Abstract
Purpose of Review
Outline the analgesic role of perineural adjuvants for local anesthetic nerve block injections, and evaluate current knowledge regarding whether adjuvants modulate the neurocytologic properties of local anesthetics.
Recent Findings
Perineural adjuvant medications such as dexmedetomidine, clonidine, buprenorphine, dexamethasone, and midazolam play unique analgesic roles. The dosing of these medications to prevent neurotoxicity is characterized in various cellular and in vivo models. Much of this mitigation may be via reducing the dose of local anesthetic used while achieving equal or superior analgesia. Dose-concentration animal models have shown no evidence of deleterious effects. Clinical observations regarding blocks with combined bupivacaine-clonidine-buprenorphine-dexamethasone have shown beneficial effects on block duration and rebound pain without long-term evidence of neurotoxicity. In vitro and in vivo studies of perineural clonidine and dexmedetomidine show attenuation of perineural inflammatory responses generated by local anesthetics.
Summary
Dexmedetomidine added as a peripheral nerve blockade adjuvant improves block duration without neurotoxic properties. The combined adjuvants clonidine, buprenorphine, and dexamethasone do not appear to alter local anesthetic neurotoxicity. Midazolam significantly increases local anesthetic neurotoxicity in vitro, but when combined with clonidine-buprenorphine-dexamethasone (sans local anesthetic) produces no in vitro or in vivo neurotoxicity. Further larger-species animal testing and human trials will be required to reinforce the clinical applicability of these findings.
Keywords: Local anesthetics, clonidine, buprenorphine, dexamethasone, midazolam
Introduction
To date, local anesthetics have typically been the mainstay of regional anesthesia. Adjuvant medications in perineural injections modulate or prolong conduction block, sometimes via mechanisms that have yet to be understood. Clonidine, an α-2 agonist, is known to primarily exerts its effects in the central nervous system for blood pressure, and is a well-established drug for intrathecal and epidural injection (also primarily via α-2 agonism). Clonidine’s α-2 effects also appear to attenuate the development of chronic pain responses1. Clonidine and another α-2 agonist, dexmedetomidine, however, exert their perineural analgesic effects via other channel mechanisms unrelated to α-2 mediation2,3. Buprenorphine, an opioid with mixed μ-agonist and κ-antagonist activity, acts at the systemic level to produce analgesic as well as anti-hyperalgesic effects, while its perineural effects include similarity to local anesthetics in blocking synaptic transmission4,5. Steroids, such as dexamethasone, have systemic anti-nausea and anti-nociceptive effects, with possible decreased C-fiber transmission with perineural administration6. Midazolam, primarily known as a GABA-A agonist, may have similar pain modulation effects by acting as an agonist in the periphery via the 18 kilodalton translocator protein (TSPO)7, formerly known as the “peripheral benzodiazepine receptor.”
The use of dexmedetomidine3,8,9, or combined clonidine-buprenorphine–dexamethasone10,11,12,13, as adjuvants to local anesthetics in peripheral nerve blocks appears to (i) clinically increase density and duration of blocks, and (ii) potentially decrease the total amount of local anesthetic required clinically. Local anesthetics are inherently toxic to neurons through disruption of signal transmission and cell membrane and sub-cellular proteins. Safety data for local anesthetics combined with the described adjuvants is otherwise not well known. This review entails the mechanisms, specific dosing, and clinical effects of adjuvants to local anesthetic-containing perineural injections.
Dexmedetomidine
Similar to clonidine, dexmedetomidine exerts its typical clinical effects via agonism at centrally-located α2-adrenergic receptors. Peripherally, however, it may exert its analgesic effects by maintaining hyperpolarization of nerve fibers and blocking synaptic transmission; peripheral addition of α-antagonists were not shown to decrease analgesic effects of dexmedetomidine3. Animal14 and clinical15,16 models have shown the safety of intrathecal administration of dexmedetomidine. Prior to a 2008 study8, no safety data for perineural use of dexmedetomidine was available. When included in sciatic nerve blocks in rats as an adjuvant to bupivacaine, dexmedetomidine was found to actually decrease perineural inflammation compared to bupivacaine alone8 (Brummett 2008). This inhibition of inflammation may be due in part to decreasing the binding activity of NF-kB17. Subsequent research utilizing this safety data have validated the clnical efficacy of dexmedetomidine9. Further studies showing dexmedetomidine in combination with other adjuvants have yet to be performed.
