Abstract
Introduction
The thumb is essential for daily activities. Unfortunately, this digit is commonly affected by trapeziometacarpal osteoarthritis (TMO), handicapping a large number of individuals. TMO constitutes an increasing human and economic burden for our society whose population is ageing. Limited access to adequate treatment is among the most important obstacles to optimal TMO management. Poor understanding of TMO characteristics, lack of knowledge about evidence-based treatments, simplistic pain management plans based solely on the patient's physical condition, absence of interprofessional communication and lack of multidisciplinary treatment guidelines contribute to inadequate TMO management. On the long term, our research project aims at improving the quality of care and services offered to patients with TMO by developing a patient-centred, evidence-based multidisciplinary management clinical pathway coordinated across the healthcare system. This proposed systematic review is a prerequisite to ensuring evidence-based practices and aims to document the efficacy of all the existing modalities for TMO management.
Methods and analysis
The protocol of the systematic review is registered with PROSPERO and will be conducted using the guidelines Cochrane Handbook for Systematic Reviews of Interventions. We will identify studies in English and French concerning TMO treatments through searches in Cochrane Central, EMBASE, MEDLINE, PsychINFO, CINHAL, PubMed, OT Seekers, PEDRO and the grey literature. 2 reviewers will independently screen study eligibility, extract data and appraise studies using published assessment tools. Meta-analyses will be undertaken where feasible; otherwise, narrative syntheses will be carried out. The robustness of evidence will be assessed using the GRADE system.
Ethics and dissemination
Ethics approval is not required for this study. A comprehensive knowledge exchange and transfer plan incorporating effective strategies will be used to disseminate the findings of this review and utilise them to optimise TMO management.
Trial registration number
PROSPERO CRD42015015623.
Keywords: PAIN MANAGEMENT, RHEUMATOLOGY
Strengths and limitations of this study.
This review is the first to carry out an extensive and comprehensive systematic review of all the existing treatments specific to trapeziometacarpal osteoarthritis (TMO) including pharmacological, non-pharmacological and surgical ones, not limited to any one discipline. Subsequently, the findings will allow us to develop and design an evidence-based multidisciplinary TMO management pathway usable for clinicians of various disciplines across the healthcare continuum.
An extensive knowledge exchange and transfer plan incorporating effective strategies to disseminate and share the results with end-users is proposed. The findings will be used in a future study aimed at developing an active collaborative partnership between researchers and end-users to optimise care for patients with TMO.
Language restriction to English and French for the literature search is a limitation of the proposed protocol such that language bias is possible.
Introduction
Trapeziometacarpal osteoarthritis: an understudied but important health problem
The most prevalent cause of chronic pain in the world is osteoarthritis (OA).1 2 Its prevalence is increasing in an alarming manner with the ageing of the population, and it is estimated it will double before the year 2020.3 This anticipated increase is somewhat frightening considering that OA is associated with numerous adverse consequences for affected individuals as well as increasing economic costs for our society.3–6 Based on the meta-analysis of Pereira et al7 on OA prevalence, hand OA is more prevalent than knee/hip OA, yet hand OA has been much less studied. Despite the fact that the thumb accounts for approximately 50% of overall hand function and is essential in our daily activities,8 relatively few studies have documented the prevalence of trapeziometacarpal osteoarthritis (TMO). Most of our knowledge comes from American and European studies which are based solely on radiographic findings: the prevalence rates of TMO ≥ grade 2 (on 4-point or 5-point severity scale) are highly variable ranging from 11.5% to 50.5%.9–13 TMO was found to be more prevalent in women than men, but the prevalence steadily increases with age in both genders. The prevalence of symptomatic TMO (as defined by the presence of clinical symptoms with or without radiographic findings) and the rates vary between 1.0% and 15.9%.14–21 Some studies have revealed that only a weak to modest association between TMO radiographic findings and clinical symptoms (pain and/or functional disability) exists10 15—that is, patients may exhibit important structural changes, yet report little or no pain; or patients may experience severe pain with little radiological evidence of TMO. Botha-Scheepers et al22 followed a group of patients with hand OA over a 2-year period and found that the progression of pain intensity and physical functioning was unrelated to X-ray findings.22 Based on the extensive clinical experience of three of the co-authors (PH, NB, TH) of this article, the above rates of symptomatic TMO are most likely to be underestimated because healthcare professionals commonly have insufficient knowledge of TMO characteristics and misdiagnose the origin of the pain (eg, tendinopathy vs TMO). As a result, these patients are referred to a hand specialist long after TMO first appears.
