Abstract
Six hybrids were subjected to chromatographic analyses by HPLC for the determination of phytochemicals such as capsaicinoid, polyphenol, and vitamin C. The dynamics of ripening of 4 of the hybrids were also characterised. Seven capsaicinoids could be separated and determined; the major compounds were nordihydrocapsaicin, capsaicin, and dihydrocapsaicin, while homocapsaicin and homodihydrocapsaicin derivatives were detected as minor constituents. Capsaicin content ranged between 95.5 ± 4.15 and 1610.2 ± 91.46 μg/g FW, and the highest value was found in Bandai (C. frutescens) at the green ripening stage. The major capsaicinoids had a decreasing tendency in Bandai and Chili 3735 hybrids, while no change was observed in Beibeihong and Lolo during ripening. Nine polyphenol compounds were detected including 8 flavonoids and a nonflavonoid compound in the pods of all hybrids. The major components were naringenin-diglucoside, catechin, and vanillic acid-derivative and luteolin-glucoside. Naringenin-diglucoside ranged from 93.5 ± 4.26 to 368.8 ± 30.77 μg/g FW. Except vanillic acid-derivative, dominant polyphenols increased or remained unchanged during ripening. As for vitamin C, its content tended to increase with the advance in ripening in all hybrids included in this study. The highest value of 3689.4 ± 39.50 μg/g FW was recorded in Fire Flame hybrid.
1. Introduction
The components evolving pungency in chili peppers have been established as a mixture of acid amides of vanillylamine and C8 to C13 fatty acids, also known as capsaicinoids [1]. Capsaicinoids are secondary metabolites and are synthesised by glands at the join of the placenta and the pod wall of pungent peppers [2]. The effect of capsaicinoids on human health has been widely investigated. For instance, it is beneficial in low concentration against gastric injuries [3], stimulates cation channels (Na+, K+, and Ca2+) in sensory receptor membrane [4], evokes pain, and activates autonomic reflexes [5]. Environmental factors and the circumstance of cultivation influence capsaicinoid content of the pods [6, 7], while probably a higher impact on pungency by the genotype is present [8, 9]. Besides, the amount and proportion of capsaicinoids are changing during the ripening process of the pods [10–13]. Flavonoids represent a significant subgroup of polyphenols [14] and naturally occur in high concentration in wild mint [15] and grape [16] while generally pungent peppers have moderate level of polyphenol content. The health protective attributions of them are mainly associated with preventing cancer through inhibiting certain enzymes and suppressing angiogenesis [17]. The polyphenol content in pungent peppers is found to be influenced by genotype and the ripening process [18–20]. Ascorbic acid, the main component of vitamin C, is very abundant in fresh Capsicum species and has been found to be beneficial in maintaining collagen synthesis and healthy immune-system and also has antitumor properties [21–23]. The content of ascorbic acid is highly varying among cultivars and ripening stages [24, 25]; in addition, the utilised agricultural techniques play significant role in the final amount of ascorbic acid in the pods [26].
Numerous cultivars of pungent pepper are nowadays available; however, many of them have not been analysed for their quality and nutritional components. The objective of the present work is to investigate capsaicinoid, polyphenol, and vitamin C content in six hybrids of chili pepper (Bandai, Beibeihong, Lolo, Chili 3735, Fire Flame, and Star Flame) using recently developed liquid chromatographic method in the determinations. In addition, characterisation of ripening stages of four hybrids was aimed.
2. Material and Methods
2.1. Plant Material
The plants were cultivated with convention horticultural practices in the experimental field of Szent István University, Gödöllő, Hungary. Bandai F1 (Bandai) and Beibeihong 695 F1 (Beibeihong) which belong to Capsicum frutescens and Lolo 736 F1 (Lolo) and Chili 3735 F1 (C3735) which belong to Capsicum annuum were all purchased from East-West Seeds Company, from Thailand, while Star Flame and Fire Flame (both Capsicum annuum) were purchased from Seminis, Hungary. The pods of Bandai, Beibeihong, Lolo, C3735, and Fire Flame are red when fully ripe, while Star Flame has vivid yellow pods. Peppers with intermediate pungency level were selected for the investigation because those have multiple utilization methods. Those peppers involved in the recent study have limited data available for breeders and growers; thus, it makes them important for research work. Star Flame and Fire Flame are commercially available in certain European countries but not yet in Hungary.
2.2. Capsaicinoid Determination
The determination of capsaicinoid content was made following the method of Daood et al. [27]. Three grams of well-blended pepper sample were crushed in a crucible mortar with quartz sand. To the macerate 50 mL of methanol (analytical grade) was added and the mixture was then transferred to a 100 mL Erlenmeyer flask. The mixture was subjected to 4 min long ultrasonication (Raypa, Turkey) and then filtered through filter paper (Munktell, Germany). The filtrate was more purified by passing through a 0.45 mm PTFE syringe filter before injection on the HPLC column.
After suitable dilution, the extract was injected to Nucleodur C18, Cross-Linked (ISIS, from Macherey-Nagel, Düren, Germany). The separation was performed with isocratic elution of 50 : 50 water-acetonitrile and a flow rate of 0.8 mL/min. Fluorometric detection of capsaicinoid was carried out at EX: 280 nm and EM: 320 nm.
Peaks referring to different capsaicinoids were identified by comparing retention times and mass data (Daood et al. [27]) of standard material (purified from pungent red pepper, with 99% purity, by Plantakem Ltd., Sándorfalva, Hungary) with those appearing on chromatogram of samples. Capsaicinoid compounds are referred as follows: nordihydrocapsaicin (NDC), capsaicin (CAP), dihydrocapsaicin (DC), homocapsaicin 1-2 (HCAP1-2), and homodihydrocapsaicin 1-2 (HDC1-2). Scoville heat unit (SHU) was calculated by the following algorithm:
| (1) |
All variables are expressed in μg/g dry weight basis [28].
2.3. Polyphenol Determination
Five grams of well-blended pepper sample were replaced into an Erlenmeyer flask and then 10 mL distilled water was added to the sample and subjected to ultrasonication force using ultrasonic bath for 30 sec. Then, 15 mL of 2% acetic acid in methanol was added to the mixture which was shaken by a mechanical shaker for 15 min. The mixture then was kept overnight at 4°C. Next day after filtrating the mixtures, a further cleanup of the filtrates was made by passing through the mixture a 0.45 μm PTFE HPLC syringe filter. That followed by injection on the HPLC column for the analysis of phenols. Nucleosil C18, 100, Protect-1 (Macherey-Nagel, Düren, Germany), 3 μm, 150 × 4.6 column was used. The gradient elution was done using 1% formic acid (A) in water, acetonitrile (B), and flow rate of 0.6 mL/min. Gradient elution started with 98% A and 2% B and changed in 10 min to 87% A and 13% B and in 5 min to 75% A and 25% B and then in 15 min to 60% A and 40% B; finally it turned in 7 min to 98% A and 2% B. The peaks that appeared on the chromatogram were identified by comparing their retention times and spectral characteristics with available standards such as catechin, quercetin-3-glucoside, kaempferol, luteolin-glucoside, and naringenin-glucoside (Sigma-Aldrich Ltd., Hungary). Quantitation of phenol components having maxima absorption at 280 nm were quantified as catechin equivalent and flavonoids were quantified as kaempferol-equivalent at 350 nm [29, 30]. The standard material was singly injected as external standard as well as being cochromatographed (spiking) with the samples.