Clonidine-Buprenorphine-Dexamethasone
In a 2009 review, Williams et al discussed the pharmacology of, and the reasons for, employing the combined nerve block adjuvants clonidine, buprenorphine, dexamethasone, and midazolam10. The authors note that, in the presence of clonidine, α-2 receptors on macrophages at the site of tissue damage create a shift towards an anti-inflammatory state1. Buprenorphine has presumed μ-agonism at the site of perineural injection, and may also inhibit voltage-gated sodium channels at the site of perineural injection far more effectively than other opioids5. Dexamethasone is also presumed to have peripheral nerve activity, through inhibition of C-fiber transmission, while causing minimal direct peripheral nerve damage compared to hydrocortisone or triamcinolone18 in clinically-relevant doses. Preservatives added to steroids such as benzyl alcohol and propylene glycol have known neurolytic effects, so it is important to avoid steroid adjuvants with any preservatives (preservative-free dexamethasone sodium phosphate is recommended by the authors). Midazolam, acting primarily as a GABA-A agonist, was previously thought to attenuate nerve blockade by acting at peripheral GABA-A receptors19, although this more recently has been attributed to TSPO activation7.
While clonidine has been approved by the FDA for intrathecal and epidural uses, its safety for use in peripheral nerve blocks can be categorized as “grandfathered” and is generally accepted in textbooks of anesthesiology. Buprenorphine has been shown to induce apoptosis in vitro in isolated neuroblastoma-glioma hybrid cells20, but at concentrations (100μM) higher than in regular clinical use (≤25 μg/mL). To our knowledge, other than in recent reports11,12, preservative-free dexamethasone sodium phosphate for perineural injection has not fully undergone proper safety testing, nor have there been any specific studies examining its co-administration with local anesthetics. Although the safety of midazolam has been examined in intrathecal animal models21 (1.7–2.5mg/mL infusions) and human clinical studies22 (2mg/injection), these generalizations cannot be directly applied to in vitro dorsal root ganglia11 (33 μg/ml) or in vivo rat peripheral nerve models12 (33 μg/mL) when much lower concentrations are used. Although these perineural adjuvants are commonly used systemically, and somewhat well-studied at the neuraxial level, the central theme is the need for further perineural animal model safety testing as dictated by extramural research funding priority, as well as better establishment of the lowest possible effective doses for off-label use23.
In a 2011 basic science report11, several adjuvants were explored regarding the neurocytotoxic effects when combined with ropivacaine. Sensory neurons (dorsal root ganglia) were isolated from rats and bathed in solutions containing various combinations of ropivacaine 0.25%, with or without buprenorphine, clonidine, dexamethasone, or midazolam, or with the 4 adjuvants alone, for 2 or 24 hours. Among many findings, it was noted that the addition of high (supraclincal) concentrations of clonidine and midazolam to ropivacaine increased neuronal death after 2 hours. At estimated clinically-used concentrations, however, there was no impact of clonidine, buprenorphine, or dexamethasone (C-B-D) on the neurotoxicity of ropivacaine. When C-B-D was combined with midazolam (and not ropivacaine) (Figure 1), there was no greater neurotoxicity than that seen after isosmolar choline control treatment. In vitro, dexamethasone at higher concentrations (133 μg/mL versus 66.6 μg/mL) as part of C-B-D did show an incremental neurotoxic effect when added to ropivacaine. Midazolam was found to increase the neurotoxicity of ropivacaine, even at low midazolam concentrations (16.6 μg/mL). There was no in vitro neuroprotective effect noted with any of these combinations of adjuvant drugs combined with ropivacaine (a drug approved by the Food and Drug Administration for perineural use). In other words, C-B-D combined with ropivacaine showed neuronal cytotoxicity primarily driven by ropivacaine and not by C-B-D (with dexamethasone at 66.6 μg/mL), while the combination of clinical concentrations of clonidine-buprenorphine-dexamethasone-midazolam showed no difference in neuronal cytotoxicity when compared with isosmolar choline control solution. To summarize, perineural analgesia that excludes local anesthetics would be potentially “relatively neuroprotective,” but none of these adjuvants at the concentrations studied were absolutely neuroprotective (in vitro) when combined with ropivacaine.
