Abstract
Introduction
Hemorrhagic shock (HS) followed by a subsequent insult (“second hit”) often initiates an exaggerated systemic inflammatory response and multiple organ failure. We have previously demonstrated that valproic acid, a pan histone deacetylase (HDAC) inhibitor, could improve survival in a rodent “two-hit” model. In present study, our goal was to determine whether selective inhibition of histone deacetylase 6 with Tubstatin A (Tub-A) could prolong survival in a 2-hit model where HS was followed by sepsis from cecal ligation and puncture (CLP).
Methods
C57Bl/6J mice were subjected to sublethal HS (30% blood loss) and then randomly divided into 2 groups (n=13/group): Tub-A group (treatment) and vehicle group (control). The Tub-A group was given an intraperitoneal injection of Tub-A (70mg/kg) dissolved in dimethyl sulfoxide (DMSO). The vehicle group was injected with 1 μl/g DMSO. After 24 h, all mice were subjected CLP followed immediately by another dose of Tub-A or DMSO. Survival was monitored for 10 days. In a parallel study, peritoneal irrigation fluid and liver tissue from Tub-A or DMSO treated mice were collected 3h after CLP. Enzyme-linked immunosorbent assay was performed to quantify activity of the myeloperoxidase (MPO) and concentrations of tumor necrosis factor-alpha (TNF-α) and interleukin -6 (IL-6) in the peritoneal irrigation fluid. RNA was isolated from the liver tissue and real-time PCR was performed to measure relative mRNA levels of TNF-α and IL-6.
Results
Treatment with Tub-A significantly improved survival compared to the control (69.2% vs. 15.4%). In addition, Tub-A significantly suppressed MPO activity (169.9 ± 8.4 ng/ml vs. 70.4 ± 17.4ng/ml; p < 0.01), and reduced levels of cytokines TNF-α and IL-6 in the peritoneal fluid (TNF-α: 105.7 ± 4.7 pg/ml vs. 7.4 ± 2.4 pg/ml; IL-6: 907.4 ± 2.3 pg/ml vs. 483.6 ± 1.6 pg/ml; p < 0.01) compared to vehicle control. Gene expression measured by real-time PCR confirmed that Tub-A inhibits transcription of TNF-α and IL-6.
Conclusion
Tubastatin A treatment significantly improves survival, attenuates inflammation and down-regulates TNF-α and IL-6 gene expression in a rodent two-hit model.
Keywords: Hemorrhagic shock, two-hit, Tubastatin A, HDAC6, mouse
INTRODUCTION
Hemorrhagic shock (HS) is a major cause of morbidity and mortality among trauma patients, while septic shock (SS) is a leading cause of mortality in the intensive care units [1]. Patients who survive the acute episode of blood loss, often exhibit a systemic inflammatory response syndrome (SIRS), which can be further complicated by immune dysfunction [2]. The combination of hemorrhage and subsequent sepsis in trauma patients (two hit insult) is considered to be a major reason for the development of multiple organ failure and death in trauma patients [3]. Despite advances in supportive treatments, the mortality and morbidity remain high with a substantial burden on the healthcare system [4].
Sepsis is classically attributed to hyperinflammatory responses that result in excessive production of cytokines, which can lead to cellular injury and organ dysfunction [5]. It has been shown that shock decreases the acetylation of nuclear and cytoplasmic proteins, which in turn impairs gene transcription and the function of multiple pathways that are involved in cell survival [6]. Inhibition of histone deacetylase (HDAC) can induce protein acetylation. It has been reported that histone hyperacetylation results in up-regulation of cell cycle inhibitors (p21Cip1, p27Kip1, and p16INK4), repression of inflammatory cytokines [interleukin (IL)-1, IL-8, tumor necrosis factor-α (TNF-α)], and down-regulation of immune stimulators (IL-6, IL-10, and CD154) [7]. Our team has previously demonstrated that treatment with valproic acid, a pan HDAC inhibitor, results in improved survival in a rodent two-hit (HS followed SS) model [8]. However, class and isoform selective inhibition of HDAC is now gaining favor as it limits the toxicity that has been observed with pan-HDAC inhibitors (HDACI). HDAC6, a member of the HDAC family, whose major substrate is a-tubulin, is being increasingly implicated in the pathogenesis of inflammatory disorders. In the present study, we tested the hypothesis that specific HDAC6 inhibition with Tubastatin A (Tub-A) would improve survival in a rodent two-hit model: HS followed by septic shock from cecal ligation and puncture (CLP).
