Abstract
Double unit cord blood (CB) transplantation (CBT) appears to augment engraftment despite only one unit engrafting in most patients. We hypothesized that superior unit quality, as measured by a higher percentage of viable cells post-thaw, would determine the engrafting unit. Therefore, we prospectively analyzed 46 double unit transplants post-thaw using flow cytometry with modified gating that included all dead cells. Using a 75% threshold (mean viability minus 2SD), 20% of units had low CD34+ cell viability, with viability varying according to the bank of origin. Further, in the 44 patients with single unit engraftment, CD34+ cell viability was higher in engrafting units (p=0.0016). While either unit engrafted if both had high CD34+ viability, units with <75% viability were very unlikely to engraft: in 16 patients that received one high and one low CD34+ viability unit, only 1/16 units with viability <75% engrafted (p=0.0006). Further, in the single patient without engraftment of either unit, both had CD34+ viability <75%. Finally, poor CD34+ viability correlated with lower CFUs (p=0.02). Our data suggests one mechanism by which double unit CBT can improve engraftment is by increasing the probability of transplanting at least one unit with adequate viability and the potential to engraft.
Introduction
Although cord blood (CB) is increasingly used as an alternative hematopoietic stem cell (HSC) source that promises to extend transplant access to patients of racial and ethnic minorities, low total nucleated cell (TNC) dose is often limiting1–4. Double unit CB transplantation (CBT) can frequently overcome the cell dose limitations of single unit grafts in adults and large children5–10. This strategy improves engraftment compared with historical controls, despite only one unit giving rise to donor hematopoiesis in most patients8. Further, preliminary data suggest that the double unit approach may be associated with a reduced risk of relapse11,12. Therefore, the mechanisms that determine engraftment and unit predominance are of great interest but have not been elucidated. Previous studies have compared the infused doses of TNC, CD34+ progenitors, or CD3+ T cells of the two units. Although one study found an association between higher CD3+ cell dose and the engrafting unit8, this variable did not predict engraftment in individual patients. We hypothesized that superior unit quality, as measured by a higher percentage of viable cells post-thaw, would determine the engrafting unit in double unit CBT. Processing, freezing, storage, transport, or thawing of a unit can damage CB cells at any point, and evaluation of cell numbers alone cannot accurately assess the degree of injury. We hypothesized that the proportion of dead cells in a unit reflects the degree of damage to the entire unit. Therefore, we evaluated the effect of viability (percent viable cells) of CB cell subpopulations (CD45+, CD34+, and CD3+ cells) post-thaw on unit engraftment, in a prospective series of 46 consecutive double unit CBT recipients.
Methods
Patients and Treatment Plan
All CBT recipients or their parents signed informed consent before transplantation. Patients had a median age of 37 years (range: 7–65) and a median weight of 72 kg (range: 22–109). All had high-risk hematologic malignancies: acute myelogenous leukemia (N=11), acute lymphoblastic leukemia (N=6), acute biphenotypic leukemia (N=2), non-Hodgkin lymphoma (N=14), Hodgkin lymphoma (N=9), chronic lymphocytic leukemia (N=3), and prolymphocytic leukemia (N=1). Conditioning was myeloablative (N=31) or non-myeloablative (N=15) according to age, diagnosis, extent of prior therapy, and co-morbidities, with all regimens including fludarabine. All patients received immunosuppression with cyclosporine-A and mycophenolate mofetil, and engraftment support with post-transplant granulocyte-colony stimulating factor (G-CSF). Recipients of a prior allogeneic transplant were not included in this study.
CB Unit Selection
CB units were selected according to TNC dose and HLA-match, and were obtained from domestic (N=71) and international CB banks (N=21 units). CB units were matched to the patient at 4–6/6 HLA-A,-B antigens, and -DRB1 alleles [6/6 (N=5, 5%), 5/6 (N=42, 46%), and 4/6 (N=45, 49%)]. HLA-matching between the two units was 6/6 (N=2), 5/6 (N=11), 4/6 (N=18), 3/6 (N=12) and 2/6 (N=3). High-resolution HLA typing at HLA-A,-B,-C,-DRB1,-DQB1 alleles was performed on all patients and donor units although the allele matching of loci other than HLA-DRB1 was usually not considered in unit selection. The donor-recipient HLA-match at high resolution ranged 2–9/10. All patients received double unit grafts. The larger unit had a median infused TNC dose of 2.5 × 107/kg (range: 1.4–4.5), and the smaller had a median TNC of 1.9 × 107/kg (range: 0.9–3.7).
