Abstract
Background
In the United States in 2012, there were 16,060 new cases of chronic lymphocytic leukemia (CLL). Often CLL is clinically occult and first detected during pathologic evaluation of the sentinel lymph node biopsy (SLNB). We reviewed our experience of patients with the coexisting diagnosis of melanoma and CLL.
Methods
An institutional review board-approved review was performed on patients with CLL and melanoma treated from 1995 to 2009 at Moffitt Cancer Center and compared with the incidence of melanoma and CLL in our tumor registry patients with breast, prostate, lung, and colon cancer.
Results
Fifty-two patients (44 males; median age, 71 years [range, 46–88]) were identified with concurrent diagnoses of melanoma and CLL. Twenty-two patients (42 %) had CLL on SLNB for their melanoma. Thirty-two patients (62 %) were diagnosed with melanoma before CLL. Concomitant or prior cancer diagnoses included nonmelanoma skin cancers (N = 29), prostate (N = 6), colorectal (N = 2), and Merkel cell carcinoma (N = 2). Five of 20 patients (25 %) had metastatic melanoma found at the time of SLNB. Patients with melanoma had a tenfold increase of CLL diagnosis compared with colorectal cancer patients, an eightfold increase compared to prostate cancer patients, and a fourfold increase compared with breast cancer patients.
Conclusions
We have confirmed an increased association of CLL and melanoma. This may be related to an underlying immunologic defect; however, there has been scant investigation into this phenomenon. Surgeons and pathologists should understand this occurrence and recognize that not all grossly enlarged or abnormal sentinel lymph nodes in melanoma patients represent melanoma.
In the United States in 2012, 16,060 new cases of low-grade lymphoproliferative disorder [chronic lymphocytic leukemia (CLL)] or small lymphocytic lymphoma were diagnosed.1 We and others have observed an increased incidence of CLL in patients with a diagnosis of melanoma and other nonmelanoma skin cancers.2–4 There is approximately twice the expected risk of second cancers in patients diagnosed with CLL. In one recent series that evaluated other malignancies in patients with CLL, there was a 7.74 standardized incidence ratio related to development of melanoma.5 Verwer et al. recently found an incidence of lymphoma in 0.3 % of patients diagnosed with melanoma, with the most common form being CLL.6
Often the lymphoproliferative disorder is clinically occult and first detected during pathologic evaluation of the sentinel lymph node biopsy performed at the time of definitive resection for the melanoma. The current standard of care for patients with melanoma with appropriate depth of invasion, ulceration, or mitotic rate includes the technique of sentinel lymph node biopsy for lymph node staging.7 During sentinel lymphadenectomy, we have recognized a subset of patients who have enlarged or hypertrophic appearing lymph node(s) at the time of operation. Subsequent pathologic review reveals evidence of CLL in the lymph node and often is the initial diagnosis of this secondary cancer. This study reviewed our experience at a tertiary referral center treating cutaneous melanoma patients with coexisting CLL and compared our findings to regional and national datasets.
METHODS
The institutional review board of the Moffitt Cancer Center approved this study. A retrospective review was performed between 1995 and 2009 to identify all melanoma and CLL patients treated at our tertiary referral center. Medical records, institutional, local, and national cancer registries were queried. We also identified a subset of patients in whom CLL was diagnosed at the time of lymph node biopsy for their melanoma. Clinical characteristics, including pathologic characteristics of the primary melanoma and the sentinel lymph node biopsy characteristics, preoperative white blood cell (WBC) count, past history of cancer, and subsequent treatments for their melanoma and CLL were evaluated. In addition, we compared the incidence of melanoma and CLL in our tumor registry patients with the national Surveillance, Epidemiology and End Results (SEER) database, the Florida State Tumor Registry database, and with the incidence of other selected cancers where regional lymph nodes are evaluated, including breast, prostate, lung, and colon cancers.
The pathologic criteria for diagnosing CLL included identification of effacement of nodal architecture and replacement by diffuse growth of small, monotonous lymphocytes expressing CD5, CD20, or CD79a. CLL tends to involve the entire lymph node and can be detected on routine H&E staining alone but requires immunohistochemical confirmation. There is a standard panel of immunohistochemical stains (CD5, CD20, CD79a) that was performed on any lymph node that was suspicious for CLL, and establishing the diagnosis does not require performing flow cytometry.