MATERIAL AND METHODS
All the research was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations related to animal experimentation. The study adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, and was approved by the Institutional Animal Care and Use Committee. Male C57BL/6J mice (18–26 g) were purchased from The Jackson Laboratory (The Jackson Laboratory, Bar Harbor, ME) and housed for 3 days before the experiment to ensure good health.
Animal Model and Experimental Design
Hemorrhage was induced as previously described by Liu et al [8]. Mice were anesthetized with 0.7% to 1.2%isoflurane (Abbott Laboratories, North Chicago, IL) mixed with air, which was administered via a nose cone scavenging system and allowed to breathe spontaneously, using a veterinary multichannel anesthesia delivery system and vaporizer (Kent Scientific Corporation, Torrington, CT). The bilateral femoral artery was cannulated with polyethylene 10 catheters (Clay Adams, Sparks, MD). The left femoral artery cannula was used for hemorrhage and fluid resuscitation, while the right arterial catheter was connected to the Ponemah Physiology Platform (Gould Instrument Systems, Valley View, OH) for continuous hemodynamic monitoring. To induce HS, baseline arterial blood samples were obtained, and then additional blood was withdrawn to a target of 30% of the estimated total blood volume (total blood volume [ml] = weight [g] * 0.07 [ml/g] over 10 minutes. After 30 minutes of unresuscitated shock, the animals were randomly assigned to three groups (n= 7–13/group) and treatment was administered via intraperitoneal injection, as follows: (a) Sham animals, instrumentation and anesthesia but no hemorrhage and no CLP (Sham) (n = 7); (b) Dimethyl sulfoxide (DMSO) (1μl/g) vehicle treated animals (VEH) (n = 13) and (c) Tubastatin A (70 mg /kg) treated animals (Tub-A) (n = 13). After 1 hour of observation, catheters were removed, vessels were ligated, and skin incisions were closed. Animals were recovered from anesthesia and returned to their cages. Twenty-four hours later, these mice were re-anesthetized with isoflurane, and polymicrobial sepsis was induced by CLP as described by Rittirsch et al [9]. In brief, the peritoneal cavity was opened under inhaled isoflurane anesthesia. Cecum was eviscerated, ligated at the designated position (75%) using a 5-0 suture, and punctured through and through (2 holes) with a 21 gauge needle. The punctured cecum was squeezed to expel a small amount of fecal material and returned to the peritoneal cavity. The abdominal incision was closed in two layers with 4-0 silk suture. A second (same as previous) dose of DMSO and Tub-A was given via intraperitoneal administration, and animals were woken from anesthesia and transferred to their cages for observation. They were monitored for 10 days to document survival. A second experiment was designed to measure the concentration of selected e pro-inflammatory cytokines in the peritoneal fluid and their mRNA expression in the liver. In this experiment, a different set of mice were subjected to the two-hit (same as experiment 1), and sacrificed 3 hours (n= 3 per group) after CLP. At the time of sacrifice, abdominal cavity was opened and irrigated with 1 ml of isotonic sodium chloride solution, and this fluid was collected, liver tissues were harvested for further assay.
Myeloperoxidase Assay
Myeloperoxidase (MPO) activity in peritoneal irrigation fluid was determined using the Myeloperoxidase Assay Kit (Cell Sciences Inc., Canton, MA) according to the manufacturer’s instructions. The peritoneal cavity was irrigated with 1 ml NS, the fluid was centrifuged at 1500g at 4°C for 10 minutes and supernatants were saved for analysis.
Cytokine measurements
TNF-α and IL-6 in peritoneal irrigation fluid were determined with commercially available Enzyme Linked Immunosorbent Assay (ELISA) kits according to the manufacturer’s instructions (R&D Systems Inc., Minneapolis, MN). The concentrations of cytokines were measured by optical densitometry at 450 nm in a SpectramaxPlus 384 microplate reader (Molecular Devices, Sunnyvale, CA). All of the analyses were performed in triplicates.