The patients with TMO reported persistent pain at the thumb base23–25 which limits their hand functions,25–27 reducing both thumb mobility28 and hand strength,29–31 thereby affecting their daily activities (eg, holding objects, preparing meals, writing).26 29 32 However, only a few studies have either quantified the severity of TMO pain and/or its impact on various aspects of daily living other than physical functioning.22 32
Management of TMO and pain-related symptoms
Despite decades of research on pain assessment and management, it is well documented that chronic pain disorders of various origins continue to be commonly undertreated, mistreated or untreated, with a large number of patients going from one doctor to another seeking pain relief.33 One of the major barriers to optimal management of persistent pain disorders including OA is the limited access to adequate healthcare services. Patients commonly have difficulty gaining timely access to appropriate pain care34–36 leading to a premature or an increased deterioration of their physical functioning, psychological well-being and health-related quality of life while waiting for treatment. Management of TMO and pain-related symptoms can be provided by different healthcare professionals including primary care physicians, rheumatologists, physiatrists, orthopaedic surgeons, plastic surgeons, radiologists, pharmacists, physical therapists and/or occupational therapists. However, these clinicians (including hand specialists) often work in silos and manage patients with TMO based on their own clinical experience rather than on well-documented scientific evidence. Other obstacles to adequate TMO management include (1) poor awareness and understanding of the characteristics of TMO (and especially in the primary sector of care), (2) lack of knowledge about evidence-based effective treatments and (3) simplistic pain management plans based solely on patients’ physical condition which do not necessarily meet all their needs. Finally, the fact that healthcare professionals commonly have insufficient knowledge and training for managing chronic pain disorders should not be neglected.37 38
Management of TMO involves various modalities including pharmacological therapy,23 39 40 corticosteroid/hyaluronic acid injections,23 25 40 hand exercises,40–42 orthoses,25 39 40 42 43 joint protection education,39 assistive devices,39 42 physical agent modality39 40 43 and surgery.40 42 44 However, the relative efficacy of these modalities remains poorly documented, some of them recommended for the treatment of hand OA in general while others are specifically for TMO. Furthermore, earlier systematic reviews examining the efficacy of TMO treatment have focused solely on one type of modality (eg, surgery, orthoses).45 46 Chronic pain disorders commonly have significant adverse consequences in various domains of a patient's life,26 39 and it is widely acknowledged that a multidisciplinary approach which takes into account the biopsychosocial components of the pain experience constitutes the ‘gold standard’ for managing this type of disorder.47 48 Therefore, there is a need to conduct a systematic review from a multidisciplinary perspective which integrates all the existing therapeutic modalities for TMO in order to (1) document their relative efficacy, and (2) examine the modalities whose efficacy for TMO is supported by scientific evidence and those which are not, without creating confusion between effective modalities with absence of documented evidence and ineffective modalities supported by evidence.
Objectives
Our ultimate aim is to improve the quality of care and delivery of services for patients with TMO by developing a patient-centred, evidence-based TMO management clinical pathway49 coupled to most optimal treatments which are evidence-based. As a prerequisite, a systematic review of the literature is needed to document the efficacy of the existing pharmacological, non-pharmacological and surgical modalities to relieve pain and improve function in patients with TMO. This paper aims at presenting the protocol for this systematic review of the literature.
Methods and analysis
The guidelines for systematic review of the literature Cochrane Handbook for Systematic Reviews of Interventions50 were referred to to prepare this protocol. The review will involve five steps (see figure 1).
Figure 1.
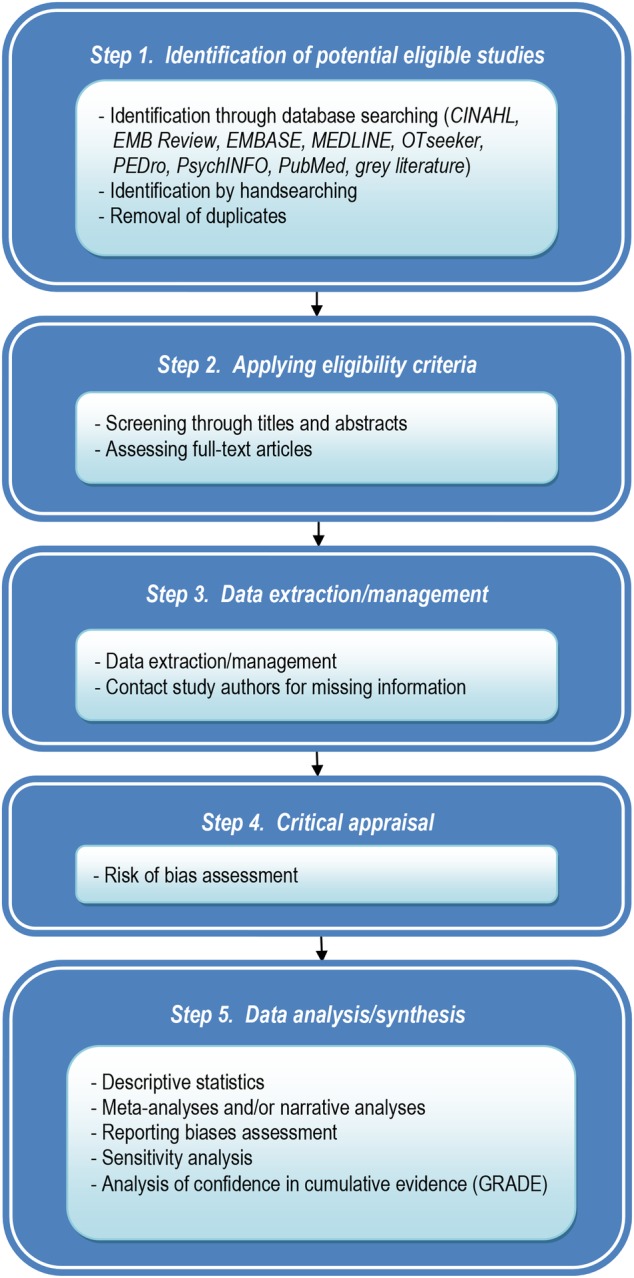
Process of the systematic review.