All cutaneous melanoma cases were reviewed by our dermatopathologist. Primary tumor characteristics were assessed and outside slides reviewed before resection of the melanoma. Sentinel lymph nodes were processed after serial sectioning with review of both hematoxylin and eosin stained sections and S-100 and Melan-A immunohistochemistry.
RESULTS
Fifty-two patients were identified with concurrent melanoma and CLL. Pathologic characteristics of the primary melanoma are listed in Table 1. Median age was 71 years (range, 46–88). The majority of patients were male (N = 44) and Caucasian (N = 50). The most common primary site was head and neck (37 %), followed by the trunk (27 %), upper extremity (19 %), and lower extremity (17 %). The majority of patients (47 %) had an intermediate thickness (1–4 mm) melanoma followed by a thick (>4 mm) melanoma (19 %), thin (<1 mm) melanoma (17 %), and unknown thickness (17 %). Twenty-two (42 %) patients were identified to have CLL on lymph node biopsy at the time of their initial staging for melanoma. Thirty-two patients (62 %) were diagnosed with melanoma before CLL.
TABLE 1.
Patient demographics
| Characteristics | N |
|---|---|
| Total patients | 52 |
| M:F | 44:8 |
| Median age (range in years) | 71 (46–88) |
| Race | |
| Caucasian | 50 (96 %) |
| Other | 2 (4 %) |
| Location | |
| Head and neck | 19 (37 %) |
| Trunk | 14 (27 %) |
| Upper extremity | 10 (19 %) |
| Lower extremity | 9 (17 %) |
| Tumor thickness (mm) | |
| < 1.0 | 9 (17 %) |
| 1–2 | 10 (19 %) |
| 2–4 | 14 (27 %) |
| > 4 | 10 (19 %) |
| Unknown | 9 (17 %) |
| Median preoperative WBC (range) | 12.1 (1.5–200) |
| Median number of SLN with CLL (range) | 3 (2–8) |
WBC white blood cell count; SLN sentinel lymph node; CLL chronic lymphocytic leukemia
Five of 20 (25 %) patients had evidence of collision of both metastatic melanoma and CLL in the sentinel lymph node (Fig. 1). The median number of sentinel lymph nodes identified with CLL was 3.0 (range 2–9). The median preoperative white blood cell count (WBC) was 12.1, with 40 % of patients having an abnormally elevated preoperative WBC count. Seventeen patients (33 %) received systemic chemotherapy for treatment of CLL.
FIG. 1.
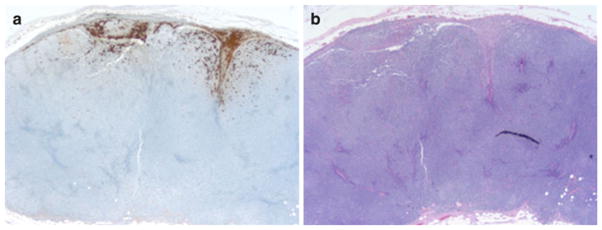
a S100 immunohistochemistry stain (×25): positive for metastatic melanoma at periphery of lymph node. b H&E stain (×25): lymph node with effacement of nodal architecture and replacement by diffuse growth of small, monotonous lymphocytes, as well as microscopic involvement by metastatic melanoma
Concomitant or prior cancer diagnoses included non-melanoma skin cancers (N = 29), prostate cancer (N = 6), colorectal cancer (N = 2), and Merkel cell carcinoma (N = 2). In Table 2, we compare the national, regional, and institution incidences of selected cancers where lymph nodes also are evaluated at the time of surgery (lung, prostate, breast, and colorectal) with melanoma and CLL. When comparing the coincidence of prostate, breast, colorectal, CLL, and melanoma in our Moffitt tumor registry, 6 % had a coincidence of CLL and melanoma (Table 3). This coincidence of CLL and melanoma was 10× higher than colorectal cancer patients, 8× higher than prostate cancer patients, and 4× higher than breast cancer patients. We also stratified this for male and female patients, comparing coincidence of prostate cancer, colorectal cancer, and CLL to melanoma for male patients. Similarly we evaluated coincidence of breast cancer, colorectal cancer, and CLL to melanoma in female patients (Table 4). In male patients, 8 % had a coincidence of CLL and melanoma compared with 1 % for prostate cancer and melanoma and 0.8 % for colorectal cancer and melanoma. In female patients, 2 % had a coincidence of CLL and melanoma compared with 0.5 % for breast cancer and melanoma and 0.8 % for colorectal cancer and melanoma.
TABLE 2.
