Abstract
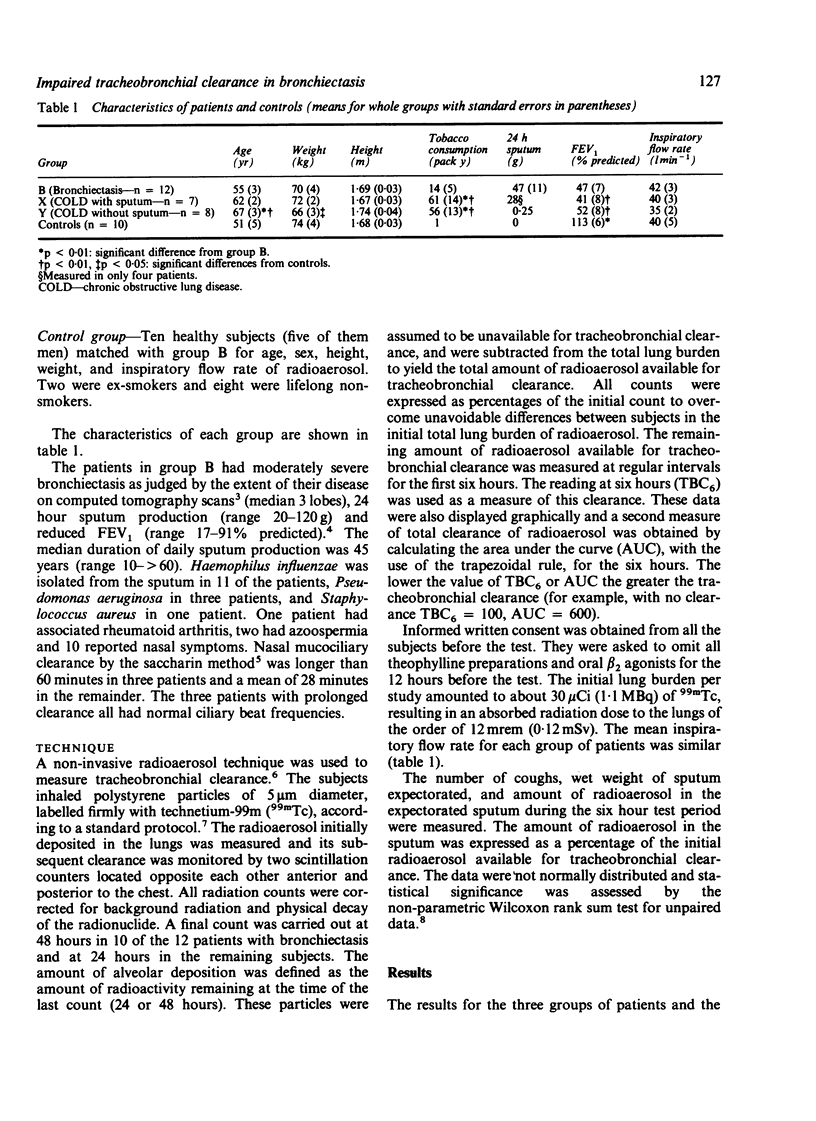
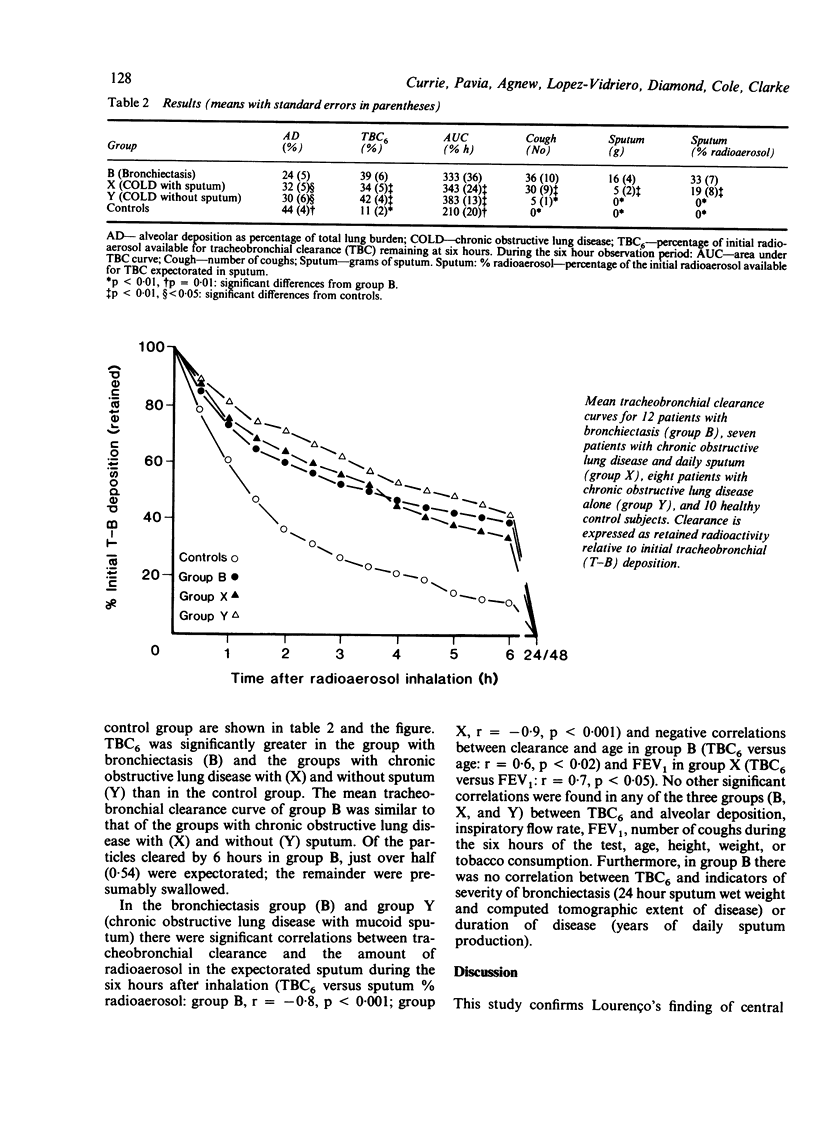
Tracheobronchial clearance was measured by a radioaerosol technique in 12 patients with bronchiectasis, seven patients with chronic obstructive lung disease expectorating mucoid sputum daily (group X), eight patients with chronic obstructive lung disease but negligible sputum expectoration (group Y), and 10 healthy subjects. The patients with bronchiectasis all expectorated purulent sputum daily (mean wet weight 47 g/day), had reduced forced expiratory volume in one second (FEV1) (mean 47.5% predicted), and were unable to avoid coughing during the six hour observation period. None of the patients with bronchiectasis or the healthy subjects were current smokers. There were five current smokers in group X and six in group Y. The mean FEV1 in group X was 41% predicted and in group Y 52% predicted, both values similar to that of the patients with bronchiectasis. Tracheobronchial clearance in the first six hours after inhalation of radioaerosol was significantly (p less than 0.01) slower in patients with bronchiectasis than in matched healthy subjects despite more proximal deposition of radioaerosol (p = 0.01) and more coughing (p less than 0.01) in the former. Tracheobronchial clearance in patients with bronchiectasis was impaired to a similar degree to that in patients with chronic obstructive lung disease but no bronchiectasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew J. E., Little F., Pavia D., Clarke S. W. Mucus clearance from the airways in chronic bronchitis--Smokers and ex-smokers. Bull Eur Physiopathol Respir. 1982 May-Jun;18(3):473–484. [PubMed] [Google Scholar]
- Dolovich M. B., Sanchis J., Rossman C., Newhouse M. T. Aerosol penetrance: a sensitive index of peripheral airways obstruction. J Appl Physiol. 1976 Mar;40(3):468–471. doi: 10.1152/jappl.1976.40.3.468. [DOI] [PubMed] [Google Scholar]
- Lourenço R. V., Loddenkemper R., Carton R. W. Patterns of distribution and clearance of aerosols in patients with bronchiectasis. Am Rev Respir Dis. 1972 Dec;106(6):857–866. doi: 10.1164/arrd.1972.106.6.857. [DOI] [PubMed] [Google Scholar]
- Mossberg B., Afzelius B. A., Eliasson R., Camner P. On the pathogenesis of obstructive lung disease. A study on the immotile-cilia syndrome. Scand J Respir Dis. 1978 Apr;59(2):55–65. [PubMed] [Google Scholar]