Research team
The team combines relevant and complementary disciplines with members in pain psychology and pharmacology (MC), epidemiology and biostatistics (LL), plastic surgery (PH), radiology (NB), physiotherapy (NG), occupational therapy (TH) and library information science (DZ). The research expertise of MC is in the field of pain assessment/management and knowledge translation. The second author's research expertise (LL) focuses on knowledge transfer on primary care clinical practices in the cardiovascular and pain fields. The third author (PH) runs the largest hand clinic in the province of Quebec (Canada) and follows about 50 patients with TMO yearly. The fourth author (NB), a radiologist and a researcher, routinely performs image-guided steroid injections. The fifth author (NG) has research expertise in systematic reviews of the literature, lower limb OA and technology assessment. The sixth author (DZ) has collaborated on a series of systematic reviews. Finally, TH, a PhD student and occupational therapist, has treated patients with TMO for over 13 years.
Step 1: Identification of potential eligible studies
Our academic librarian informationist (DZ) will search through bibliographic electronic databases CINAHL (from 1937 onwards), EMB Review (from 1991 onwards), EMBASE (from 1974 onwards), MEDLINE (from 1946 onwards), OTseeker, PEDro, PsychINFO (from 1806 onwards), PubMed and the grey literature (CADTH, Clinical Trials, National Guideline Clearing House, National Institute for Health and Care Excellence (NICE), MedNar, Google Scholar, OAIster and Open Grey). The first search will combine words and expressions for three conceptual groups: trapeziometacarpal joint, OA and treatment. To ensure that psychotherapeutic modalities for TMO will be picked up, the following keywords will be added: cognitive therapy, cognitive behavior therapy, relaxation, biofeedback, supportive psychotherapy, group therapy and counseling. For the second search, the first two conceptual groups will be the same while the third group will focus on ‘pain’ (see online supplementary annex 1 for details on the search strategy for MEDLINE). For each database, we will use words and expressions from controlled vocabulary (MeSH, EMTREE and others) and free-text searching. The searches will be restricted to articles published in English and French. Handsearching will also be used to identify other references (TH and MC). A pilot search through the CINAHL, EMB Review, EMBASE, MEDLINE, OTseeker, PEDro, PsychINFO and PubMed have identified approximately 2000 references, demonstrating the study's feasibility.
Step 2: Applying eligibility criteria
Once the results from multiple searches will be merged by the librarian (DZ) using the reference management software EndNote, duplicate records will be removed (DZ and TH). Titles and abstracts of studies will be screened independently by two reviewers for eligibility (MC and TH). Agreement between the two reviewers will be established using κ statistic.50 Full-text copies of potentially relevant reports will be retrieved (TH). They will be analysed against eligibility criteria and the results will be recorded in part 1 (General Information) and part 2 (Eligibility) of the Cochrane Effective Practice and Organisation of Care Group (EPOC) Data Abstraction Form50 by the two screeners. In the cases where no consensus is reached by the two reviewers, a third reviewer (PH) will determine the eligibility of the study. Part 1 of the EPOC form includes study identification (surname of first author and year of first full report of study), date form completed, name of person extracting data, report title, publication type, study funding source and possible conflicts of interest. Part 2 consists of study characteristics (type of study, participants, types of intervention/outcome measure).