National, regional, and institution incidence of selected cancers
| Cancer type | United States (N) 2008 | Florida (N) 2008 | Moffitt Cancer Center (N) 1986–2006 |
|---|---|---|---|
| Lung | 215,020 | 17,360 | – |
| Prostate | 186,320 | 11,380 | 7,116 |
| Breast (female) | 182,460 | 11,850 | 10,881 |
| Colorectal | 108,070 | 10,920 | 3,783 |
| Melanoma | 62,480 | 4,430 | 7,239 |
| CLL | 15,110 | 3190 | 739 |
CLL chronic lymphocytic leukemia
TABLE 3.
Coincidence of selected cancers: Moffitt Tumor Registry 1986–2006
| Cancer type | Patients with both (N) | Percentage with both (%) |
|---|---|---|
| Prostate and melanoma | 72 | 1 |
| Breast and melanoma | 52 | 0.5 |
| Colorectal and melanoma | 24 | 0.6 |
| CLL and melanoma | 41 | 6 |
CLL chronic lymphocytic leukemia
TABLE 4.
Coincidence of selected cancers by gender
| Cancer type | Patients with both (N) | Percentage with both (%) |
|---|---|---|
| Male patients | ||
| Prostate and melanoma | 72 | 1 |
| Colorectal and melanoma | 16 | 0.8 |
| CLL and melanoma | 36 | 8 |
| Female patients | ||
| Breast and melanoma | 52 | 0.5 |
| Colorectal and melanoma | 8 | 0.8 |
| CLL and melanoma | 5 | 2 |
CLL chronic lymphocytic leukemia
DISCUSSION
Although previous studies have described the association between the incidence of CLL and melanoma, there has been scant investigation into this phenomenon. There are multiple case reports and small series in the literature related to this codiagnosis.2,8 The coincidence of cancers may possibly be related to an underlying immunologic defect, yet this defect still remains undefined. We herein report the one of the largest series of patients diagnosed with both CLL and cutaneous melanoma, especially with those diagnosed with CLL at the time of sentinel lymph node biopsy for their melanoma.
In 1995, Adami et al. reported on the secondary occurrences of skin cancer in patients with lymphoma in Denmark and Sweden. They reported a relative risk for melanoma of 3.1 for patients with known CLL.9 Landgren et al. assessed the risk of second cancers in 7,764 patients with CLL and reported a 1.19 relative risk of secondary diagnosis of melanoma.10 Similarly, Brennan et al. initially published in 2000 evaluating second malignancies in patients with NHL from the New South Wales central cancer registry in Australia and found a twofold increased risk of melanoma in these patients.11 Travis et al. also have confirmed an association with NHL and skin cancers.12 In 2005, Brennan et al. expanded their studies to 109,451 patients and demonstrated an overall standardized incidence ratio of 1.92 for melanoma as a second malignancy in patients with NHL.13
In 2001, Hisada et al. evaluated the incidence of subsequent tumors after CLL using the SEER database.14 In their study, they evaluated observed/expected (O/E) ratios and specifically found an O/E ratio of 3.18 for melanoma in this patient population. Riou et al. evaluated the incidence of associated neoplasms in a database of 664 patients diagnosed with melanoma at Yale-New Haven Hospital.15 They reported that 8.1 % of patients initially diagnosed with melanoma had an additional malignant neoplasm, with lymphomas being the most prevalent, and CLL was diagnosed in two patients in their series. One of the most recent series from Tsimberidou et al. reviewed patients treated for CLL at MD Anderson and compared second malignancies with the number of expected cases from the SEER database.16 They identified an 8 % incidence of subsequent melanoma on their patient population, with an O/E ratio of 6.17.