- Pavia D., Bateman J. R., Sheahan N. F., Agnew J. E., Clarke S. W. Tracheobronchial mucociliary clearance in asthma: impairment during remission. Thorax. 1985 Mar;40(3):171–175. doi: 10.1136/thx.40.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia D., Bateman J. R., Sheahan N. F., Agnew J. E., Newman S. P., Clarke S. W. Techniques for measuring lung mucociliary clearance. Eur J Respir Dis Suppl. 1980;110:157–177. [PubMed] [Google Scholar]
- Pavia D., Sutton P. P., Agnew J. E., Lopez-Vidriero M. T., Newman S. P., Clarke S. W. Measurement of bronchial mucociliary clearance. Eur J Respir Dis Suppl. 1983;127:41–56. [PubMed] [Google Scholar]
- Pavia D., Thomson M. L., Clarke S. W., Shannon H. S. Effect of lung function and mode of inhalation on penetration of aerosol into the human lung. Thorax. 1977 Apr;32(2):194–197. doi: 10.1136/thx.32.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman C. M., Forrest J. B., Ruffin R. E., Newhouse M. T. Immotile cilia syndrome in persons with and without Kartagener's syndrome. Am Rev Respir Dis. 1980 Jun;121(6):1011–1016. doi: 10.1164/arrd.1980.121.6.1011. [DOI] [PubMed] [Google Scholar]
- Smallman L. A., Hill S. L., Stockley R. A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984 Sep;39(9):663–667. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., MacWilliam L., Greenstone M., Mackay I., Cole P. Efficacy of a saccharin test for screening to detect abnormal mucociliary clearance. Br J Dis Chest. 1984 Jan;78(1):62–65. [PubMed] [Google Scholar]
- Tegner H., Ohlsson K., Toremalm N. G., von Mecklenburg C. Effect of human leukocyte enzymes on tracheal mucosa and its mucociliary activity. Rhinology. 1979 Sep;17(3):199–206. [PubMed] [Google Scholar]
- Thomson M. L., Pavia D. Particle penetration and clearance in the human lung. Results in healthy subjects and subjects with chronic bronchitis. Arch Environ Health. 1974 Oct;29(4):214–219. doi: 10.1080/00039896.1974.10666571. [DOI] [PubMed] [Google Scholar]
- Thomson M. L., Short M. D. Mucociliary function in health, chronic obstructive airway disease, and asbestosis. J Appl Physiol. 1969 May;26(5):535–539. doi: 10.1152/jappl.1969.26.5.535. [DOI] [PubMed] [Google Scholar]
- Waite D. A., Wakefield S. J., Mackay J. B., Ross I. T. Mucociliary transport and ultrastructural abnormalities in Polynesian bronchiectasis. Chest. 1981 Dec;80(6 Suppl):896–898. doi: 10.1378/chest.80.6.896. [DOI] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Currie D., Cole P. J. Beat frequency of cilia from sites of purulent infection. Thorax. 1986 Jun;41(6):453–458. doi: 10.1136/thx.41.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]