Criteria for considering studies for this review
Types of studies
Meta-analyses, systematic reviews of the literature, randomised controlled trials (RCT) will be included. If there are no RCT, non-RCTs, controlled before-after studies, interrupted time series (ITS) and repeated measures studies will be considered as well as observational studies (cohort, case–control).40 39 Case series, review articles, editorials and commentaries will be excluded. The studies with higher evidence will be prioritised to determine the efficacy of therapeutic modalities. Results of most recent systematic reviews and those of reviews including more studies will be prioritised if there is more than one systematic review on a given intervention.
Types of participants
Studies conducted among TMO adults who had received treatment to decrease pain and/or improve function will be included. Studies on diseases other than primary TMO (eg, traumatic OA, rheumatoid arthritis), on OA other than the trapeziometacarpal joint or on animals will be excluded. Studies including OA of different joints will be included if the data of TMO are separately presented.
Types of interventions
All the existing therapeutic modalities for TMO treatments (eg, pharmacological, non-pharmacological, surgical) to reduce pain and improve function will be included. The possible interventions are ‘drug therapy’, ‘surgery’, ‘manual therapy’, ‘psychotherapy’, ‘orthoses’, ‘acupuncture’, ‘hand exercises’, ‘assistive devices’, ‘education’, ‘joint injections’, ‘joint protection’, ‘laser therapy’ and ‘thermotherapy’. The comparators are another intervention or a non-exposed control group.
Type of outcomes
Primary outcomes are pain and function, considered core outcomes for OA clinical trials according to the international consensus group Outcome Measures in Rheumatology (OMERACT).51 52 Secondary outcomes are patients’ psychological well-being, health-related quality of life and treatment satisfaction.
Step 3: Data extraction/management
Data will be independently extracted by two persons (MC and TH) using part 3 of the EPOC data abstract form50 (Population and Setting) which explores population description, setting, inclusion criteria, exclusion criteria and methods of recruitment. Part 4 (Methods) looks at aims of study, design, unit of allocation, start date, end date and duration of participation. Part 5 (Risk of bias) will be used at step 4. Part 6 (Participants) considers total number of participants, withdrawals and exclusion, severity of illness, comorbidities, other treatment, relevant sociodemographics, and subgroups. Part 7 (Intervention group) takes into account description of intervention, duration of treatment period and others. Part 8 (Outcomes) records outcome name, time points measured/reported, outcome definition, person measuring/reporting, unit of measurement, scales and others. Part 9 (Results) varies according to study design and nature of outcome (dichotomous/continuous). It mainly concerns comparison, outcome, subgroup, results, baseline data, number of missing participants, statistical methods and appropriateness of these methods, and others. Part 10 (Applicability) questions if important populations have been excluded from the study, if the intervention is likely to be aimed at disadvantaged groups and if the study directly addresses the review question. Part 11 (Other information) includes key conclusions, references to other relevant studies, correspondence required for further study information and others. In cases where data are missing, study authors will be contacted.
Step 4: Critical appraisal
Risk of bias in individual studies will be separately assessed by two reviewers (MC and TH). In the cases of disagreement, discussion will take place to achieve consensus. If necessary, the third one (PH) will appraise the study. Different assessment tools will be used depending on study design: Assessment of Multiple Systematic Reviews (AMSTAR) for systematic reviews of the literature,53 EPOC Risk of Bias Tool for controlled studies and for ITS studies,54 Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies for cohort studies or case–control study.55
Assessment of Multiple Systematic Reviews
The questionnaire is composed of 11 items.53 It examines the methodological quality of a systematic review including double review, exhaustive research strategy, heterogenic analysis and publication bias. It scores each criterion on four scales ‘yes’, ‘no’, ‘can’t answer’ and ‘not applicable’, and total score on seven scales. Its inter-rater reliability for each item is moderate to perfect (0.51<κ<1.00) and excellent for the global score (κ=0.84, 95% CI 0.67 to 1.00). Its construct validity (Pearson coefficient) is 0.72 (95% CI 0.53 to 0.84). The minimal detectable difference is 0.64.56
EPOC Risk of Bias Tool for studies with a separate control group
This tool includes the five domains of bias determined by the Cochrane Risk of Bias Tool55 57—selection (random sequence generation and allocation concealment), performance, attrition (method addressing incomplete outcome), detection and reporting (selective outcome reporting)—and two other criteria regarding ‘similarity of baseline outcome measurements between experimental and control groups’ and ‘similarity of baseline characteristics between experimental and control groups’. Each item is scored ‘yes’ for high risk, ‘no’ for low risk and ‘unclear’ if not specified in the paper.
EPOC Risk of Bias Tool for ITS studies
This tool examines four domains of risks of bias determined by the Cochrane Risk of Bias Tool54 57 (performance, attrition, detection and reporting bias) and three risks of bias associated with the ITS study design; ‘was the intervention independent of other changes?’, ‘was the shape of the intervention effect prespecified?’ and ‘was the intervention unlikely to affect data collection?’