2.4. Ascorbic Acid Determination
Five grams of well-homogenised sample were disrupted in a crucible mortar with quartz sand. To the macerate 50 mL of metaphosphoric acid (analytical grade) was gradually added and the mixture was then transferred to a 100 mL Erlenmeyer flask closed with stopper and then filtered. The filtrate was purified in addition by passing through a 0.45 mm PTFE syringe filter before injection on HPLC column. The analytical determination of ascorbic acid was performed on C18 Nautilus, 100-5, 150 × 4.6 mm (Macherey-Nagel, Düren, Germany) column with gradient elution of 0.01 M KH2PO4 (A) and acetonitrile (B). The gradient elution started with 1% B in A and changed to 30% B in A in 15 min; then; it turned to 1% A in B in 5 min. The flow rate was 0.7 mL/min. The highest absorption maxima of ascorbic acid under these conditions were detected at 265 nm. For quantitative determination of ascorbic acid standard materials (Sigma-Aldrich, Budapest, Hungary) were used. Stock solutions and then working solutions were prepared for each compound to make the calibration between concentration and peak area.
2.5. HPLC Apparatus
A Hitachi Chromaster HPLC instrument, which consists of a Model 5110 Pump, a Model 5210 Auto Sampler, a Model 5430 Diode Array detector, and a Model 5440 Fluorescence detector, was used for the determination of all compounds.
2.6. Validation of Applied Methods
Since the methods used in the different chromatographic determinations are derived from the literature (validated protocols) we dealt with only measuring the limit of detection (LOD) and quantification (LOQ) and linearity curves of different compounds under the conditions of our laboratories. The LOD and LOQ were calculated from standard solutions and samples as the concentrations of analytes at peak/noise of 3 times and 10 times, respectively. Linearity curves were made plotting concentration in μg/mL against peak areas.
2.7. Dry Matter Determination
Three grams of fresh pepper samples were dried at 65°C until constant weight. The dry matter content was measured as a proportion of fresh and dried fruit weight.
2.8. Statistical Analyses
For each independent variable a one-way linear model (LM) was fitted, where “ripening stage” was set as explanatory (factor) variable. Prior to model fitting assumptions were checked by plot diagnosis. In the analysis of the major compounds (SHU, CAP, naringenin-diglucoside, ascorbic acid, and dry matter) among the six hybrids another LM was made, where “hybrid” was set as explanatory (factor) variable. Post hoc comparison was made by Tukey HSD test. All statistical analyses were performed in IBM SPSS 22 software (IBM Co., USA) and Microsoft Excel (Microsoft Co., USA). α was set at 0.05 in the entire study.
3. Results and Discussion
To adapt the applied chromatographic protocols under the conditions of our laboratories, certain parameters such as LOD, LOQ, and linearity curve were studied. The values depicted in Table 1 show that the used methods are accurate enough to carry on precise and sensitive determination of polyphenols, capsaicinoids, and ascorbic acid. This statement is based on the low levels of LOQ, LOD found for all tested compounds. The concentration of such compounds in our samples is much higher than the levels of LOQ and LOD. Moreover, values obtained for regression coefficient indicated that the methods can be applied at wide range of concentrations for different compounds in chili samples.
Table 1.
Some validation parameters for the HPLC determinations of the major polyphenols, ascorbic acid, and capsaicinoids.
| LOD μg/mL | LOQ μg/mL | Linearity range μg/mL | Linearity curve | R 2 | |
|---|---|---|---|---|---|
| Catechin | 2.625 | 8.75 | 0–50 | y = 0.331x − 2.5895 | 0.899 |
| Naringenin-diglucoside | 0.0318 | 0.106 | 0–50 | y = 0.3906x − 3.0556 | 0.899 |
| Quercetin-3-glucoside | 1.083 | 3.61 | 0–50 | y = 0.2188x − 0.781 | 0.983 |
| Luteolin-glucoside | 1.018 | 3.39 | 0–50 | y = 0.1912x − 0.381 | 0.979 |
| Kaempferol-derivative | 0.0208 | 0.069 | 0–50 | y = 0.4402x − 3.444 | 0.899 |
| Ascorbic acid | 2.500 | 0.750 | 30–120 | y = 0.2736x − 2.4305 | 0.997 |
| Nordihydrocapsaicin∗ | 0.003∗ | 0.008∗ | 0–0.07–1.1∗ | y = 2000 + 07x + 3000 + 06∗ | 0.997∗ |
| Capsaicin∗ | 0.004∗ | 0.01∗ | 0.1–5∗ | y = 2000 + 07x + 3000 + 06∗ | 0.998∗ |
| Dihydrocapsaicin∗ | 0.002∗ | 0.007∗ | 0.3–6∗ | y = 2000 + 07x + 3000 + 06∗ | 0.998∗ |
∗From previously published research work on HPLC determination of capsaicinoids by Daood et al. [27].
3.1. Pungency
The major components evolving pungency in our hybrids are NDC, CAP, and DC. Besides, we could identify the homologues of CAP and DC which are HCAP1, HCAP2 and HDC1, HDC2, respectively (Figure 1). All of them are branched-chain alkyl vanillylamides. Kozukue et al. [31] detected the 7 compounds, in addition to nonivamide which is a straight-chain nonoyl vanillylamide analog of CAP [1]. In Beibeihong advance in ripening did not affect the major capsaicinoids (CAP, NDC, and DC shown in Table 2), while it influenced HCAP1 and HDC1 (both p ≤ 0.032) including a slight decrease from green to colour-break stage and then a low increase at the final stage. In Bandai, unlike Beibeihong the ripening affected the major and minor capsaicinoids as well (all p ≤ 0.027). The changing of CAP included a notable decrease between the initial stage and the colour-break stage. On DC, NDC, and HDC2 a gradual decrease was measured. A straight increasing of HDC1 was observed, while on HPC1 the same tendency like that in HPC1 of Beibeihong was observed.
Figure 1.
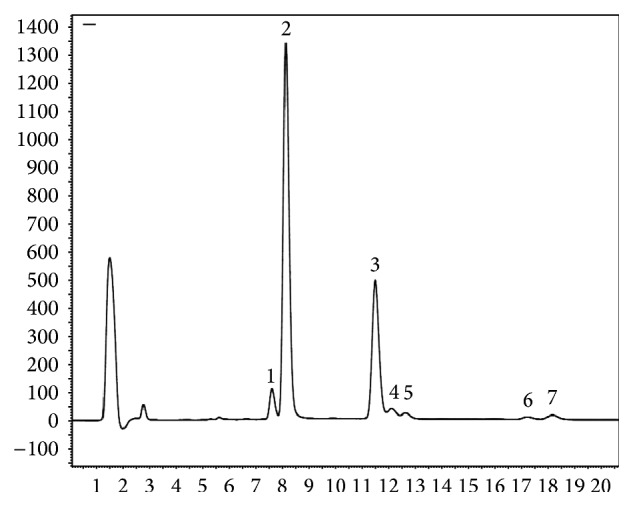
HPLC profile of capsaicinoid components separated from red stage of Bandai hybrid using cross-linked C18 column with acetonitrile-water elution and fluorescence detection. 1: NDC, 2: CAP, 3: DC, 4: HCAP1, 5: HCAP2, 6: HDC1, and 7: HDC2. For more information see text.