In 2010, Verwer et al. observed in their cohort that lymphoma was diagnosed subsequent to melanoma in 41.8 % of patients and before melanoma in 12.7 %; CLL was the most common subtype: 49.1 %.6
Agnew et al. have evaluated 40 patients with CLL who developed 125 skin manifestations; 18 patients had basal or squamous cell carcinoma, melanoma (N = 2), and Merkel cell carcinoma (N = 2).17 McKenna et al. concluded that regardless of age, the risk of developing CLL after melanoma is increased by 130 %, but it was only statistically significant in patients older than 50 years at the time of diagnosis (P > 0.05).18 The risk of developing subsequent primary cancers is substantially higher after invasive melanoma and was elevated for developing an additional invasive melanoma, thyroid cancer, non-Hodgkin lymphoma, and CLL. Such risk appears to be slightly higher in women than in men.19 In a SEER population-based study, the overall survival in patients with melanoma and Merkel cell carcinoma with a history of CLL remained shorter than expected compared with melanoma patients without CLL regardless of Breslow thickness or Clark level.20
CONCLUSIONS
Our study evaluated the codiagnosis of melanoma and CLL; 42 % of the 52 patients were diagnosed with CLL at the time of their initial surgery for their melanoma by lymph node biopsy. Surgeons and pathologists should be aware of this occurrence and recognize that not all grossly enlarged or abnormal sentinel lymph nodes in melanoma patients represent metastatic melanoma. Further investigation is warranted to determine the clinical significance with hopes of identifying possible etiologies, including underlying immunologic and genetic mechanisms related to this phenomenon, both to improve our understanding of the disease processes and offer the potential of future clinical treatments.
Footnotes
DISCLOSURE None.
References
- 1.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Cahill RA, McGreal G, Neary P, Redmond HP. Synchronous high-risk melanoma and lymphoid neoplasia. Melanoma Res. 2001;11(5):517–22. doi: 10.1097/00008390-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232(3):267–9. [PubMed] [Google Scholar]
- 4.Greene MH, Hoover RN, Fraumeni JF., Jr Subsequent cancer in patients with chronic lymphocytic leukemia: a possible immunologic mechanism. J Natl Cancer Inst. 1978;61(2):337–40. [PubMed] [Google Scholar]
- 5.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105(7):1076–81. doi: 10.1038/bjc.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verwer N, Murali R, Winstanley J, et al. Lymphoma occurring in patients with cutaneous melanoma. J Clin Pathol. 2010;63(9):777–81. doi: 10.1136/jcp.2010.077768. [DOI] [PubMed] [Google Scholar]
- 7.Ross MI, Thompson JF, Gershenwald JE. Sentinel lymph node biopsy for melanoma: critical assessment at its twentieth anniversary. Surg Oncol Clin N Am. 2011;20(1):57–78. doi: 10.1016/j.soc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 8.El Demellawy D, Ross C, Sur M, Alowami S. Synchronously diagnosed lymph nodal collision tumor of malignant melanoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: case report. Diagn Pathol. 2007;2:34. doi: 10.1186/1746-1596-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;310(6993):1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landgren O, Pfeiffer RM, Stewart L, et al. Risk of second malignant neoplasms among lymphoma patients with a family history of cancer. Int J Cancer. 2007;120(5):1099–102. doi: 10.1002/ijc.22414. [DOI] [PubMed] [Google Scholar]
- 11.Brennan P, Coates M, Armstrong B, Colin D, Boffetta P. Second primary neoplasms following non-Hodgkin’s lymphoma in New South Wales, Australia. Br J Cancer. 2000;82(7):1344–7. doi: 10.1054/bjoc.1999.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis LB, Curtis RE, Glimelius B, et al. Second cancers among long-term survivors of non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1993;85(23):1932–7. doi: 10.1093/jnci/85.23.1932. [DOI] [PubMed] [Google Scholar]
- 13.Brennan P, Scelo G, Hemminki K, et al. Second primary cancers among 109 000 cases of non-Hodgkin’s lymphoma. Br J Cancer. 2005;93(1):159–66. doi: 10.1038/sj.bjc.6602654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisada M, Biggar RJ, Greene MH, Fraumeni JF, Jr, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98(6):1979–81. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- 15.Riou JP, Ariyan S, Brandow KR, Fielding LP. The association between melanoma, lymphoma, and other primary neoplasms. Arch Surg. 1995;130(10):1056–61. doi: 10.1001/archsurg.1995.01430100034007. [DOI] [PubMed] [Google Scholar]
- 16.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(6):904–10. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agnew KL, Ruchlemer R, Catovsky D, Matutes E, Bunker CB. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150(6):1129–35. doi: 10.1111/j.1365-2133.2004.05982.x. [DOI] [PubMed] [Google Scholar]
- 18.McKenna DB, Stockton D, Brewster DH, Doherty VR. Evidence for an association between cutaneous malignant melanoma and lymphoid malignancy: a population-based retrospective cohort study in Scotland. Br J Cancer. 2003;88(1):74–8. doi: 10.1038/sj.bjc.6600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balamurugan A, Rees JR, Kosary C, Rim SH, Li J, Stewart SL. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5 Suppl 1):S69–77. doi: 10.1016/j.jaad.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30(8):843–9. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]