EPHPP Quality Assessment Tool for Quantitative Studies
This tool will be used to assess cohort and case–control studies.55 It includes the items defined by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.58 It includes 21 items from eight categories (selection, study design, confounders, blinding, data collection methods, withdrawals and drop-outs, intervention integrity and analyses). This tool is considered one of the best tools for systematic review.59 Content validity and construct validity, and inter-rater and intra-rater reliability have been demonstrated (κ=0.74, intraclass correlation coefficient=0.77).55 60 Administration time is 10–15 min, and its ease of use has been reported.55 59
Step 5: Data analysis/synthesis
Characteristics of included studies
Descriptive statistics will present features of included studies in terms of study design, clinical and sociodemographic characteristics of participants, studied TMO treatments and their results.
Efficacy analysis of each therapeutic modality
Meta-analyses will be undertaken using the Cochrane Group's Review Manager software (RevMan V.5.1)61 unless heterogeneity among studies is demonstrated by the I2 statistic, that is, I2≥50%.62 For continuous outcomes, mean differences and standardised mean differences will be used for meta-analysis. For dichotomous outcomes, ORs, risk ratios, absolute risk reduction and number needed to treat will be computed. For longitudinal studies, risk ratios or HRs will be calculated; for case–control studies, ORs will be computed. In the presence of substantial variation among studies, narrative syntheses will be favoured and studies will be classified in logical categories.63 In cases where data are missing, study authors will be contacted; otherwise, participant attrition will be treated by intention-to-treat analysis.50 Missing statistics (eg, SD) will be calculated from available data (eg, SE will be reported from p values or 95% CIs).50
Reporting biases assessment and sensitivity analyses
Reporting biases across studies will be analysed by funnel plots when feasible—that is, at least 10 studies are included in the meta-analysis to ensure the power of the tests.50 Sensitivity analyses will be undertaken in case the eligibility of some studies in the meta-analysis is doubtful (eg, low-quality studies).50
Confidence in cumulative evidence
The robustness of evidence will be assessed by using the GRADE classification64–77 and its software GRADEpro.78 Two tables will be dressed for each therapeutic modality. ‘Clinical Evidence Profile’ tables present quality of evidence for each outcome, while ‘Clinical Evidence Summary of Findings’ tables will provide end-users (administrators, healthcare professionals, patients) with key information helping them with decision-making in choosing the right treatments.64
Ethics and dissemination
Ethics approval is not required for this study. Once completed, the systematic review findings will be presented to a group of stakeholders during a 1-day workshop where researchers, clinicians from various disciplines, managers/decision-makers and patients will work together to elaborate a TMO management clinical pathway. This partnership between researchers and end-users will contribute to effective knowledge exchange and transfer.79 With regard to our end-of-project knowledge transfer plan, we will draw on three key principles: (1) developing communication vehicles adapted to the target audience; (2) presenting concise messages; and (3) creating settings for exchange and discussion.80 We consider the target audiences to be the: (1) scientific community, (2) healthcare professionals, (3) general public including patients with TMO or those afflicted with other types of OA or chronic pain disorders, and (4) administrators. In addition to traditional vehicles (eg, scientific meetings, publications), we will also create a module tab on the website of the Quebec Pain Research Network and on the Centre hospitalier de l'Université de Montréal (CHUM) website where the results of the project will be made accessible to the different targeted audiences. The final product (TMO management clinical pathway) will be made available in the form of a twofold pamphlet, one will be specifically for healthcare professionals, while the other for patients with TMO (ie, patient decision aids), elaborated by following the recommendations of the International Patient Decision Aids Standards Collaboration.81 82 They will be duly delivered and subsequently presented to different institutions from the primary to tertiary sectors of care.
Discussion
TMO is a chronic and degenerative disease which can seriously handicap patients, hence affecting their quality of life. However, TMO management is far from optimal due to several obstacles including limited access to adequate healthcare services. Developing a patient-centred, evidence-based multidisciplinary treatment algorithm for TMO is paramount to improving the quality of care to this patient clientele. It will help guide the decision-making process of clinicians and patients with TMO in choosing the most suitable therapeutic modalities. To do so, a systematic review is a prerequisite, and to our knowledge, we are the first to propose the conduct of an extensive and comprehensive literature review of all the existing treatments for TMO including pharmacological, non-pharmacological and surgical modalities, not limited to any one discipline. Language restriction to English and French for the literature search is a limitation of the proposed protocol such that language bias is possible. However, the obtained findings will be crucial in developing a TMO treatment algorithm useful to all stakeholders across the healthcare continuum.
Acknowledgments
The authors are grateful to Mr Denis Bélanger who reviewed and edited the present manuscript.