Table 2.
Change in content of capsaicinoid compounds in chili hybrids as a function of ripening. The values represent means in μg/g fresh base weight ± standard deviation (n = 3).
| Hybrid | Ripening stage | NDC (μg/g) | CAP (μg/g) | DC (μg/g) | HCAP1 (μg/g) | HCAP2 (μg/g) | HDC1 (μg/g) | HDC2 (μg/g) |
|---|---|---|---|---|---|---|---|---|
| Beibeihong | Green | 51.8 ± 3.90a | 294.5 ± 19.72a | 326.5 ± 51.20a | 3.5 ± 2.01ab | 20.0 ± 2.37a | 10.6 ± 3.01ab | 26.3 ± 2.60a |
| Colour-breaker | 60.6 ± 15.01a | 254.6 ± 31.90a | 263.4 ± 25.92a | 1.8 ± 0.70a | 25.3 ± 5.13a | 9.0 ± 0.82a | 29.5 ± 6.42a | |
| Orange | 61.7 ± 6.65a | 261.9 ± 26.12a | 269.3 ± 14.06a | 3.6 ± 0.97ab | 28.5 ± 4.64a | 10.7 ± 0.50ab | 26.7 ± 2.09a | |
| Red | 63.2 ± 15.12a | 311.8 ± 63.25a | 272.7 ± 74.99a | 5.7 ± 0.51b | 23.7 ± 3.62a | 13.9 ± 0.5b | 23.9 ± 3.79a | |
| F-value | 0.61 | 1.44 | 1.12 | 5.41 | 2.26 | 4.89 | 0.94 | |
| p value | 0.626 | 0.302 | 0.394 | 0.025 | 0.158 | 0.032 | 0.464 | |
|
| ||||||||
| Bandai | Green | 102.9 ± 14.17ab | 1610.2 ± 91.46b | 780 ± 36.03b | 13.1 ± 5.61ab | 8.9 ± 0.99a | 12.8 ± 1.22a | 30.2 ± 2.29ab |
| Colour-breaker | 102.2 ± 1.21ab | 1182.2 ± 82.56a | 725.2 ± 32.03ab | 6.3 ± 1.83a | 18.5 ± 3.69b | 12.7 ± 0.57a | 31 ± 3.41ab | |
| Orange | 115.6 ± 5.26b | 1104.9 ± 77.27a | 635.2 ± 32.36a | 11.5 ± 0.51ab | 27.2 ± 3.33c | 15 ± 0.46ab | 37.9 ± 3.41b | |
| Red | 81.5 ± 6.91a | 1176.1 ± 112.1a | 600.4 ± 87.11a | 20.1 ± 6.24b | 14.3 ± 2.39ab | 16.3 ± 2.06b | 27.3 ± 3.34a | |
| F-value | 8.59 | 18.93 | 7.40 | 5.27 | 22.70 | 5.95 | 6.10 | |
| p value | 0.007 | 0.001 | 0.011 | 0.027 | <0.001 | 0.020 | 0.018 | |
|
| ||||||||
| Lolo | Green | 18.7 ± 2.42a | 222.5 ± 69.33a | 139.2 ± 50.97a | 0.5 ± 0.23b | 0.4 ± 0.08a | 1.8 ± 0.15a | 9.2 ± 1.19a |
| Colour-breaker | 26.2 ± 3.94a | 95.5 ± 4.15a | 96.7 ± 9.29a | 0.4 ± 0.04b | 2.4 ± 0.77b | 1.9 ± 0.08a | 12.6 ± 1.31a | |
| Red | 22.2 ± 5.14a | 197 ± 92.13a | 119.8 ± 53.03a | 0 ± 0a | 3.2 ± 0.15b | 1.9 ± 0.27a | 12.5 ± 3.27a | |
| F-value | 2.69 | 3.05 | 0.74 | 12.12 | 29.94 | 0.86 | 2.54 | |
| p value | 0.146 | 0.122 | 0.516 | 0.008 | 0.001 | 0.467 | 0.159 | |
|
| ||||||||
| C3735 | Green | 31.3 ± 1.46b | 259.6 ± 39.15b | 183.4 ± 23.27b | UDL | 7 ± 0.79a | 1.6 ± 0.21b | 8.2 ± 0.58ab |
| Colour-breaker | 35.9 ± 1.64b | 168.9 ± 33.86ab | 148.2 ± 24.21b | UDL | 12.6 ± 4.16a | 1.9 ± 0.08b | 9.8 ± 1.01b | |
| Red | 18.2 ± 6.21a | 126.3 ± 35.95a | 88.9 ± 6.38a | UDL | 12.6 ± 3.7a | 1.3 ± 0.07a | 7.2 ± 1.31a | |
| F-value | 17.39 | 10.50 | 17.58 | — | 3.00 | 15.03 | 5.00 | |
| p value | 0.003 | 0.011 | 0.003 | — | 0.125 | 0.005 | 0.053 | |
|
| ||||||||
| Fire Flame | Red | 15.5 ± 3.28 | 234.3 ± 45.23 | 109.7 ± 19.9 | 1.2 ± 0.13 | 1 ± 0.23 | 0.9 ± 0.13 | 5.9 ± 1.24 |
|
| ||||||||
| Star Flame | Yellow | 21.9 ± 5.36 | 440.8 ± 17.22 | 135.9 ± 20.28 | 2.5 ± 0.08 | 0.2 ± 0.08 | 1.4 ± 0.25 | 6.4 ± 1.10 |
The same letter indicates no significant difference in capsaicinoid content between ripening stages in the given hybrid according to Tukey HSD post hoc test; UDL: under detection limit.
Focusing on the major compounds of capsaicinoids, Bandai hybrid could be characterised with pungency loss, while in Beibeihong those compounds did not change during ripening. In the study by Gnayfeed et al. [12] CAP reached the highest value in F-03 cultivar (C. annuum) at the initial green stage, similarly found in Bandai, but its content in F-03 did not change significantly with ripening. The obtained results suggest even in the same species (C. frutescens) that the hybrids have a different characteristic in ripening regarding capsaicinoid contents. It is in accordance with findings of Merken and Beecher [30] who also measured the maximal capsaicinoid content in 3 different C. frutescens peppers in 3 variant times after flower budding.
In Lolo the ripening slightly affected but not significantly CAP, while it increased HCAP2 (p = 0.001). After the colour-break stage the amount of HCAP1 decreased (p = 0.008) to undetectable level. In C3735 ripening decreased NDC, CAP, DC, HDC1 (all p ≤ 0.011), and nonmarginally HDC2, while HCAP1 was absent or under detection limit at all ripening stages. Therefore, most of the compounds showed a decreasing tendency during ripening of C3735, so a remarkable pungency loss was observed. On the contrary, those compounds remained unchanged in Lolo.