CB Unit Thaw and Infusion
Initially units were thawed with albumin-dextran dilution followed by centrifugation13. However, in an effort to reduce cell loss and unit manipulation, we subsequently changed our thaw strategy to a dilution without centrifugation for CBT recipients ≥20 kg14. Hence, the majority of CB units (N=83, 90%) were thawed using the “no wash” technique, whereas 9 CB units were thawed and washed with centrifugation. Units were infused within 2 hours of thawing with an interval of less than 45 minutes between the infusion of the first and second unit.
Post-thaw Evaluations
Post-thaw TNC
Post-thaw TNC count was obtained using an automated hematology Coulter counter. Nucleated cell viability was assessed by trypan blue exclusion as per standard laboratory procedures.
Flow cytometric evaluation
Four color flow cytometric evaluation of the CB units was performed using a FACS Calibur flow cytometer (Becton Dickinson Biosciences, San Jose, CA) and CellQuest Pro software (BD Biosciences). Samples for flow cytometric analysis were taken from the final product prior to its release to the transplant floor and were stained within one hour of thaw. Duplicate aliquots containing 0.5 × 106 CB cells were incubated with anti-CD45 FITC (BD #340664), anti-CD34 PE (Beckman Coulter #IM1871), and anti-CD3 APC (BD #340661) at room temperature in the dark for 20 minutes. Red blood cells (RBC) were lysed with fixative-free ammonium chloride (10X NH4Cl Lysing Solution, Beckman Coulter #IM3514, diluted with reagent grade water to 1X). 7-amino-actinomycin D (7AAD) (Beckman Coulter #IM3422) was then added. Samples were vortexed, kept at room temperature in the dark for 15 minutes, and then stored for <1 hour on wet ice in the dark until acquisition. Because most units underwent albumin-dextran dilution, and cells were not washed during the staining, dead cells were not removed at any step during sample preparation.
Flow cytometric evaluation was performed using the International Society of Hematotherapy and Graft Engineering (ISHAGE) gating strategy15 with several modifications to maximize the detection of live and dead cells. Compensations were set to avoid spectral overlap for CD34 PE and 7AAD. This was achieved by initially determining the appropriate photo multiplier tube (PMT) and compensation settings and then periodically checking the compensation settings with single stains. Heat killed or lysed cells were used to establish the 7AAD compensation, and compensation settings were confirmed with each sample by checking two-color dot plots.
While the essentials of the ISHAGE sequential gating strategy were retained, we lowered the forward scatter (FSC) threshold to include all 7AAD-positive dead cells and viewed the 7AAD versus FSC instead of the traditional 7AAD versus side scatter (SSC) dot plot. More debris was acquired with the lowered FSC threshold but this was easily excluded with the modified 7AAD versus FSC dot plot because debris is 7AAD-negative with a lower FSC than cells. Therefore, a “Not Debris” region can be set so that debris is excluded from all subsequent dot plots. Figure 1A, Plot 1 shows the FSC versus SSC dot plot in which the FSC primary threshold was adjusted to exclude most debris and RBC, but not the dead cells. Similarly, the FL1 secondary threshold was adjusted on the CD45 FITC versus SSC dot plot to exclude most red cells, platelets, and other debris but not any CD45+ cells (not shown). The sequential Boolean gating strategy as defined by ISHAGE was then applied to identify CD34+ cells. Specifically, we gated on CD34+ antigen expression, followed by a gate on CD45+ low to intermediate antigen expression with fluorescence intensity characteristic of blast cells, and finally we set a FSC versus SSC gate with low SSC and low to intermediate FSC characteristic of lymphocytes and blast cells. We assured that the lower boundary of the lymph-blast gate included the smallest lymphocytes. In this sequential gating strategy, monocytes are excluded because they express high levels of CD45 and increased SSC in comparison with true CD34+ cells, whereas granulocytes are excluded since they exhibit markedly higher SSC characteristics.