Footnotes
Twitter: Follow Daniela Ziegler at @danielamihu
Contributors: TH conceived the protocol and drafted the manuscript under MC’s supervision. DZ developed the literature search strategies and made substantial contributions to the section regarding information sources and literature search. All authors contributed to the preparation of the manuscript, read and approved the final version of this systematic review protocol.
Funding: TH was supported by a doctoral scholarship from the CHUM Foundation—Hand Surgery Branch and from MC's internal funds of the CHUM Research Center. TH is now a recipient of a Doctoral training award of the Fonds de recherche du Québec—Santé (FRQS). LL holds Sanofi Aventis Endowment Chair in Ambulatory Pharmaceutical Care. NB is a recipient of FRQS Research Scholar.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag 2011;16:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 3.Creamer P, Hochberg MC. Osteoarthritis. Lancet 1997;350:503–8. 10.1016/S0140-6736(97)07226-7 [DOI] [PubMed] [Google Scholar]
- 4.Kraus VB. Pathogenesis and treatment of osteoarthritis. Med Clin North Am 1997;81:85–112. 10.1016/S0025-7125(05)70506-X [DOI] [PubMed] [Google Scholar]
- 5.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 6.Dibonaventura M, Gupta S, McDonald M et al. Evaluating the health and economic impact of osteoarthritis pain in the workforce: results from the National Health and Wellness Survey. BMC Musculoskelet Disord 2011;12:83 10.1186/1471-2474-12-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira D, Peleteiro B, Araujo J et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270–85. 10.1016/j.joca.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 8.Ladd AL, Weiss AP, Crisco JJ et al. The thumb carpometacarpal joint: anatomy, hormones, and biomechanics. Instr Course Lect 2013;62:165–79. [PMC free article] [PubMed] [Google Scholar]
- 9.Haara MM, Heliovaara M, Kroger H et al. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am 2004;86-A:1452–7. [DOI] [PubMed] [Google Scholar]
- 10.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ et al. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis 2005;64:682–7. 10.1136/ard.2004.023564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodha S, Ring D, Zurakowski D et al. Prevalence of osteoarthrosis of the trapeziometacarpal joint. J Bone Joint Surg Am 2005;87:2614–18. 10.2106/JBJS.E.00104 [DOI] [PubMed] [Google Scholar]
- 12.Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage 2006;14:953–7. 10.1016/j.joca.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 13.Becker SJ, Briet JP, Hageman MG et al. Death, taxes, and trapeziometacarpal arthrosis. Clin Orthop Relat Res 2013;471:3738–44. 10.1007/s11999-013-3243-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillon CF, Hirsch R, Rasch EK et al. Symptomatic hand osteoarthritis in the United States: prevalence and functional impairment estimates from the third U.S. National Health and Nutrition Examination Survey, 1991–1994. Am J Phys Med Rehabil 2007;86:12–21. 10.1097/PHM.0b013e31802ba28e [DOI] [PubMed] [Google Scholar]
- 15.Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br 1994;19:340–1. 10.1016/0266-7681(94)90085-X [DOI] [PubMed] [Google Scholar]
- 16.Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis 1966;25:1–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Niu J, Kelly-Hayes M et al. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham Study. Am J Epidemiol 2002;156:1021–7. 10.1093/aje/kwf141 [DOI] [PubMed] [Google Scholar]
- 18.Wolf JM, Turkiewicz A, Atroshi I et al. Prevalence of doctor-diagnosed thumb carpometacarpal joint osteoarthritis: an analysis of Swedish health care. Arthritis Care Res 2014;66:961–5. 10.1002/acr.22250 [DOI] [PubMed] [Google Scholar]
- 19.Niu J, Zhang Y, LaValley M et al. Symmetry and clustering of symptomatic hand osteoarthritis in elderly men and women: the Framingham Study. Rheumatology (Oxford) 2003;42:343–8. 10.1093/rheumatology/keg110 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Xu L, Nevitt MC et al. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum 2003;48:1034–40. 10.1002/art.10928 [DOI] [PubMed] [Google Scholar]
- 21.Haugen IK, Englund M, Aliabadi P et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis 2011;70:1581–6. 10.1136/ard.2011.150078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botha-Scheepers S, Riyazi N, Watt I et al. Progression of hand osteoarthritis over 2 years: a clinical and radiological follow-up study. Ann Rheum Dis 2009;68:1260–4. 10.1136/ard.2008.087981 [DOI] [PubMed] [Google Scholar]
- 23.Dias R, Chandrasenan J, Rajaratnam V et al. Basal thumb arthritis. Postgrad Med J 2007;83:40–3. 10.1136/pgmj.2006.046300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman RD. Pain relief in osteoarthritis: the rationale for combination therapy. J Rheumatol 2004;31:5–7. [PubMed] [Google Scholar]
- 25.Bijsterbosch J, Visser W, Kroon HM et al. Thumb base involvement in symptomatic hand osteoarthritis is associated with more pain and functional disability. Ann Rheum Dis 2010;69:585–7. 10.1136/ard.2009.104562 [DOI] [PubMed] [Google Scholar]
