infected gastric mucosa. Pathology International. 2002;52(4):265–271. doi: 10.1046/j.1440-1827.2002.01347.x. [DOI] [PubMed] [Google Scholar]
- 5.Bagheri N., Azadegan-Dehkordi F., Shirzad H., Rafieian-Kopaei M., Rahimian G., Razavi A. The biological functions of IL-17 in different clinical expressions of Helicobacter pylori-infection. Microbial Pathogenesis. 2015;81:33–38. doi: 10.1016/j.micpath.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nature Reviews Gastroenterology and Hepatology. 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres L. E., Melián K., Moreno A., et al. Prevalence of vacA, cagA and babA2 genes in Cuban Helicobacter pylori isolates. World Journal of Gastroenterology. 2009;15(2):204–210. doi: 10.3748/wjg.15.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci V., Giannouli M., Romano M., Zarrilli R. Helicobacter pylori gamma-glutamyl transpeptidase and its pathogenic role. World Journal of Gastroenterology. 2014;20(3):630–638. doi: 10.3748/wjg.v20.i3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song X., Chen H.-X., Wang X.-Y., et al. H. pylori-encoded CagA disrupts tight junctions and induces invasiveness of AGS gastric carcinoma cells via Cdx2-dependent targeting of Claudin-2. Cellular Immunology. 2013;286(1-2):22–30. doi: 10.1016/j.cellimm.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Covacci A., Censini S., Bugnoli M., et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amjad N., Osman H. A., Razak N. A., Kassian J., Din J., Abdullah N. B. Clinical significance of Helicobacter pylori cagA and iceA genotype status. World Journal of Gastroenterology. 2010;16(35):4443–4447. doi: 10.3748/wjg.v16.i35.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paziak-Domańska B., Chmiela M., Jarosińska A., Rudnicka W. Potential role of cagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cellular Immunology. 2000;202(2):136–139. doi: 10.1006/cimm.2000.1654. [DOI] [PubMed] [Google Scholar]
- 13.Backert S., Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori . Trends in Microbiology. 2010;18(11):479–486. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Raghavan S., Quiding-Järbrink M. Immune modulation by regulatory T cells in Helicobacter pylori-associated diseases. Endocrine, Metabolic and Immune Disorders—Drug Targets. 2012;12(1):71–85. doi: 10.2174/187153012799278974. [DOI] [PubMed] [Google Scholar]
- 15.Lamb A., Chen L.-F. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. Journal of Cellular Biochemistry. 2013;114(3):491–497. doi: 10.1002/jcb.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backert S., Tegtmeyer N., Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: the master key hypothesis. Helicobacter. 2010;15(3):163–176. doi: 10.1111/j.1523-5378.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 17.Amieva M. R., El-Omar E. M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134(1):306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Wen S., Moss S. F. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Letters. 2009;282(1):1–8. doi: 10.1016/j.canlet.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera V., Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clinical Microbiology and Infection. 2009;15(11):971–976. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 20.Park S. A., Ko A., Lee N. G. Stimulation of growth of the human gastric pathogen Helicobacter pylori by atmospheric level of oxygen under high carbon dioxide tension. BMC Microbiology. 2011;11, article 96 doi: 10.1186/1471-2180-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrar J. D., Asnagli H., Murphy K. M. T helper subset development: roles of instruction, selection, and transcription. Journal of Clinical Investigation. 2002;109(4):431–435. doi: 10.1172/jci200215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García Jacobo R. E., Serrano C. J., Enciso Moreno J. A., et al. Analysis of Th1, Th17 and regulatory T cells in tuberculosis case contacts. Cellular Immunology. 2014;289(1-2):167–173. doi: 10.1016/j.cellimm.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Murphy K. M., Reiner S. L. The lineage decisions of helper T cells. Nature Reviews Immunology. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 24.Harrington L. E., Hatton R. D., Mangan P. R., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 25.Park H., Li Z., Yang X. O., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan C., Ramaswamy M., Shi G., Vistica B. P., Siegel R. M., Gery I. Inflammation-inducing Th1 and Th17 cells differ in their expression patterns of apoptosis-related molecules. Cellular Immunology. 2011;271(2):210–213. doi: 10.1016/j.cellimm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal S., Ghilardi N., Xie M.-H., De Sauvage F. J., Gurney A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. Journal of Biological Chemistry. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 28.Iwakura Y., Ishigame H., Saijo S., Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Qi W., Huang X., Wang J. Correlation between Th17 cells and tumor microenvironment. Cellular Immunology. 2013;285(1-2):18–22. doi: 10.1016/j.cellimm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Nakae S., Nambu A., Sudo K., Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. Journal of Immunology. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 31.Komiyama Y., Nakae S., Matsuki T., et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. Journal of Immunology. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 32.Mangan P. R., Harrington L. E., O'Quinn D. B., et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang X., Yang Z., Zhang R., et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cellular Immunology. 2011;266(2):208–217. doi: 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunology. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 35.