Figure 1. Flow cytometric evaluation of CB units post-thaw.


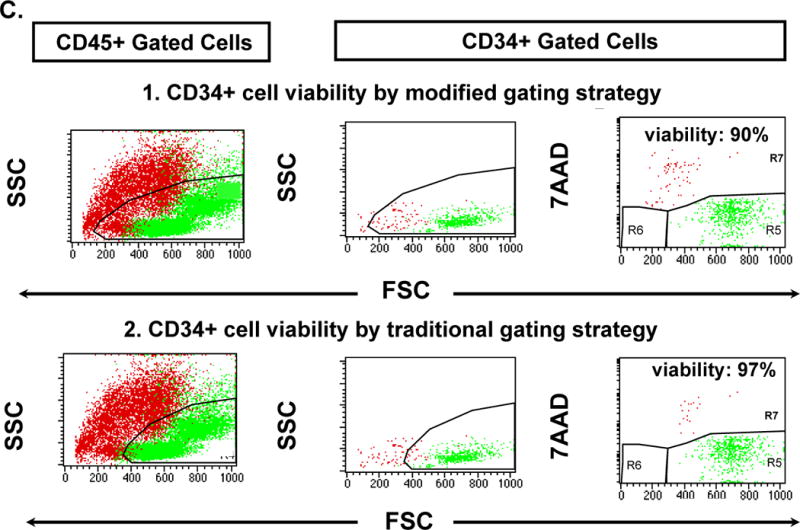
Live cells are shown in green, dead cells are shown in red, and debris is shown in black.
A. Modified gating strategy. Plot 1: The lower FSC primary threshold excludes most debris but no dead cells. Plot 2: Viability is assessed in the FSC versus 7AAD dot plot. Debris is shown in R6 and is gated out in all subsequent plots. Dead cells (7AAD-positive) are in R7 whereas live cells (7AAD-negative) are in R5. Plot 3: For comparison traditional gating cannot adequately distinguish debris from viable cells in the 7AAD versus SSC dot plot. Live and dead cells are shown for CD45+ (Plot 4), CD34+ (Plot 5), and CD3+ (Plot 6) cell populations. Viability was calculated from the total debris-free sample for CD45+ cells (Plot 7), CD34+ cells (Plot 8), and CD3+ cells (Plot 9). An average of 300 events (range: 100–1000) were acquired per sample for the CD34+ cell analysis.
B. Traditional gating strategy. The same plots as in 1A are shown with a high FSC threshold that excludes most dead cells, as frequently used in traditional gating. In CB units with a high percentage of dead cells the traditional methodology over-estimates cell viability compared with our modified gating.
C. Comparison of CD34+ cell viability using modified and traditional gating in CB units with a small percentage of dead cells. The difference between the two gating strategies is small.
Viability was then evaluated on the FSC versus 7AAD dot plot (Figure 1A, Plot 2) instead of the traditional SSC versus 7AAD plot (shown in Figure 1A, Plot 3 for comparison). In our modified gating strategy shown in Figure 1A, Plot 2, to more clearly distinguish dead cells from debris, events were gated to encompass all 7AAD-negative (viable) cells in region 5 (R5), all 7AAD-positive (dead) cells in R7, and all low FSC 7AAD-negative debris in R6. Live and dead cells were evaluated as shown for CD45+ (Figure 1A, Plot 4), CD34+ (Figure 1A, Plot 5), and CD3+ (Figure 1A, Plot 6) cell populations. Notably, Figure 1A, Plot 5, clearly shows that the CD34+ cells are low SSC. Viability was calculated from the total debris-free sample for CD45+ cells (Figure 1A, Plot 7), CD34+ cells (Figure 1A, Plot 8), and CD3+ cells (Figure 1A, Plot 9). Initially, we performed isoclonic (CD34+) and isotype (CD3+) controls, but due to the multi-parameter gating using Boolean logic they were found to be redundant, and were abandoned as recommended by Gratama et al16 and Kenney et al17. Periodically, we stain controls to assure that non-specific staining is minimal.