- 26.Bellamy N, Campbell J, Haraoui B et al. Dimensionality and clinical importance of pain and disability in hand osteoarthritis: development of the Australian/Canadian (AUSCAN) Osteoarthritis Hand Index. Osteoarthritis Cartilage 2002;10:855–62. 10.1053/joca.2002.0837 [DOI] [PubMed] [Google Scholar]
- 27.Hislop Lennie K, Barbosa Boucas S, Adams J et al. Patient and public involvement (PPI) in informing the osteoarthritis of the thumb therapy (OTTER) pilot trial: what matters most to people with thumb base osteoarthritis otter collaborations. Ann Rheum Dis 2013;72:A778 10.1136/annrheumdis-2013-eular.2301 [DOI] [Google Scholar]
- 28.Gehrmann SV, Tang J, Li ZM et al. Motion deficit of the thumb in CMC joint arthritis. J Hand Surg 2010;35:1449–53. 10.1016/j.jhsa.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 29.Kjeken I, Dagfinrud H, Slatkowsky-Christensen B et al. Activity limitations and participation restrictions in women with hand osteoarthritis: patients’ descriptions and associations between dimensions of functioning. Ann Rheum Dis 2005;64:1633–8. 10.1136/ard.2004.034900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominick KL, Jordan JM, Renner JB et al. Relationship of radiographic and clinical variables to pinch and grip strength among individuals with osteoarthritis. Arthritis Rheum 2005;52:1424–30. 10.1002/art.21035 [DOI] [PubMed] [Google Scholar]
- 31.Van Den Ende C, Stukstette M, Dekker J et al. Thumb base involvement in established hand osteoarthritis. Ann Rheum Dis 2013;72(Suppl 3):A784. [Google Scholar]
- 32.Kwok WY, Vliet Vlieland TP, Rosendaal FR et al. Limitations in daily activities are the major determinant of reduced health-related quality of life in patients with hand osteoarthritis. Ann Rheum Dis 2011;70:334–6. 10.1136/ard.2010.133603 [DOI] [PubMed] [Google Scholar]
- 33.Sessle B. Unrelieved pain: a crisis. Pain Res Manag 2011;16:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veillette Y, Dion D, Altier N et al. The treatment of chronic pain in Quebec: a study of hospital-based services offered within anesthesia departments. Can J Anaesth 2005;52:600–6. 10.1007/BF03015769 [DOI] [PubMed] [Google Scholar]
- 35.Lynch ME, Campbell FA, Clark AJ et al. Waiting for treatment for chronic pain—a survey of existing benchmarks: toward establishing evidence-based benchmarks for medically acceptable waiting times. Pain Res Manag 2007;12:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng P, Choiniere M, Dion D et al. Challenges in accessing multidisciplinary pain treatment facilities in Canada. Can J Anaesth 2007;54:977–84. 10.1007/BF03016631 [DOI] [PubMed] [Google Scholar]
- 37.Lalonde L, Leroux-Lapointe V, Choiniere M et al. Knowledge, attitudes and beliefs about chronic noncancer pain in primary care: a Canadian survey of physicians and pharmacists. Pain Res Manag 2014;19:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watt-Watson J, McGillion M, Hunter J et al. A survey of prelicensure pain curricula in health science faculties in Canadian universities. Pain Res Manag 2009;14:439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hochberg MC, Altman RD, April KT et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Doherty M, Leeb BF et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:377–88. 10.1136/ard.2006.062091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valdes K, von der Heyde R. An exercise program for carpometacarpal osteoarthritis based on biomechanical principles. J Hand Ther 2012;25:251–62; quiz 263 10.1016/j.jht.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 42.Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg 2008;16:140–51. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien VH, McGaha JL. Current practice patterns in conservative thumb CMC joint care: survey results. J Hand Ther 2014;27:14–22. 10.1016/j.jht.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 44.Khorashadi L, Ha AS, Chew FS. Radiologic guide to surgical treatment of first carpometacarpal joint osteoarthritis. AJR Am J Roentgenol 2012;198:1152–60. 10.2214/AJR.11.7387 [DOI] [PubMed] [Google Scholar]
- 45.Egan MY, Brousseau L. Splinting for osteoarthritis of the carpometacarpal joint: a review of the evidence. Am J Occup Ther 2007;61:70–8. 10.5014/ajot.61.1.70 [DOI] [PubMed] [Google Scholar]
- 46.Wajon A, Vinycomb T, Carr E et al. Surgery for thumb (trapeziometacarpal joint) osteoarthritis. Cochrane Database Syst Rev 2015;2:CD004631 10.1002/14651858.CD004631.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cousins M, Breivik H, Cardosa M, et al. 2014. Desirable characteristics of National Pain Strategies: recommendations by the International Association for the Study of Pain. Secondary desirable characteristics of National Pain Strategies: recommendations by the International Association for the Study of Pain 27 September 2014. http://www.iasp-pain.org/Advocacy/Content.aspx?ItemNumber=1473.