Real-time Polymerase Chain Reaction (real-time PCR)
RNA was isolated from liver tissue and converted into cDNA with High Capacity cDNA Reverse Transcription kit, following the manufacturer’s protocol (Applied Biosystems, Foster City, CA). Equal amounts of cDNA were subjected to the PCR in the presence of SYBR green Master Mix, forward and reverse primers, and the ABI PRISM 7300 Real Time PCR detection machine. Primers were purchased from RealTimePrimer.com (Elkins Park, PA). PCR was performed with 40 cycles of 15 seconds at 95 °C, and 1 minute at 59 °C. GAPDH was used as an internal control. Each sample was run in triplicates. Relative mRNA expression was calculated using the parameter threshold cycle (CT) values. The ΔCT was the difference in the CT values derived from the specific gene being assayed and the GAPDH mRNA. ΔΔCT represented the difference between the paired samples, as calculated by the formula ΔCT of a sample – ΔCT of reference (the average ΔCT of sham samples). The fold change was calculated as 2−ΔΔCT. Primers for TNF-α, Forward: 5′-CCC ACT CTG ACC CCT TTA CT-3′, Reverse: 5′-TTT GAG TCC TTG ATG GTG GT-3′; IL-6, Forward: 5′-CTA CCC CAA TTT CCA ATG CT -3′, Reverse 5′-ACC ACA GTG AGG AAT GTC CA-3′:GAPDH, Forward: 5′-GGA GCG AGA CCC CAC TAA CA-3′, Reverse: 5′-ACA TAC TCA GCA CCG GCC TC-3′.
Statistical analysis
Survival rates were compared by Kaplan–Meier log-rank test. Data are presented as group means ± standard error of mean (SEM). Statistical differences were determined by analysis of variance (ANOVA) for multiple-group comparisons, and p value of less than 0.05 was considered to be statistically significant. Data were analyzed using GraphPad Prism (version 5.0 for Windows, GraphPad Software, San Diego, CA).
RESULT
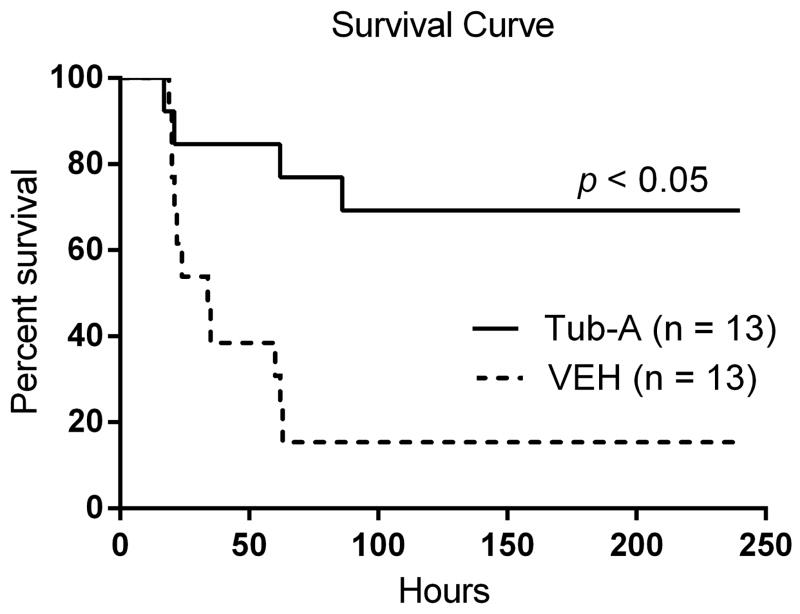
Tub-A improves survival in a mouse two-hit model of hemorrhagic and septic shock
The first hit (30% blood volume hemorrhage) was intentionally kept sub-lethal to ensure that all the animals survival until the second hit. As shown in Fig 1, animals started dying after the CLP insult and only 15.4% of mice in the control group survived for 10 days, with most of the deaths within the first 24 h. In contrast, Tub-A treated animals displayed a significantly better long-term survival rate (69.2% survived > 10 days). These results indicate that administration of Tub-A significantly improves survival in this two-hit model (p < 0.05).