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annual Review of Immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 36.Heiber J. F., Geiger T. L. Context and location dependence of adaptive Foxp3+ regulatory T cell formation during immunopathological conditions. Cellular Immunology. 2012;279(1):60–65. doi: 10.1016/j.cellimm.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curotto de Lafaille M. A., Lafaille J. J. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Rahimian G., Sanei M. H., Shirzad H., et al. Virulence factors of Helicobacter pylori vacA increase markedly gastric mucosal TGF-beta1 mRNA expression in gastritis patients. Microbial Pathogenesis. 2014;67-68(1):1–7. doi: 10.1016/j.micpath.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Terrés A. M., Pajares J. M. An increased number of follicles containing activated CD69+ helper T cells and proliferating CD71+ B cells are found in H. pylori-infected gastric mucosa. The American Journal of Gastroenterology. 1998;93(4):579–583. doi: 10.1016/s0002-9270(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 40.Di Tommaso A., Xiang Z., Bugnoli M., et al. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infection and Immunity. 1995;63(3):1102–1106. doi: 10.1128/iai.63.3.1102-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagheri N., Rahimian G., Salimzadeh L., et al. Association of the virulence factors of Helicobacter pylori and gastric mucosal interleukin-17/23 mRNA expression in dyspeptic patients. EXCLI Journal. 2013;12:5–14. [PMC free article] [PubMed] [Google Scholar]
- 42.Caruso R., Pallone F., Monteleone G. Emerging role of IL-23/IL-17 axis in H pylori-associated pathology. World Journal of Gastroenterology. 2007;13(42):5547–5551. doi: 10.3748/wjg.v13.i42.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Elios M. M., Manghetti M., De Carli M., et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. Journal of Immunology. 1997;158(2):962–967. [PubMed] [Google Scholar]
- 44.Serrano C., Wright S. W., Bimczok D., et al. Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunology. 2013;6(5):950–959. doi: 10.1038/mi.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bontems P., Aksoy E., Burette A., et al. NF-kappaB activation and severity of gastritis in Helicobacter pylori-infected children and adults. Helicobacter. 2014;19(3):157–167. doi: 10.1111/hel.12118. [DOI] [PubMed] [Google Scholar]
- 46.Harris P. R., Wright S. W., Serrano C., et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134(2):491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Bhuiyan T. R., Islam M. M. T., Uddin T., et al. Th1 and Th17 responses to Helicobacter pylori in Bangladeshi infants, children and adults. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0093943.e93943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freire de Melo F., Rocha G. A., Rocha A. M. C., et al. Th1 immune response to H. pylori infection varies according to the age of the patients and influences the gastric inflammatory patterns. International Journal of Medical Microbiology. 2014;304(3-4):300–306. doi: 10.1016/j.ijmm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Cho K. Y. Y., Cho M. S., Seo J. W. FOXP3+ regulatory T cells in children with Helicobacter pylori infection. Pediatric and Developmental Pathology. 2012;15(2):118–126. doi: 10.2350/11-06-1046-oa.1. [DOI] [PubMed] [Google Scholar]
- 50.Freire de Melo F., Rocha A. M. C., Rocha G. A., et al. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes and Infection. 2012;14(4):341–347. doi: 10.1016/j.micinf.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez C., Serrano C., Einisman H., et al. Peptic ulcer disease in Helicobacter pylori-infected children: clinical findings and mucosal immune response. Journal of Pediatric Gastroenterology and Nutrition. 2014;59(6):773–778. doi: 10.1097/MPG.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 52.Chen W., Jin W., Hardegen N., et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of Experimental Medicine. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ivanov I. I., McKenzie B. S., Zhou L., et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 54.Cheng H.-H., Tseng G.-Y., Yang H.-B., Wang H.-J., Lin H.-J., Wang W.-C. Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma. World Journal of Gastroenterology. 2012;18(1):34–43. doi: 10.3748/wjg.v18.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimura A., Naka T., Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou L., Lopes J. E., Chong M. M. W., et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujimoto M., Nakano M., Terabe F., et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. Journal of Immunology. 2011;186(1):32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 58.Zhang G., Zeng F., Yan K. Topological sequence entropy of operators on function spaces. Journal of Applied Functional Analysis. 2010;5(3):325–329. [Google Scholar]
- 59.Kao J. Y., Zhang M., Miller M. J., et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138(3):1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bimczok D., Clements R. H., Waites K. B., et al. Human primary gastric dendritic cells induce a Th1 response to H. pylori . Mucosal Immunology. 2010;3(3):260–269. doi: 10.1038/mi.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bimczok D., Grams J. M., Stahl R. D., Waites K. B., Smythies L. E., Smith P. D. Stromal regulation of human gastric dendritic cells restricts the Th1 response to Helicobacter pylori . Gastroenterology. 2011;141(3):929–938. doi: 10.1053/j.gastro.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luzza F., Parrello T., Monteleone G., et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in helicobacter pylori-infected human gastric mucosa. Journal of Immunology. 2000;165(9):5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]