Iwai found the peak 40 days after flowering and then a gradual decrease of capsaicinoid content in a C. annuum pepper. Because of the different scale used by Iwai et al. [32], it is difficult to compare to our data, but probably the 40 days after flowering is roughly equal to the green stage we used. Gnayfeed et al. [12] observed in C. annuum cultivars that capsaicinoids reached maximum level at the colour-break stage and then started declining in Hungarian spice pepper (C. annuum), which is a characteristic of pungency change that we did not observe. The change in capsaicin content during ripening of pepper may relate to activity of some enzymes that interfere in the ripening dynamics. The amount of capsaicinoids has been investigated in relation with several enzymes [10, 33, 34]. Contreras-Padilla and Yahia [10] showed that peroxidase activity started increasing, when the amount of capsaicinoid started to decrease in Habanero and de Arbol, while in Piquin it began to increase before the decrease of capsaicinoid. They concluded that peroxidase enzyme is involved in capsaicinoid degradation and that attribution is a genotypic characteristic. Iwai et al. [32] found higher phenylalanine ammonia-lyase activity in green stage than in red stage. In addition, Bernal et al. [33] observed that the operation of capsaicinoid synthetase enzyme is more influenced by the availability of precursors and the conditions of forming than its substrate specificity. The capsaicinoid composition and content are the result of the above referred enzymes.
A study concerning the maturation of Habanero (C. chinense) proved that green pod contains four times less capsaicin than ripe red ones [13], while we found less difference and even more capsaicin in green stage (e.g., Bandai); however, none of our investigated hybrids belong to C. chinense. They also reported that DC content is seven times less in green pods as compared to red ones, while we found only a slight decrease of DC between the green and red stages.
3.2. Polyphenols
Since there is no available standard for myricetin and vanillic acid in our laboratory, they were tentatively identified based on comparison of their spectral characteristics and retention behaviour on the HPLC column with those found in the literature.
Due to the high content of vanillic acid-derivative, catechin, and naringenin-diglucoside, those compounds were found to be the dominant polyphenols, which have maxima absorption at 280 nm (Figure 2). The minor compounds were luteolin-rutinoside, quercetin-glucoside, quercetin-glycosides, myricetin, and kaempferol-derivative; all were detected with maxima absorption at 350 nm and also luteolin-glucoside occurs in higher concentration and is detected at 350 nm (Figure 3).
Figure 2.
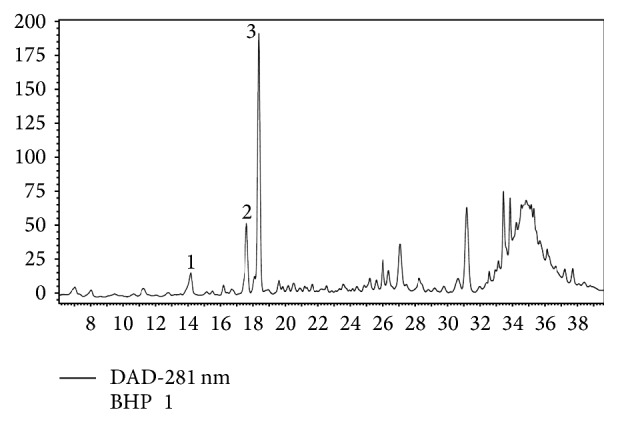
HPLC profile of polyphenols detected separated on Protect-1 C18 column and detected at 280 nm. 1: vanillic acid-derivative, 2: catechin, and 3: naringenin-diglucoside.
Figure 3.
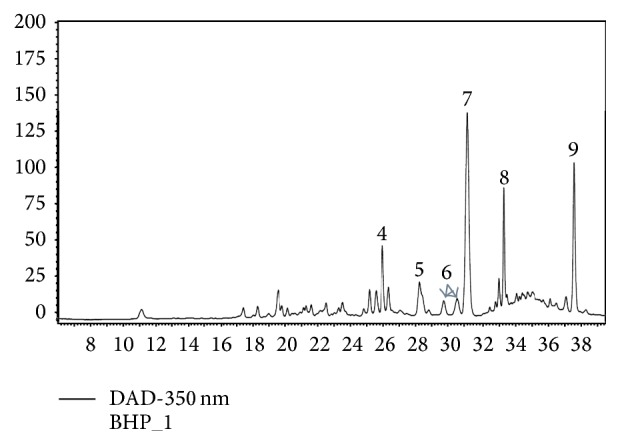
HPLC profile of polyphenols detected separated on Protect-1 C18 column and detected at 350 nm. 4: luteolin-rutinoside, 5: quercetin-glucoside, 6: quercetin-glycosides (the sum of these compounds is used in Table 3), 7: luteolin-glucoside, 8: myricetin, and 9: kaempferol-derivative.
In Beibeihong, ripening increased catechin, luteolin-rutinoside, quercetin compounds, myricetin, and kaempferol-derivative (all p ≤ 0.02 shown in Table 3), while it decreased vanillic acid content (p < 0.001). In Bandai ripening increased all compounds (all p ≤ 0.002) except vanillic acid and luteolin-rutinoside which statistically remained unchanged during ripening stages. In quercetin-glucoside, myricetin, and kaempferol-derivative the highest values were measured in the middle of the ripening. Most of the studies regarding polyphenol constitution of pungent pepper focus on the green (initial) and red (final) ripe stages but omit the intermediate or colour-break stage. Howard et al. [20] found that quercetin decreased, while luteolin did not change with ripening of Tabasco (C. frutescens). On the contrary, we found an increase of quercetin-related compounds in both C. frutescens hybrids and also an increase of luteolin-rutinoside in Beibeihong and of luteolin-glucoside in Bandai.
Table 3.
Change in content of polyphenol compounds in different chili hybrids as a function of ripening. The values represent means in μg/g fresh weight base ± standard deviation (n = 3).