Our modified gating strategy allowed the acquisition of all dead (7AAD-positive) cells and viable (7AAD-negative) cells so that viability was determined from the total, debris-free populations. Figure 1B demonstrates the overestimation of the percent viability by traditional gating compared with the modified gating strategy in Figure 1A. This is especially evident in units with many dead cells as shown in this example. In contrast, both traditional and modified gating strategies resulted in similar CD34+ viabilities in CB units with few dead cells, as shown in Figure 1C. Absolute CD34+ and CD3+ cell numbers were determined using the two-platform method.
Colony-forming unit (CFU) assays
CFU assays were performed on all CB units using a total of 1 × 105 cells plated in duplicate. Colony growth was evaluated by light microscopy at 14 days.
Engraftment, Donor Chimerism, and Unit Predominance
Neutrophil engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count (ANC) ≥0.5 × 109/L after the post-transplant nadir. Serial sampling of the bone marrow and/or peripheral blood at days 21, 28, 60, 100, 180, and 360 after transplantation determined donor chimerism using quantitative polymerase chain reaction of informative polymorphic DNA short tandem repeats (STR) in recipient and donor units (GenePrint Fluorescent STR Marker Kit, Promega)8. The engrafting unit contributed >50% of the total donor chimerism in serial testing.
Statistical Analysis
Statistical analyses were performed using SPSS (v12.0, Chicago, IL) or Excel software. A p value <0.05 was significant. Differences between categorical variables were estimated by chi-square and between means by the Student t test (paired).
Results
Engraftment and Donor Chimerism
Forty-five of the 46 patients (98%) had donor engraftment in the bone marrow by DNA analysis on day 21 post-transplant. One unit engrafted in 44 of these 45 patients while a single patient had sustained engraftment of both units. The single patient without engraftment of either unit received non-myeloablative conditioning and had autologous recovery.
The median donor chimerism of the engrafting unit was 100% in recipients of myeloablative conditioning at all time points tested. Recipients of non-myeloablative conditioning initially had mixed donor-host chimerism due to transient recovery of autologous hematopoiesis but attained 100% donor with the engrafting unit by day 60. While a minority of patients had a small contribution from the non-engrafting unit early after transplant, the median percent chimerism of the non-engrafting unit was 0% at all time points with both types of conditioning.
Of the 45 patients with donor engraftment by DNA analysis, 43 had donor-derived neutrophil recovery with a median time to ANC ≥0.5 × 109/L of 25 days (range: 13–38) after myeloablative and 11 days (range: 7–36) after non-myeloablative conditioning. The two patients who engrafted by DNA analysis but did not have sustained neutrophil recovery received myeloablative conditioning and suffered lethal infections early post-transplant. One developed Staphylococcus aureus septicemia on day 7, and the other had cytomegalovirus disease at day 20. One additional patient, also a recipient of myeloablative conditioning, had secondary graft failure in the setting of human herpes virus-6 infection.
Post-thaw CD34+ Cell Viability and Unit Engraftment
Figure 2 depicts the flow cytometric cell viabilities of the engrafting and non-engrafting units in the 44 patients with single unit engraftment in the bone marrow. The mean CD34+ cell viability of the 44 units that engrafted in these patients was 88% (SD=6.5%). CD34+ cell viability was significantly higher in the engrafting units (p=0.0016), and units with a high proportion of dead cells (i.e. low viability) did not engraft. The numbers of engrafting and non-engrafting units were compared at various viability thresholds using a chi-squared analysis (Table 1). A clear distinction was seen at a CD34+ viability threshold of 75% which represented two standard deviation (SD) below the mean of the overall distribution. Among the 44 patients (88 units) with engraftment of a single unit, 16 received grafts consisting of one high and one low viability unit. In these 16 patients only one of 16 (6%) units with CD34+ cell viability <75% engrafted. In the other 28 patients both units had viability over 75% and either engrafted. Thus, in these patients with engraftment of a single unit, 43 of 72 (60%) engrafted with a unit with high viability (Table 2). Notably, the single patient without evidence of any donor hematopoiesis post-transplant received two units, each of which had CD34+ viability <75% (74% and 36%, respectively). Thus, overall, only 1 of 18 units (6%) with CD34+ viability <75% engrafted. The patient with stable engraftment of both units received two units with CD34+ viability ≥75% (both were 86%).