Figure 1. Tubastatin A (Tub-A) protects mice against hemorrhagic shock (HS) and septic shock (SS) induced lethality.
Male C57BL/6J mice (18–26 g) were subjected to sublethal HS and then randomized into two groups (n = 7–13): Tub-A and vehicle (VEH) control. The Tub-A group was injected with Tub-A (70mg/kg, i.p.). VEH group given (DMSO) (1μl/g, i.p.). After 24 h, all mice received CLP followed immediately by injection of the same dose of Tub-A (Tub-A group) or DMSO (VEH group). Survival was monitored for 10 days. The Kaplan-Meier curve illustrates survival over the 10-day observation period. Treatment with Tub-A significantly improved long-term survival compared to VEH group (69.2% vs. 15.4).
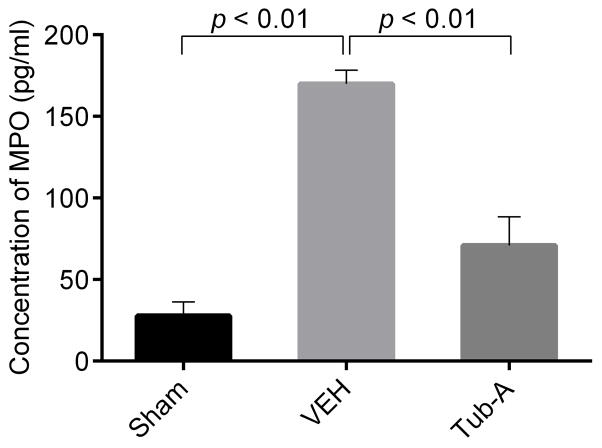
Tub-A decreases CLP-induced myeloperoxidase activity
As a marker for neutrophil-mediated oxidative damage, MPO activity in the peritoneal fluid was measured to determine the degree of local inflammation. As shown in Fig 2, MPO activity was low (27.8 ± 4.9, p < 0.01) in the sham group, whereas CLP resulted in a significant increase in the MPO activity (169.9 ± 8.4 ng/ml, p < 0.01). In contrast, Tub-A treatment was associated with a significant attenuation in the MPO activity (70.4 ± 17.4ng/ml, p < 0.01).
Figure 2. Tubastatin A attenuates myeloperoxidase (MPO) activity in peritoneal fluid.
The peritoneal fluid from different treatment were collected 3 h after CLP and assayed for MPO activity by Enzyme-Linked Immunosorbent Assay activity (Group means ± SEM, n = 3). Tub-A: Tubastatin A; VEH: vehicle control.
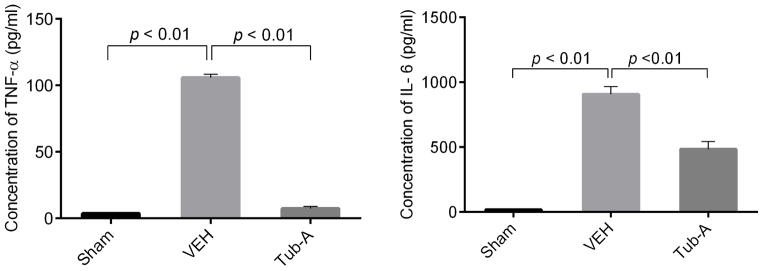
Tub-A suppresses production of pro-inflammatory cytokines TNF-α and IL-6
Peritoneal fluid collected at 3 hours after CLP was examined for these pro-inflammatory cytokines. In the sham groups, the levels of TNF-α and IL-6were very low, whereas the vehicle treated group showed a significant elevation in the levels of TNF-α (105.7 ± 4.7 pg/ml, p < 0.01) and IL-6 (907.4 ± 2.3 pg/ml, p < 0.01) in the peritoneal fluid. Administration of Tub A attenuated these changes significantly (TNF-α: 7.4 ± 2.4 pg/ml; IL-6: 483.6 ±1.6 pg/ml; p< 0.01) (Fig 3).
Figure 3. Tubastatin A attenuates levels of TNF-α and IL-6 in peritoneal fluid.
The peritoneal fluid and blood were collected at 3 h after CLP and assayed for TNF-α and IL-6 levels by ELISA kits (Group means ± SEM, n = 3). Tub-A: Tubastatin A; VEH: vehicle control.