| Hybrid | Ripening stage | Vanillic acid-derivative (μg/g) |
Catechin (μg/g) | Naringenin-diglucoside (μg/g) |
Luteolin-rutinoside (μg/g) |
Quercetin-glucoside (μg/g) |
Quercetin-glycosides (μg/g) |
Luteolin-glucoside (μg/g) |
Myricetin (μg/g) | Kaempferol-derivative (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Beibeihong | Green | 109.5 ± 9.84b | 50.4 ± 1.86a | 349.5 ± 13.09a | 11.3 ± 0.53a | 12.5 ± 2.07a | 3.7 ± 0.14a | 62.9 ± 2.78a | 10.3 ± 0.74a | 22.6 ± 1.13a |
| Colour-breaker | 145.7 ± 9.71c | 135.3 ± 3.97b | 477.4 ± 52.69b | 8.5 ± 0.73a | 13.4 ± 1.19a | 4.7 ± 0.12ab | 79 ± 2.78a | 23.5 ± 1.13b | 67.3 ± 5.26c | |
| Orange | 114.9 ± 9.66b | 153.5 ± 5.56c | 431.3 ± 39.72ab | 9.7 ± 0.67a | 9.1 ± 0.07a | 4.9 ± 0.62b | 84.6 ± 13.18a | 24.9 ± 0.46b | 69.7 ± 3.86c | |
| Red | 79.2 ± 11.08a | 132.7 ± 3.85b | 368.8 ± 30.77a | 17.0 ± 3.37b | 21.1 ± 2.69b | 5.5 ± 0.68b | 90.1 ± 16.97a | 31.9 ± 6.77b | 51.1 ± 5.22b | |
| F-value | 21.88 | 390.61 | 7.54 | 13.47 | 23.64 | 7.62 | 3.45 | 20.45 | 79.18 | |
| p value | <0.001 | <0.001 | 0.01 | 0.02 | <0.001 | 0.01 | 0.071 | <0.001 | <0.001 | |
|
| ||||||||||
| Bandai | Green | 96.3 ± 0.25a | 96.9 ± 7.67a | 130.3 ± 4.82a | 13.2 ± 1.06a | 4.6 ± 0.66a | 5.6 ± 0.24a | 84.6 ± 2.37a | 25.7 ± 1.58a | 62.8 ± 3.33a |
| Colour-breaker | 89.7 ± 7.98a | 134.6 ± 17.02b | 202 ± 17.77b | 15.1 ± 3.96a | 14.2 ± 0.61ab | 7.8 ± 0.91ab | 91.4 ± 13.75a | 117.1 ± 8.21b | 289.5 ± 45.59bc | |
| Orange | 92 ± 14.56a | 166.4 ± 17.16b | 254.2 ± 38.95b | 14.8 ± 3.68a | 17.4 ± 2.99c | 10.2 ± 1.38bc | 107.3 ± 23.49a | 110.4 ± 19.41b | 307.8 ± 53.01c | |
| Red | 101.6 ± 5.67a | 175.2 ± 12.32c | 276.5 ± 16.65c | 21.6 ± 10.69a | 12.8 ± 0.97b | 11.9 ± 1.15c | 157.8 ± 15.26b | 52.9 ± 12.94a | 200.7 ± 20.83b | |
| F-value | 1.05 | 19.03 | 23.73 | 1.15 | 33.24 | 22.39 | 13.41 | 38.70 | 28.15 | |
| p value | 0.42 | <0.001 | <0.001 | 0.385 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | |
|
| ||||||||||
| Lolo | Green | 72.2 ± 0.85c | 45 ± 2.71a | 116.8 ± 7.28a | 2.1 ± 0.49a | 5.7 ± 0.83b | 5 ± 0.78b | 25.7 ± 5.05a | 2 ± 0.51a | UDL |
| Colour-breaker | 50.3 ± 2.85a | 51.4 ± 3.09a | 160.8 ± 14.93b | 3.2 ± 0.36a | 2.8 ± 0.08a | 2.9 ± 1.03a | 53.4 ± 4.52b | 4.8 ± 0.62b | UDL | |
| Red | 64.2 ± 4.15b | 171.5 ± 6.14b | 117.8 ± 7.18a | 7.3 ± 1.22b | 6.1 ± 1.28b | 4.5 ± 0.28ab | 75.9 ± 2.48c | 8.9 ± 0.57c | 9.1 ± 3.77 | |
| F-value | 42.59 | 836.79 | 17.35 | 37.55 | 12.72 | 6.49 | 108.95 | 113.35 | — | |
| p value | <0.001 | <0.001 | 0.003 | <0.001 | 0.007 | 0.032 | <0.001 | <0.001 | — | |
|
| ||||||||||
| C3753 | Green | 73.1 ± 5.46b | 22.6 ± 1.28a | 123.6 ± 6.23a | 2 ± 0.7a | 1.7 ± 0.39a | 16.8 ± 2.38a | 17 ± 2.57a | 3.8 ± 0.96a | UDL |
| Colour-breaker | 51 ± 8.55a | 64.2 ± 10.49b | 148.3 ± 40.01ab | 1.5 ± 0.37a | 1.3 ± 0.24a | 17.3 ± 3.44a | 13.5 ± 2.66a | 10 ± 1.09b | 21 ± 1.71a | |
| Red | 56 ± 6.67a | 124.5 ± 6.18c | 217.2 ± 8.36b | UDL | 1.1 ± 0.23a | 19.8 ± 2.55a | 17.5 ± 2.78a | 11.3 ± 0.94b | 16.4 ± 2.85a | |
| F-value | 13.06 | 157.47 | 8.32 | 0.44 | 2.84 | 0.95 | 1.99 | 48.91 | 6.007 | |
| p value | 0.007 | <0.001 | 0.019 | 0.661 | 0.135 | 0.44 | 0.12 | <0.001 | 0.070 | |
|
| ||||||||||
| Fire Flame | Red | 27.8 ± 2.07 | 26.6 ± 1.40 | 141.6 ± 4.17 | 2.8 ± 0.27 | 2.7 ± 0.16 | 4.2 ± 0.22 | 44.4 ± 2.76 | 18.2 ± 0.43 | UDL |
|
| ||||||||||
| Star Flame | Yellow | 24.2 ± 1.37 | 17.6 ± 0.24 | 93.5 ± 4.33 | 9.5 ± 0.47 | 2.0 ± 0.14 | 4.1 ± 0.62 | 60.6 ± 1.34 | 18.4 ± 1.29 | UDL |
The same letter indicates no significant difference in polyphenol content between ripening stages in the given hybrid according to Tukey HSD post hoc test; UDL: under detection limit.
In Lolo the ripening significantly decreased vanillic acid (p < 0.001) but increased catechin, luteolin-rutinoside, luteolin-glucoside, and myricetin (all p < 0.001). In C3735 vanillic acid decreased (p = 0.007) while catechin, naringenin-diglucoside, and myricetin increased (all p ≤ 0.019). Howard et al. stated that quercetin had either increasing or decreasing tendency depending on cultivar; also no change was observed during maturity stages of certain cultivars on C. annuum peppers. We could only confirm the last statement that none of the quercetin-related compounds changed when the pods changed from green to red in C. annuum peppers studied.
According to Materska and Perucka [19] the most abundant flavonoid compounds in the green stage were quertecin-3-O-L-rhamnoside and luteolin-related compounds, and with ripening those phytochemicals decreased. In the present work particularly in red stage contained higher amounts of luteolin-related in Lolo, while in quercetin-glycoside content no change was detected in both C. annuum hybrids.
The disappearances of flavonoids are parallel to capsaicinoids accumulation [35] because the synthesis of flavonoids may converge with the capsaicinoid pathways [36]. The only nonflavonoid phenolic acid detected in our peppers is vanillic acid, and it is the only polyphenol compound which decreased or stayed unchanged during ripening, while the flavonoids mostly increased with advance of ripening. At the same time the major capsaicinoids generally decreased or did not change even with ripening. Kawada and Iwai [37] found a direct relation between DC and vanillic acid; they fed rats with DC and then detected vanillic acid in a notable amount in the urine of the rats. This experiment may also support our findings that vanillic acid is certainly related to capsaicinoids and has similar dynamics during ripening in pungent pepper.