Figure 2. Post-thaw CD34+, CD3+ and CD45+ cell viability and unit engraftment in 44 double unit CB grafts.

The distribution of post-thaw viabilities of the CD34+, CD3+, and CD45+ cells are shown for the engrafting (in closed symbols) and non engrafting units (open symbols) for the 44 patients with single unit engraftment.
Table 1.
Analysis of CD34+ and CD3+ cell viability thresholds for unit engraftment.
| CD34+ Cell Viability of Engrafting CB Unit | Viability Threshold | P value |
|---|---|---|
| Mean viability | 88.0% | 0.56 |
| Mean − SD | 81.5% | 0.016 |
| Mean − 2SD | 75.0% | 0.0006 |
| Mean − 3SD | 68.5% | 0.048 |
| CD3+ Cell Viability of Engrafting CB Unit | Viability Threshold | P value |
| Mean viability | 84.0% | 0.15 |
| Mean − SD | 73.4% | 0.039 |
| Mean − 2SD | 62.8% | 0.11 |
| Mean − 3SD | 52.2% | 0.048 |
Table 2.
Post-thaw CD34+ cell viability and unit engraftment in 44 recipients (88 units) of double unit CB grafts who engrafted with a single unit.
| % CD34+ Cell Viability | Engrafting CB Unit (N, %) | Non-Engrafting CB Unit (N, %) |
|---|---|---|
| <75% | 1 (6%) | 15 (94%) |
| ≥75% | 43 (61%) | 29 (39%) |
| All units | 44 (50%) | 44 (50%) |
The mean post-thaw CD34+ cell viability for the engrafting units was 88%, SD was 6.5%. Using CD34+ viability of 75% (i.e., mean − 2SD), all but one of the engrafting units had CD34+ cell viability ≥75% (p=0.0006). The single patient with engraftment of both units (both with high viability) is excluded.
CD3+ cell viability correlated with CD34+ cell viability (R2=0.47, p<0.01) and with unit engraftment (p=0.0077). The mean CD3+ cell viability of the engrafting units was 84% (SD=10.6%). An analysis was performed comparing the number of engrafting and non-engrafting CB units, using similar viability thresholds of CD3+ cells as for CD34+ cells, but the results were not highly significant (Table 1). CD45+ viability correlated weakly with CD34+ cell viability (R2=0.3, p<0.01). There was no difference in CD45+ cell viability between the units that engrafted and those that did not (p=0.07). Moreover, post-thaw evaluation of nucleated cells by trypan blue exclusion had no correlation with CD34+ cell viability (R2=0.008) or unit engraftment (p=NS).
Post-thaw CD34+ cell viability correlated with post-thaw total CFU output. CB units with CD34+ cell viability ≥75% had a significantly higher median total CFU of 224 (range: 18–722) as compared to units with CD34+ cell viability <75% that had a lower median total CFU of 118 (range: 1–493) (p=0.02).
Figure 3 shows the post-thaw CD34+ cell viability of all CB units used in the study (N=92) according to the CB bank of origin. While units of high viability were obtained from both domestic and international banks, viability of units varied between banks with some banks (both domestic and international) providing a disproportionate number of low viability units. We did not detect a difference in CD34+ viability according to the method of thaw (data not shown).
Figure 3. Post-thaw CD34+ cell viability and CB bank of origin (N=92 units).

CB units were obtained from domestic (N=71), and international banks (INT, N=21). Each group depicts the distribution of viability for the respective units from each bank (for the domestic) or each country (for the international ones).
Other Graft Characteristics and Unit Engraftment
Infused cell and CFU doses were examined for their relationship with engraftment in the 44 patients with engraftment of a single unit (Table 3). As in previous analyses of double unit engraftment8, the absolute numbers of infused TNC/kg and infused viable CD34+ progenitor cells/kg were not associated with unit engraftment in univariate analyses. Engrafting units had significantly higher infused viable CD3+ cells/kg (p=0.03), although the absolute CD3+ cells/kg dose did not always predict the engrafting unit in individual patients. CFU/kg and CFU-GM/kg were not associated with the engrafting unit except in one patient where one of the two units had no CFU growth and failed to engraft18.
Table 3.