- 48.Gatchel RJ, Peng YB, Peters ML et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- 49.Kinsman L, Rotter T, James E et al. What is a clinical pathway? Development of a definition to inform the debate. BMC Med 2010;8:31 10.1186/1741-7015-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. http://handbook.cochrane.org/ (accessed 14 May 2014).
- 51.Boers M, Kirwan JR, Tugwell P et al. The OMERACT handbook. Amsterdam, Netherlands: OMERACT, 2014. [Google Scholar]
- 52.National Institute for Health and Care Excellence. Osteoarthritis: care and management in adults. London, UK: National Institute for Health and Care Excellence, 2014. [Google Scholar]
- 53.Shea BJ, Grimshaw JM, Wells GA et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10 10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochrane Effective Practice and Organization of Practice (EPOC) Risk of Bias Tool. 2008. http://epoc.cochrane.org/fr/epoc-resources (accessed 15 June 2014).
- 55.Thomas BH, Ciliska D, Dobbins M et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176–84. 10.1111/j.1524-475X.2004.04006.x [DOI] [PubMed] [Google Scholar]
- 56.Shea BJ, Bouter LM, Peterson J et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS ONE 2007;2:e1350 10.1371/journal.pone.0001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higgins J, Altman D, Gotzsche P et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 59.Deeks JJ, Dinnes J, D'Amico R et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii–x, 1–173 10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- 60.Armijo-Olivo S, Stiles CR, Hagen NA et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 2012;18:12–18. 10.1111/j.1365-2753.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 61.Review Manager (RevMan) Version 5.1.; 2014 (November 15). http://ims.cochrane.org/revman (accessed 3 Jun 2014).
- 62.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. www.cochrane-handbook.org. [Google Scholar]
- 63.Grimshaw J. A knowledge synthesis chapter. 2010. http://www.cihr-irsc.gc.ca/e/41382.html (accessed 14 Oct 2014).
- 64.Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 65.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395–400. 10.1016/j.jclinepi.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 66.Guyatt GH, Oxman AD, Schunemann HJ et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 67.Balshem H, Helfand M, Schünemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64: 401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 68.Guyatt GH, Oxman AD, Vist G et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 69.Guyatt GH, Oxman AD, Montori V et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol 2011;64:1277–82. 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 70.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011;64:1283–93. 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 71.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 2011;64:1294–302. 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 72.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 2011;64:1303–10. 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 73.Guyatt GH, Oxman AD, Sultan S et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311–16. 10.1016/j.jclinepi.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 74.Guyatt G, Oxman AD, Sultan S et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66:151–7. 10.1016/j.jclinepi.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 75.Guyatt GH, Thorlund K, Oxman AD et al. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles—continuous outcomes. J Clin Epidemiol 2013;66:173–83. 10.1016/j.jclinepi.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 76.Andrews J, Guyatt G, Oxman AD et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–25. 10.1016/j.jclinepi.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 77.Guyatt GH, Oxman AD, Santesso N et al. GRADE guidelines: 12. Preparing Summary of Findings tables—binary outcomes. J Clin Epidemiol 2013;66:158–72. 10.1016/j.jclinepi.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 78.McMaster University. GRADEpro. [Computer program on www.gradepro.org]. 2014. http://tech.cochrane.org/revman/other-resources/gradepro/about-gradepro (accessed 22 Dec 2014).
- 79.Canadian Institutes of Health Research. Guide to knowledge translation planning at CIHR: Integrated and End-of-Grant Approaches. 2012. http://www.cihr-irsc.gc.ca/e/45321.html (accessed 24 Oct 2014).
- 80.Baumbusch JL, Kirkham SR, Khan KB et al. Pursuing common agendas: a collaborative model for knowledge translation between research and practice in clinical settings. Res Nurs Health 2008;31:130–40. 10.1002/nur.20242 [DOI] [PubMed] [Google Scholar]
- 81.Elwyn G, O'Connor A, Stacey D et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006;333:417 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.International Patient Decision Aids Standards Collaboration. IPDAS Checklist for judging the quality of patient decision aids. 2006. http://ipdas.ohri.ca/resources.html (accessed 6 Mar 2015).