According to Tsao [14], flavonols (kaempferol, quercetin, and myricetin) consist of highly conjugated bindings and a 3-hydroxy group, whose attributions are considered very important in evolving high antioxidant activity. In our hybrids the highest levels of the latter flavonoids were obtained at the orange or red stage that makes the pepper of higher nutritive value.
3.3. Ascorbic Acid
By the applied HPLC method only L-ascorbic acid was found in the extract of all hybrids (Figure 4). It was found that ascorbic acid increased during ripening in all hybrids (p ≤ 0.001 shown in Table 4). In Beibeihong and Bandai after green stage a more notable increase was observed than after the colour-break stage where the ascorbic acid gradually increased, while in Bandai at the red stage the average of ascorbic acid was less than in orange stage. In Lolo the green and colour-break stage did not differ significantly, while the red stage contained the most. In C3735 a straight increase was observed. The increasing tendency in the investigated hybrids is in accordance with that found in previous works [12, 20, 24, 25] which concluded that the more ripened the pods were, the more ascorbic acid could be measured from them. With ripening the pepper pods store more reducing sugars [36], which are the precursors of L-ascorbic acid [38], and that explains the increasing vitamin C content with ripening in all hybrids included in our study. On the contrary, Shaha et al. [18] showed a different dynamics of the ascorbic acid accumulation, because they found the highest level in yellow (intermediate) stage and the declining level in the red mature stage. That agrees with our finding in Bandai, where the highest average values (1005.2 ± 100.73 μg/g) were observed in the orange or colour-break stage (937.9 ± 78.04 μg/g), although these are not significantly higher than that determined in red stage (787.4 ± 131.21 μg/g). Probably it is also due to the high standard deviation present in the red stage.
Figure 4.
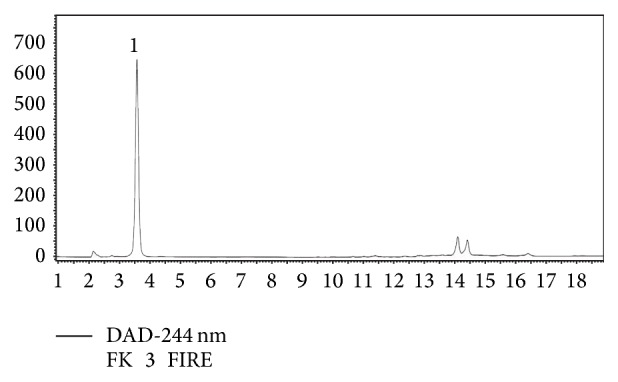
HPLC profile of vitamin C determination. The separation was performed on C18 Nautilus column with PDA detection at 244 nm. 1: L-ascorbic acid.
Table 4.
Change in content of ascorbic acid in different chili hybrids as a function of ripening. The values represent means in μg/g fresh weight base ± standard deviation (n = 3).
| Hybrid | Ripening stage | Ascorbic acid (μg/g) |
|---|---|---|
| Beibeihong | Green | 355 ± 64.85a |
| Colour-breaker | 1503.4 ± 358.31b | |
| Orange | 2085.7 ± 252.2bc | |
| Red | 2483.8 ± 570.74c | |
| F-value | 19.74 | |
| p value | <0.001 | |
|
| ||
| Bandai | Green | 329.5 ± 58.88a |
| Colour-breaker | 937.9 ± 78.04b | |
| Orange | 1005.2 ± 100.73b | |
| Red | 787.4 ± 131.21b | |
| F-value | 30.09 | |
| p value | <0.001 | |
|
| ||
| Lolo | Green | 111.3 ± 14.01a |
| Colour-breaker | 451.5 ± 115.56a | |
| Red | 1940.9 ± 533.57b | |
| F-value | 28.57 | |
| p value | 0.001 | |
|
| ||
| C3735 | Green | 315.1 ± 59.91a |
| Colour-breaker | 1522.5 ± 127.47b | |
| Red | 2468.2 ± 58.93c | |
| F-value | 449.65 | |
| p value | <0.001 | |
|
| ||
| Fire Flame | Red | 3689.4 ± 39.50 |
|
| ||
| Star Flame | Yellow | 3154.8 ± 160.61 |
The same letter indicates no significant difference in ascorbic acid content between ripening stages in the given hybrid according to Tukey HSD post hoc test.
The recommended daily allowance (RDA) is 60 μg FW; according to Dias [39] 100 g fresh chili provides about 143.7 μg vitamin C. Focusing on the hybrids of the recent study at the green stage all of them failed to reach this value, while at colour-break stage Beibeihong and C3735 reached it and finally at the red stage all of them achieved the RDA.
3.4. Comparison of Major Compounds among the 6 Hybrids
The comparison among the hybrids has been done on the main parameters: CAP, ascorbic acid, naringenin-diglucoside, Scoville heat unit, and dry matter (shown in Table 5), at the final stage of the hybrids, which is generally considered as the most valuable in nutrition and having the most processing possibility. A higher dry matter signifies a better fruit quality and also a higher nutritional concentration when fresh weight basis is used to express nutritional parameters. We measured 25–30% dry matter content in C. frutescens, which produces more seeds and smaller pods, while in the peppers belonging to C. annuum this value lessens to 14.1–15.8%.
Table 5.
Capsaicin, ascorbic acid, and naringenin-diglucoside content (μg/g fresh weight base), pungency unit of Scoville, and dry matter of different chili hybrids. The values represent means ± standard deviation (n = 3).
| Hybrid | Capsaicin (μg/g) | Scoville heat unit | Ascorbic acid (μg/g) | Naringenin-diglucoside (μg/g) | Dry matter |
|---|---|---|---|---|---|
| Beibeihong | 311.8 ± 63.25ab | 37999.8 ± 5761.66a | 2483.8 ± 570.74bc | 368.8 ± 30.77f | 25.8 ± 0.82d |
| Bandai | 1176.1 ± 112.1c | 98090.8 ± 9920.74c | 787.4 ± 131.21a | 276.5 ± 16.65e | 30.2 ± 0.41c |
| Lolo | 197 ± 92.13a | 33188.2 ± 5229.83a | 1940.9 ± 533.57b | 117.8 ± 7.18ab | 14.0 ± 0.61a |
| C3735 | 126.3 ± 35.95a | 23730.9 ± 3174.95a | 2468.2 ± 58.93bc | 217.2 ± 8.36d | 15.8 ± 0.93b |
| Fire Flame | 234.3 ± 45.23a | 40417.3 ± 7830.33a | 3689.4 ± 160.61d | 141.6 ± 4.19c | 14.1 ± 0.34ab |
| Star Flame | 440.8 ± 17.22b | 66201.2 ± 7132.51b | 3154.8 ± 160.61cd | 93.5 ± 4.26a | 14.4 ± 0.58ab |
| F-value | 94.64 | 48.43 | 27.62 | 146.17 | 357.48 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
The same letter indicates no significant difference in the major components between the fully ripe stages of the 6 hybrids according to Tukey HSD post hoc test.