Post-thaw mean cell doses in engrafting and non-engrafting units of the 44 double unit CBT recipients with single unit engraftment.
| Engrafting CB Unit | Non-Engrafting CB Unit | p | |
|---|---|---|---|
| Infused TNC/kg (×107) | 2.2 | 2.2 | 0.90 |
| Infused viable CD34+/kg (×105) | 1.1 | 0.9 | 0.13 |
| Infused viable CD3+/kg (×105) | 38.6 | 31.4 | 0.03 |
| CFU/kg (×104) | 3.7 | 3.2 | 0.31 |
| CFU-GM/kg (×104) | 1.6 | 1.5 | 0.90 |
Also as previously reported8, donor-recipient HLA-matching at HLA-A,-B antigens and -DRB1 alleles was not associated with unit engraftment in this study. In the 44 patients with single unit engraftment, 12 received units with different HLA-match levels, and the better matched unit engrafted in only 3 cases. Each unit differed in the donor-recipient HLA-A,-B,-C,-DRB1, and -DQ allele match in 25 patients. The better HLA-matched unit engrafted in only 10 of them (p=NS). Notably, however, the patient with sustained engraftment of both donors received units that were each 9/10 HLA-matched to the recipient and to each other (each had a single mismatch at HLA-A). This was the only patient who received units so closely matched by high resolution HLA typing.
Finally, the order of unit infusion did not predict the engrafting unit. Of the 44 patients who had donor hematopoiesis from a single unit, the engrafting unit was infused first in 23 and second in 21 patients (p=NS).
Discussion
We prospectively investigated the correlates of donor engraftment after double unit CBT in 46 consecutive patients. In contrast to the traditional gating where dead cells can be excluded15 we used modified flow cytometric gating that excludes only erythrocytes and debris to evaluate the number of live and dead cells. With this methodology, an approach that is appropriate for the assessment of all cryopreserved hematopoietic products, all dead cells in the graft are counted and therefore the proportions of dead and live cells can be calculated precisely. Using this modified gating strategy we showed significant differences in the post-thaw viabilities of CD34+ and CD3+ cells between engrafting and non-engrafting CB units. Although it is unclear how widely flow based viability has been adopted by transplant centers, the modification of traditional gating to incorporate a 7AAD viability analysis has previously been reported19,20. Further, the potential clinical importance of measuring the viable cell dose has been appreciated when administering hematopoietic grafts such as donor lymphocytes21. However, the significance of the percentage of viable cells in a cryopreserved product as a surrogate of product quality is a novel finding. We found the percentage of viable CD34+ cells as measured by the modified gating, but not the infused CD34+ dose/kg, proved to be the most critical determinant of the engraftment potential of a CB unit in double unit CBT (Figure 2, and Tables 1 and 2). This concept can be explained by the example in Figure 4 where two units with similar absolute CD34+ cell doses/kg differ widely in their percentages of viable CD34+ cells and thus their quality. Units with a low percentage of viable CD34+ cells have had a significant proportion of the CD34+ cells destroyed. The remaining cells, although viable as determined by 7AAD staining, are likely also damaged, thereby compromising the engraftment potential of the entire unit. The correlation of percent CD34+ cell viability with the respective values of CD45+ and CD3+ cell viability support this hypothesis, as does the association between lower CFU counts and units with poor CD34+ cell viability.
Figure 4. Schema depicting the difference between the dose of viable CD34+ cells/kg versus the percentage of viable CD34+ cells in two CB units.

The infused viable CD34+ cells per kg of the recipient weight (CD34+ cell dose) and the percent CD34+ cell viability (7AAD-positive CD34+ cells divided by the total CD34+ cells in the unit expressed as a percentage) represent two different graft characteristics. In this example the two CB units have the same total number of viable CD34+ cells (and would have an identical infused CD34+ cell dose). However, the percentage of viable CD34+ cells is markedly different. Based on the results of this study unit #1 is of poor quality and we would predict that unit #2 would engraft in the double unit setting.