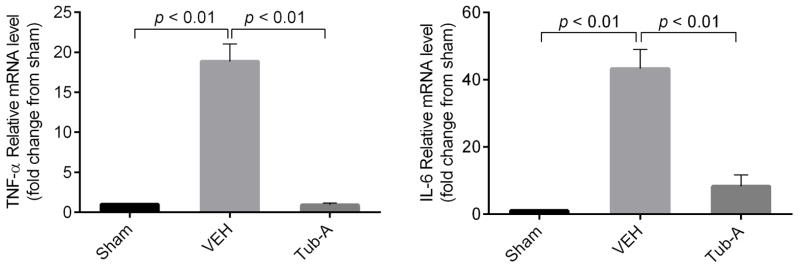
Tub-A suppresses transcription of TNF-α and IL-6
To determine whether Tub-A could affect transcription of TNF-α and IL-6 genes, the real time PCR analysis was performed. Compared to the sham group, mRNA levels of TNF-α and IL-6 in the liver tissue were 18.9 ± 1.3 and 43.3 ± 5.8 folds higher in the vehicle group respectively, whereas Tub-A treatment suppressed this up regulation (p < 0.01) (Fig 4).
Figure 4. Tubastatin A inhibits mRNA expression levels of both TNF-α and IL-6 in liver tissue.
Real-time PCR data showing the decrease of mRNA fold changes in Tub-A group compare to VEH group, Sham animal fold change expressed as 1. (Group means ± SEM, n = 3). Tub-A: Tubastatin A; VEH: vehicle control.
DISCUSSION
In this study we have shown that inhibition of HDAC6 with Tub-A significantly increases survival in a rodent two-hit model, along with an attenuation of peritoneal myeloperoxidase activity and down regulation of pro-inflammatory cytokine (TNF-α and IL-6) gene and protein expression.
SIRS caused by trauma and hemorrhage can be particularly lethal and often is complicated by a second injury such as sepsis. The initial injury acts as the first hit, which disturbs the immune system, making the host vulnerable to subsequent infections. The concept of two-hit insult has been increasingly accepted as an explanation for the development of multiple organ dysfunction syndrome in the trauma patients [3]. In recent years, we have moved away from aggressive crystalloid resuscitation for the treatment of hemorrhagic shock due to accumulating pre-clinical and clinical data about its adverse consequences [10–12]. The contemporary trauma care now is based upon the concept of Damage Control Resuscitation, which limits use of crystalloids, promotes early blood product use, and prompt hemorrhage control [13, 14]. In contrast to hemorrhage, early treatment of sepsis continues to emphasize goal directed crystalloid resuscitation to replace the intravascular fluid that is lost due to leaky capillaries [15]. But even in these patients, excessive administration of fluids is associated with adverse outcomes [16]. A practical problem for the pre-hospital environment, especially the battlefield setting, is the limited availability of resources and supplies, such as fluids and blood products. Therefore, the focus of our research team has been to develop logistically practical pharmacological treatments that can keep the injured alive under austere circumstances and serve as a bridge to definitive care. HDACIs have emerged as a promising group of drugs as they have potent pro-survival and anti-inflammatory properties, and possess numerous attractive features such as quick onset, reversible cellular changes, and easy availability (many are approved for clinical use in non-trauma conditions)[17]. Preclinical data have confirmed that treatment with a pan-HDACI agent (e.g. valproic acid) improves survival in small and large animal models of HS, suppresses inflammation, activates pro-survival pathways, attenuates tissue damage, prevents distant organ injury, and prevents death in models of septic shock as well as in two-hit rodent models [8, 18–20]. Although pan HDACI are effective, they often require very large doses to exert the desired effect, which raises concerns about potential toxicity. A phase I dose optimization clinical trial is currently underway in healthy volunteers and trauma patients (ClinicalTrials.gov Identifier: NCT01951560) to identify the maximum safe dose. Another potential approach is to use isoform selective HDACI instead that have shown promising results [21–23] and may have better safety profiles.