The CAP content was found to be statistically the same in all red coloured C. annuum hybrids, while the yellow hybrid Star Flame (234.3 ± 45.23 μg/g) contained more, and Bandai (1176.1 ± 112.1 μg/g) the most (p < 0.001). Our findings roughly agree with the result of Sanatombi and Sharma [40] who showed that the cultivars belonging to C. annuum contain less capsaicin than others of Capsicum frutescens. Beibeihong was an exception, because it statistically contained the same amount as C. annuum hybrids. Focusing on the Scoville heat units, the highest CAP value in Bandai corresponds to the highest SHU (98090.8 ± 9920.74) observed among the hybrids investigated. Bernal et al. [33] measured 87300–276500 SHU in ripe C. frutescens peppers, but in Bandai hybrid the value found was close to the lower level determined by the authors. Among C. annuum hybrids, Star Flame was found to be a prominent pepper regarding SHU (66201.2 ± 7132.51) comparing to the measurements of Topuz and Ozdemir [41] 9720 ± 2061.8 and Giuffrida et al. [42] 21034 ± 3579. Beibeihong and Bandai have not been investigated by pungency profiles before. Comparing to Tabasco (also belonging to C. frutescens) the SHU measured by Giuffrida et al. [42] (21348 ± 867) is below our values of the latter hybrids, although CAP content determined by Giuffrida et al. [42] (917 ± 34 μg/g) is between the values measured in Bandai (1176.1 ± 112.1 μg/g) and Beibeihong (311.8 ± 63.25 μg/g). Interestingly, Bandai hybrid had the highest CAP content at the same time; it also had the lowest ascorbic acid amount. Topuz and Ozdemir [41] described in pungent peppers that the content of ascorbic acid and capsaicinoid is positively related, which we could not underline in case of Bandai. The highest ascorbic acid was measured in ripe Fire Flame (3689.4 ± 160.61 μg/g) and this value is well above the one measured in Hungarian spice pepper where approximately 1800 μg/g converted to fresh weight basis [12], and it is more than the one detected in New Mexican-type chili peppers 2766 μg/g [25].
Naringenin-diglucoside content ranged from 93.5 ± 4.26 to 368.8 ± 30.77 μg/g and had higher values in C. frutescens hybrids compared to C. annuum hybrids, probably because of the higher dry matter content of such peppers. Naringenin (belonging to flavanones), being an initial compound in the chain of flavonoid synthesis [14], explains the high content present in our samples. Other studies found also naringenin-glucosides as a dominant flavonoid in peel of pungent pepper [43] and in sweet pepper alike [44].
4. Conclusion
The investigated new hybrids can be regarded to be good sources of phytochemicals for future applications. We recommend using the red coloured hybrid Fire Flame to produce chili products with high content of vitamin C. On the other hand, when heat principles (capsaicinoid) for food and pharmaceutical industries are required, the use of Star Flame and Bandai can be suggested, as they contain a level of capsaicin around 440.8 ± 17.22 μg/g and 1610.2 ± 91.46 μg/g, respectively. In order to get the maximum level of the bioactive phytochemicals such as vitamin C, capsaicinoid, and polyphenol it is important to characterize the ripening dynamics of each of these new hybrids. For example, the highest level of capsaicin could be found in the green stage of ripening of Bandai and C3735 hybrids, while in the other hybrids pungency was similar in all ripening stages.
Acknowledgment
This study was funded in part by Research Centre of Excellence: 8526-5/2014/TUDPOL Szent István University and KTIA_AIK_12-1-2012-0012 projects.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mózsik G., Dömötör A., Past T., et al. Capsaicinoids. Budapest, Hungary: Akadémiai Publishing; 2009. Chemical taxonomy of the functional parts of the Capsicums . [Google Scholar]
- 2.DeWitt D., Bosland P. W. The Complete Chile Pepper Book. 1st. London, UK: Timber Press; 2009. Capsaicin and the quest for the world's hottest pepper. [Google Scholar]
- 3.Mózsik G., Szolcsányi J., Rácz I. Gastroprotection induced by capsaicin in healthy human subjects. World Journal of Gastroenterology. 2005;11(33):5180–5184. doi: 10.3748/wjg.v11.i33.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood J. N., Winter J., James I. F., Rang H., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. The Journal of Neuroscience. 1988;8(9):3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachtel R. E. Capsaicin. Regional Anesthesia and Pain Medicine. 1999;24(4):361–363. doi: 10.1097/00115550-199924040-00015. [DOI] [PubMed] [Google Scholar]
- 6.Medina-Lara F., Echevarría-Machado I., Pacheco-Arjona R., Ruiz-Lau N., Guzmán-Antonio A., Martinez-Estevez M. Influence of nitrogen and potassium fertilization on fruiting and capsaicin content in Habanero pepper (Capsicum chinense Jacq.) HortScience. 2008;43(5):1549–1554. [Google Scholar]
- 7.Sung Y., Chang Y. Y., Ting N. L. Capsaicin biosynthesis in water-stressed hot pepper fruits. Botanical Bulletin of Academia Sinica. 2005;46:35–42. [Google Scholar]
- 8.Gurung T., Techawongstien S., Suriharn B., Techawongstien S. Stability analysis of yield and capsaicinoids content in chili (Capsicum spp.) grown across six environments. Euphytica. 2012;187(1):11–18. doi: 10.1007/s10681-012-0672-6. [DOI] [Google Scholar]
- 9.Harvell K. P., Bosland P. W. The environment produces a significant effect on pungency of chiles. Hortscience. 1997;32:p. 1292. [Google Scholar]
- 10.Contreras-Padilla M., Yahia E. M. Changes in capsaicinoids during development, maturation, and senescence of chile peppers and relation with peroxidase activity. Journal of Agricultural and Food Chemistry. 1998;46(6):2075–2079. doi: 10.1021/jf970972z. [DOI] [Google Scholar]
- 11.Shan-Han C., Shen-Kui H. E., Wen-Bin C., Yan-Ge W. U. Detection of capsaicin and dihydrocapsaicin content and analysis of pungency degree in different pepper genotypes. Natural Science Journal of Hainan University. 2009;1:p. 009. [Google Scholar]
- 12.Gnayfeed M. H., Daood H. G., Biacs P. A., Alcaraz C. F. Content of bioactive compounds in pungent spice red pepper (paprika) as affected by ripening and genotype. Journal of the Science of Food and Agriculture. 2001;81(15):1580–1585. doi: 10.1002/jsfa.982.abs. [DOI] [Google Scholar]
- 13.Menichini F., Tundis R., Bonesi M., et al. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chemistry. 2009;114(2):553–560. doi: 10.1016/j.foodchem.2008.09.086. [DOI] [Google Scholar]