We, therefore, propose that double unit CBT increases the probability that the patient will receive at least one unit with adequate viability and thus with the potential to engraft. To facilitate clinical decisions, however, an appropriate viability “threshold” is needed. Using 75% viability, which represented the mean of the CD34+ distribution minus 2 SD, had clinical utility as units with CD34+ cell viability below 75% had a low probability of engraftment (p=0.0006). In fact, only one of the 18 units with CD34+ cell viability <75% engrafted. Using this threshold a further major finding of our study was that low viability units were not uncommon: 18/92 (20%) had CD34+ cell viability <75%. This percentage is similar to the incidence of graft failure reported in many series of single unit CBT (reviewed in22,23). Therefore, while the significance of viability in single unit CBT cannot be determined from this study, the correlation of viability and engraftment in single unit CBT is of great interest. We are not able to evaluate this as in an attempt to augment engraftment, and possibly protect against relapse11,12, we have only performed CBT using double unit grafts to date.
It is intriguing to hypothesize that the only unit with CD34+ cell viability <75% that engrafted in our study may have sustained a different type of injury than the others. It is also likely that engraftment may be influenced by additional factors such as the infused TNC dose. Units with a very high TNC dose may engraft despite low CD34+ cell viability if they contain sufficient numbers of unaffected cells. Our patients received only a mean TNC dose of 2.2 × 107/kg (SD=0.69). Thus, the effect of low CD34+ cell viability on the engraftment of high cell dose units could not be evaluated.
Our findings highlight the importance of CB unit quality and have significant implications for both CB banks and transplant centers. Notably, not only did we find variable viability from unit to unit, we also found marked differences in the CD34+ cell viability of units obtained from different individual CB banks (Figure 3) with some banks providing a disproportionate number of low viability units. This was despite the fact that units were stored in a uniform manner at our center and likely indicates the extent to which varying bank practices may alter product quality, and could not be attributed to shipping distance since many international units had high viability. What specific banking practices could lead to this finding could not be analyzed as this information is not provided to the transplant centers. Future studies involving both banks and transplant centers need to investigate the events that could adversely affect viability during CB collection, processing, cryopreservation, long-term storage, and transport, as well as short-term storage at the transplant center. Criteria of product quality also merit standardization and implementation to optimize the global CB inventory.
These findings also have multiple ramifications for transplant centers. Centers must determine how they will assess unit quality and what threshold is acceptable. Based on the results of this study we now define an adequate graft as two units with at least one with CD34+ cell viability ≥75%. Our findings have prompted us to perform double unit transplants exclusively at MSKCC to date as the risk of having a single unit graft with poor viability on the day of transplant is not acceptable given the challenging logistics of urgently obtaining a second unit which may not be readily available.
While this analysis demonstrated that units with low CD34+ viability are very unlikely to engraft, it does not elucidate the mechanism of CB unit predominance when both units of a double unit graft have high viability. In this scenario we postulate that either unit has the potential to engraft and that immune mediated phenomena may dictate engraftment. This is supported by the engrafting unit having a higher CD3+ dose/kg in this analysis, as previously reported, as well as preliminary results of transplants in immunodeficient mice given aliquots from each unit of our patient’s grafts as single unit grafts and in combination24. Further, it is interesting that the single patient with stable mixed donor engraftment was the only one who received two units with high viability that were both also unusually highly matched at 9/10 HLA-alleles to the patient and to each other. We propose that in this case each unit had engraftment potential, but each was tolerant of the other permitting co-engraftment.
In summary, units with low viability, and thus poor quality, are very unlikely to engraft in double unit CBT. Centers should ensure that at least one of the units of the graft has a significant proportion of viable CD34+ and CD3+ cells, without depending on trypan blue exclusion or traditional ISHAGE gating for evaluation of unit quality. Our viability assay is easily performed and available on transplant day, in contrast to CFU assay results that require two additional weeks. In the future, it would be preferable that a similar assay could be used to evaluate viability post-cryopreservation but prior to unit thaw, using the cells from an attached segment for example. Development of such an approach should be a major priority. Moreover, our results merit investigation in larger patient series of both single and double unit transplants in the multi-center setting. Important aspects of these studies will be the standardization of the CD34+ cell viability assay, the effect of the thaw method (wash versus no wash) on the accurate calculation of dead versus viable cells, and whether transplantation with high quality units speeds neutrophil and platelet engraftment.