In this study, we used a severe yet nonlethal blood loss (without resuscitation) as the first hit, followed by CLP (a widely used model for polymicrobial infection) to induce sepsis. We selected HDAC6 inhibitor Tub-A for testing, based on two recent findings. First, we have shown that Tub-A treatment can improve survival in a rodent model of lethal CLP-induced septic shock [24]. Second, we have found that selective inhibition of HDAC6 with Tub-A can improve survival following lethal HS (data accepted as an oral presentation for the 45th Annual Meeting of Western Trauma Association, 2015). We therefore reasoned that Tub-A treatment should improve survival in a combined model of hemorrhage and subsequent sepsis. Results from the present study support this hypothesis.
There are some theoretical advantages to using isoform selective HDACI rather than pan-inhibitors (e.g. VPA). While VPA has been shown to suppress inflammation, attenuate tissue damage, prevent distant organ injury, and death in models of septic shock as well as two-hit insults [8, 18], the non-specific inhibition of HDAC can be problematic. VPA inhibits class I (HDAC 1, 2, 3 and 8) and class II HDACs (HDAC4, 5 7, and 9) [25], which can have undesirable effects. For example, Class I HDACs repress TNF-induced NF-kB-dependent gene expression and promote interferon signaling [26]. Inhibiting Class I HDAC may lead to toxicity in immune cells, lymphocyte development impairment, amplified production of TLR/NF-kB-inducible inflammatory mediators, and compromised anti-microbial responses. Classical HDACs such as HDAC1 and HDAC2 have been shown to promote B cell proliferation, and their inhibition leads to the arrest of pre-B cells in the G1 phase of development, accompanied by increased apoptotic death. In addition, T cell development and genomic stability in mice are dependent on HDAC1 and HDAC2 [27]. Conditional deletion of HDAC1 in T cells results in increased Th2 cytokine production as well as heightened airway inflammation [28]. In contrast, HDAC6-deficient mice showed normal lymphoid development, with only moderately-affected immune responses, and HDAC6 deletion has not been found to be detrimental to normal mammalian development [29]. Our own studies have recently demonstrated that Tub-A can prevent immune cells apoptosis in a model of septic shock [21]. Taken together, these data suggest that isoform specific inhibition of HDAC6 with newer pharmacological agents may be a better approach than non-selective HDAC inhibition.
This study has certain limitations that must be acknowledged. For logistical reasons, we only measured selected cytokines. Many more mechanisms and factors (e.g. oxidative stress-induced signal transduction pathway, serum cytokines, etc.) are likely to be influenced by Tubastatin A treatment. Further molecular mechanisms underlying phagocytosis and apoptosis changes after inhibition of HDAC6 need to be explored. While the sample size was statistically adequate, it may not have been large enough to show smaller intergroup differences. Similarly, to minimize repeated blood draws (that can be lethal) and to reduce the total number of animals, we did not obtain serial samples. Because this was a proof-of-concept study, we only tested a single dose of the drug, and it was given as an intra-peritoneal injection, which although commonly used in mice studies, is not clinically realistic.
In conclusion, we have demonstrated that Tubastatin A, an inhibitor of HDAC6, can effectively reduce MPO levels, inhibit pro-inflammatory cytokines TNF-α and IL-6, suppress TNF-α and IL-6 gene transcription, and improve survival in a rodent model of HS followed by CLP. Although the fundamental molecular and cellular signaling events still require further investigation, HDAC6 may represent a novel and promising therapeutic target for post hemorrhage sepsis.
Acknowledgments
This work was funded by a grant from NIH RO1 GM084127 to HBA. Data presented at 10th Annual Academic Surgical Congress, Las Vegas, Nevada (February, 2015).