- 14.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal T., Hussain A. I., Chatha S. A., Naqvi S. A., Bokhari T. H. Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (Mentha longifolia) from the Pakistani Flora. Journal of Analytical Methods in Chemistry. 2013;2013:6. doi: 10.1155/2013/536490.536490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández-Jiménez A., Gil-Muñoz R., Ruiz-García Y., López-Roca J. M., Martinez-Cutillas A., Gómez-Plaza E. Evaluating the polyphenol profile in three segregating grape (Vitis vinifera L.) populations. Journal of Analytical Methods in Chemistry. 2013;2013:9. doi: 10.1155/2013/572896.572896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harborne J. B., Williams C. A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 18.Shaha R. K., Rahman S., Asrul A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Annals of Biological Research. 2013;4(8):27–34. [Google Scholar]
- 19.Materska M., Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.) Journal of Agricultural and Food Chemistry. 2005;53(5):1750–1756. doi: 10.1021/jf035331k. [DOI] [PubMed] [Google Scholar]
- 20.Howard L. R., Talcott S. T., Brenes C. H., Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. Journal of Agricultural and Food Chemistry. 2000;48(5):1713–1720. doi: 10.1021/jf990916t. [DOI] [PubMed] [Google Scholar]
- 21.Sauberlich H. E. Pharmacology of vitamin C. Annual Review of Nutrition. 1994;14(1):371–391. doi: 10.1146/annurev.nutr.14.1.371. [DOI] [PubMed] [Google Scholar]
- 22.Lutsenko E. A., Carcamo J. M., Golde D. W. Vitamin C prevents DNA mutation induced by oxidative stress. The Journal of Biological Chemistry. 2002;277(19):16895–16899. doi: 10.1074/jbc.m201151200. [DOI] [PubMed] [Google Scholar]
- 23.Frei B., Lawson S. Vitamin C and cancer revisited. Proceedings of the National Academy of Sciences. 2008;105(32):11037–11038. doi: 10.1073/pnas.0806433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae H., Jayaprakasha G. K., Crosby K., et al. Ascorbic acid, capsaicinoid, and flavonoid aglycone concentrations as a function of fruit maturity stage in greenhouse-grown peppers. Journal of Food Composition and Analysis. 2014;33(2):195–202. doi: 10.1016/j.jfca.2013.11.009. [DOI] [Google Scholar]
- 25.Osuna-García J. A., Wall M. M., Waddell C. A. Endogenous levels of tocopherols and ascorbic acid during fruit ripening of New Mexican-type chile (Capsicum annuum L.) cultivars. Journal of Agricultural and Food Chemistry. 1998;46(12):5093–5096. doi: 10.1021/jf980588h. [DOI] [Google Scholar]
- 26.Lee S. K., Kader A. A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology. 2000;20(3):207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- 27.Daood H. G., Halász G., Palotás G., Palotás G., Bodai Z., Helyes L. HPLC determination of capsaicinoids with cross-linked C18 column and buffer-free eluent. Journal of Chromatographic Science. 2014;53(1):135–143. doi: 10.1093/chromsci/bmu030. [DOI] [PubMed] [Google Scholar]
- 28.Ziino M., Condurso C., Romeo V., Tripodi G., Verzera A. Volatile compounds and capsaicinoid content of fresh hot peppers (Capsicum annuum L.) of different calabrian varieties. Journal of the Science of Food and Agriculture. 2009;89(5):774–780. doi: 10.1002/jsfa.3511. [DOI] [Google Scholar]
- 29.Wu S., Dastmalchi K., Long C., Kennelly E. J. Metabolite profiling of jaboticaba (Myrciaria cauliflora) and other dark-colored fruit juices. Journal of Agricultural and Food Chemistry. 2012;60(30):7513–7525. doi: 10.1021/jf301888y. [DOI] [PubMed] [Google Scholar]
- 30.Merken H. M., Beecher G. R. Measurement of food flavonoids by high-performance liquid chromatography: a review. Journal of Agricultural and Food Chemistry. 2000;48(3):577–599. doi: 10.1021/jf990872o. [DOI] [PubMed] [Google Scholar]
- 31.Kozukue N., Han J., Kozukue E., et al. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry. 2005;53(23):9172–9181. doi: 10.1021/jf050469j. [DOI] [PubMed] [Google Scholar]
- 32.Iwai K., Suzuki T., Fujiwake H. Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuun var. annuun cv. karayatsubusa at different growth stages after flowering. Agricultural and Biological Chemistry. 1979;43(12):2493–2498. doi: 10.1271/bbb1961.43.2493. [DOI] [Google Scholar]
- 33.Bernal M. A., Calderon A. A., Pedreno M. A., Munoz R., Ros Barcelo A., de Caceres F. M. Capsaicin oxidation by peroxidase from Capsicum annuum (variety Annuum) fruits. Journal of Agricultural and Food Chemistry. 1993;41(7):1041–1044. doi: 10.1021/jf00031a004. [DOI] [Google Scholar]
- 34.Gao Y. P., He L. L., Chen J. Q., Gao S., Li X. W., Dong X. K. Effects of shading on capsaicin and relevant enzymes of fruit in pepper. Acta Agriculturae Boreali-Sinica. 2008;3:p. 33. [Google Scholar]
- 35.Sukrasno N., Yeoman M. Phenylpropanoid metabolism during growth and development of Capsicum frutescens fruits. Phytochemistry. 1993;32(4):839–844. doi: 10.1016/0031-9422(93)85217-f. [DOI] [Google Scholar]
- 36.Diaz J., Bernal A., Merino F., Barcelo R. A. Phenolic metabolism in Capsicum annuum L. Recent Research Developments in Phytochemistry. 1998;2:155–169. [Google Scholar]
- 37.Kawada T., Iwai K. In vivo and in vitro metabolism of dihydrocapsaicin, a pungent principle of hot pepper, in rats. Agricultural and Biological Chemistry. 1985;49(2):441–448. doi: 10.1271/bbb1961.49.441. [DOI] [Google Scholar]
- 38.Stone I. The natural history of ascorbic acid in the evolution of the mammals and primates and its significance for present-day man. Orthomolecular Psychiatry. 1972;1(2-3):82–89. [Google Scholar]
- 39.Dias J. S. Nutritional quality and health benefits of vegetables: a review. Food and Nutrition Sciences. 2012;3(10):1354–1374. doi: 10.4236/fns.2012.310179. [DOI] [Google Scholar]
- 40.Sanatombi K., Sharma G. J. Capsaicin content and pungency of different Capsicum spp. cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2008;36(2):89–90. [Google Scholar]
- 41.Topuz A., Ozdemir F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. Journal of Food Composition and Analysis. 2007;20(7):596–602. doi: 10.1016/j.jfca.2007.03.007. [DOI] [Google Scholar]
- 42.Giuffrida D., Dugo P., Torre G., et al. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chemistry. 2013;140(4):794–802. doi: 10.1016/j.foodchem.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 43.Xin X., Fan R., Gong Y., Yuan F., Gao Y. On-line HPLC-ABTS•+ evaluation and HPLC-MSn identification of bioactive compounds in hot pepper peel residues. European Food Research and Technology. 2014;238(5):837–844. [Google Scholar]
- 44.Morales-Soto A., Gómez-Caravaca A. M., García-Salas P., Segura-Carretero A., Fernández-Gutiérrez A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Research International. 2013;51(2):977–984. doi: 10.1016/j.foodres.2013.02.022. [DOI] [Google Scholar]