Acknowledgments
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Society, the Translational and Integrative Medicine Research Grant, and P01 CA23766 from the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
A.S. performed the research, analyzed and interpreted the data and wrote the manuscript, K.S, R.H., S.L., A.S., M.A., N.A.K. performed the research, J.W.Y. wrote the manuscript and J.N.B. directed and supervised the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
References
- 1.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 4.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JN, Verfaillie CM, McGlave P, et al. Creation of a Double Chimera by Transplantation of Two Unrelated Donor Umbilical Cord Blood Units. Blood. 2000;96:207a. [Google Scholar]
- 6.De Lima M, St John LS, Wieder ED, et al. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–776. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 8.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of Two Partially HLA-Matched Umbilical Cord Blood Units To Enhance Engraftment in Adults with Hematologic Malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 9.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein C, Barker JN, Weisdorf DJ, et al. Umbilical Cord Blood Transplantation after Non-myeloablative Conditioning: Impact on Transplant Outcomes in 110 Adults with Hematological Disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 12.Verneris MR, Brunstein C, Barker JN, MacMillan M, DeFor TE, McKenna D, Burke M, Blazar B, Miller JS, McGlave P, Weisdorf D, Wagner JE. Relapse Risk After Umbilical Cord Blood Transplantation: Enhanced Graft Versus Leukemia Effect in Recipients of Two Units. Blood. 2009 doi: 10.1182/blood-2009-05-220525. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker JN, Abboud M, Rice RD, Hawke R, Schaible A, Heller G, La Russa V, Scaradavou A. A “No-wash” Albumin-Dextran Dilution Strategy for Cord Blood Unit Thaw: High Rate of Engraftment and a Low Incidence of Serious Infusion Reactions. Biol Blood Marrow Transplant. 2009 doi: 10.1016/j.bbmt.2009.08.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 16.Gratama JW, Orfao A, Barnett D, et al. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells. European Working Group on Clinical Cell Analysis. Cytometry. 1998;34:128–142. doi: 10.1002/(sici)1097-0320(19980615)34:3<128::aid-cyto3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Keeney M, Gratama JW, Chin-Yee IH, Sutherland DR. Isotype controls in the analysis of lymphocytes and CD34+ stem and progenitor cells by flow cytometry–time to let go! Cytometry. 1998;34:280–283. [PubMed] [Google Scholar]
- 18.Yoo KH, Lee SH, Kim HJ, et al. The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant. 2007;39:515–521. doi: 10.1038/sj.bmt.1705629. [DOI] [PubMed] [Google Scholar]
- 19.Keeney M, Sutherland DR. Stem cell enumeration by flow cytometry: current concepts and recent developments in CD34+ cell enumeration. Cytotherapy. 2000;2:395–402. doi: 10.1080/146532400539242. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland DR, Nayyar R, Acton E, Giftakis A, Dean S, Mosiman VL. Comparison of two single-platform ISHAGE-based CD34 enumeration protocols on BD FACSCalibur and FACSCanto flow cytometers. Cytotherapy. 2009:1–11. doi: 10.1080/14653240902923161. [DOI] [PubMed] [Google Scholar]
- 21.Koehl U, Bochennek K, Esser R, et al. ISHAGE-based single-platform flow cytometric analysis for measurement of absolute viable T cells in fresh or cryopreserved products: CD34/CD133 selected or CD3/CD19 depleted stem cells, DLI and purified CD56+CD3- NK cells. Int J Hematol. 2008;87:98–105. doi: 10.1007/s12185-007-0018-7. [DOI] [PubMed] [Google Scholar]
- 22.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 23.Chan KW, Grimley MS, Taylor C, Wall DA. Early identification and management of graft failure after unrelated cord blood transplantation. Bone Marrow Transplant. 2008;42:35–41. doi: 10.1038/bmt.2008.40. [DOI] [PubMed] [Google Scholar]
- 24.Eldjerou LK, Chaudhury S, He M, et al. Graft-Vs-Graft Immune Interaction Is the Likely Mechanism of Absolute Unit Dominance in Double Unit Cord Blood (DCB) Transplantation Using Patient DCB Grafts: An in Vivo but Not in Vitro Phenomenon. Blood. 2008;112:1195a. [Google Scholar]