Footnotes
Conflict of Interest Disclosure: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Authors’ contribution: Y.L. and H.B.A. designed this study. X.C and Z.L. performed experiments, collected and analyzed data. T. Z. and B.L provided experimental support. X.C and Y.L. wrote the manuscript, which was critically revised by Y.L. and H.B.A. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. Jama. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012;366:2122–2124. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Alam HB. Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock. Advances in experimental medicine and biology. 2012;710:107–133. doi: 10.1007/978-1-4419-5638-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung YL, Lee MY, Wang AJ, Yao LF. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol Ther. 2003;8:707–717. doi: 10.1016/s1525-0016(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Li Y, Chong W, Deperalta DK, Duan X, et al. Creating a prosurvival phenotype through a histone deacetylase inhibitor in a lethal two-hit model. Shock. 2014;41:104–108. doi: 10.1097/SHK.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan I, Bunn F, Roberts I. Committee WHOP-HTCS Timing and volume of fluid administration for patients with bleeding. The Cochrane database of systematic reviews. 2003:CD002245. doi: 10.1002/14651858.CD002245. [DOI] [PubMed] [Google Scholar]
- 11.Alam HB, Stanton K, Koustova E, Burris D, Rich N, et al. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60:91–99. doi: 10.1016/j.resuscitation.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33:229–241. doi: 10.1097/SHK.0b013e3181c30f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. The Surgical clinics of North America. 2012;92:877–891. viii. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Butler FK, Holcomb JB, Schreiber MA, Kotwal RS, Jenkins DA, et al. Fluid Resuscitation for Hemorrhagic Shock in Tactical Combat Casualty Care: TCCC Guidelines Change 14-01 - 2 June 2014. Journal of special operations medicine: a peer reviewed journal for SOF medical professionals. 2014;14:13–38. doi: 10.55460/DPOC-JWIY. [DOI] [PubMed] [Google Scholar]
- 15.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 16.Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiology intensive therapy. 2014;46:361–380. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Liu B, Gu X, Kochanek AR, Fukudome EY, et al. Creating a “pro-survival” phenotype through epigenetic modulation. Surgery. 2012;152:455–464. doi: 10.1016/j.surg.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwabejire JO, Lu J, Liu B, Li Y, Halaweish I, et al. Valproic acid for the treatment of hemorrhagic shock: a dose-optimization study. The Journal of surgical research. 2014;186:363–370. doi: 10.1016/j.jss.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butt MU, Sailhamer EA, Li Y, Liu B, Shuja F, et al. Pharmacologic resuscitation: cell protective mechanisms of histone deacetylase inhibition in lethal hemorrhagic shock. The Journal of surgical research. 2009;156:290–296. doi: 10.1016/j.jss.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Fukudome EY, Kochanek AR, Li Y, Smith EJ, Liu B, et al. Pharmacologic resuscitation promotes survival and attenuates hemorrhage-induced activation of extracellular signal-regulated kinase 1/2. The Journal of surgical research. 2010;163:118–126. doi: 10.1016/j.jss.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao T, Li Y, Bronson RT, Liu B, Velmahos GC, et al. Selective histone deacetylase-6 inhibition attenuates stress responses and prevents immune organ atrophy in a lethal septic model. Surgery. 2014;156:235–242. doi: 10.1016/j.surg.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao T, Li Y, Liu B, Halaweish I, Mazitschek R, et al. Selective inhibition of histone deacetylase 6 alters the composition of circulating blood cells in a lethal septic model. The Journal of surgical research. 2014 doi: 10.1016/j.jss.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao TLY, Liu B, Bronson RT, Halaweish I, Alam HB. Histone deacetylase III as a potential therapeutic target for the treatment of lethal sepsis. J Trauma and Acute Care Surg. 2014 doi: 10.1097/TA.0000000000000347. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao T, Li Y, Liu B, Halaweish I, Mazitschek R, et al. Selective inhibition of histone deacetylase 6 alters the composition of circulating blood cells in a lethal septic model. The Journal of surgical research. 2014;190:647–654. doi: 10.1016/j.jss.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends in neurosciences. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YS, Jeong S. PI3-kinase and PDK-1 regulate HDAC1-mediated transcriptional repression of transcription factor NF-kappaB. Mol Cells. 2005;20:241–246. [PubMed] [Google Scholar]
- 27.Dovey OM, Foster CT, Conte N, Edwards SA, Edwards JM, et al. Histone deacetylase 1 and 2 are essential for normal T-cell development and genomic stability in mice. Blood. 2013;121:1335–1344. doi: 10.1182/blood-2012-07-441949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suliman BA, Xu D, Williams BR. HDACi: molecular mechanisms and therapeutic implications in the innate immune system. Immunol Cell Biol. 2012;90:23–32. doi: 10.1038/icb.2011.92. [DOI] [PubMed] [Google Scholar]
- 29.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer letters. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]