Figure 1.
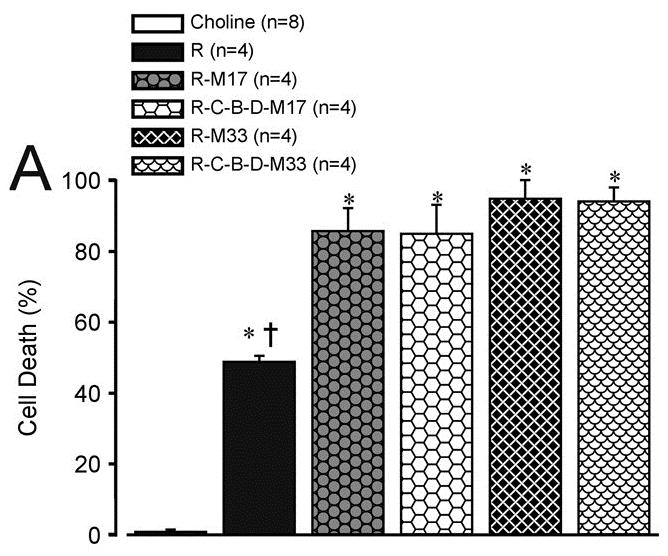
Cell death as a function of treatment after 24 hours exposure to ropivacaine alone (RPV) and clonidine(C)-buprenorphine(B)-dexamethasone(D)-midazolam(MDZ) adjuvant combinations.
Panel A shows all RPV-M combinations. Panel B shows the cytotoxicity outcomes in clinical concentrations with and without RPV 2.5mg/mL.
Source: Figure 3A, Williams, BA, Hough, KA, Tsui BY et al. Neurotoxicity of Adjuvants Used in Perineural Anesthesia and Analgesia in Comparison with Ropivacaine. Reg Anesth Pain Med. 2011 May–Jun;36(3):225–230
The study11 was limited (e.g., resources were available to explore in vitro effects on ropivacaine, but not in combination with other local anesthetics), but establishes data that has broad implications. Medications injected perineurally (in vivo) can diffuse into surrounding tissues, and may be metabolized or absorbed by the surrounding vasculature, potentially lowering neurotoxic effects produced by direct contact. These mitigating factors are not subject to analysis in an in vitro model. Perineural local anesthetics in clinical scenarios primarily act at the axon of nerves, whereas in this study11 the primary sensory neuron (i.e., cell body) was exposed to the drug/combination. Drug concentrations used in portions of this study11 were commonly well-above clinical concentrations, rendering neurons in this model more sensitive to potential neurotoxic effects. Because these drugs were neither metabolized nor absorbed by surrounding vasculature, the neurons were exposed to higher net concentrations of the adjuvants (with or without ropivacaine, specifically in the 24-hour exposure experiments). These factors theoretically provide a higher margin for safety, given the lack of neurotoxic findings with C-B-D adjuvants added to ropivacaine, or with C-B-D added to midazolam. The dosing parameters established by this study are clinically relevant, based on doses used in previous clinical reports, and the dose-concentrations deemed safe in vitro were used in subsequent in vivo work. The article11 emphasizes the need for further investigation, especially regarding time- and concentration-dependent neurotoxicity of combinations of dexamethasone and local anesthetics. The authors also suggest that their findings indicate that midazolam should not be combined with any local anesthetic for perineural injections until further safety data can be gathered.
Advancing beyond the cellular model, a recent 2015 report12 evaluated the effect of bupivacaine combined with the aforementioned C-B-D adjuvants on rat perineural and dorsal root ganglia tissue. Also evaluated was the chemical compatibility and solubility of such combinations for storage and injection. For the in vivo peripheral nerve and dorsal root ganglia toxicity study, combinations of saline control versus bupivacaine-C-B-D or midazolam-C-B-D were injected perineurally (sciatic) in rats. Behavioral observation of all animals was carried out, and after 15 days neural tissue was harvested and evaluated for dorsal and ventral root fiber degeneration, gliosis, and neuronal vacuolization. The results revealed no behavioral changes with these single injections of any combination of the above drugs at either 1 or 15 days. There were also no major non-reversible motor or proprioceptive differences between the control group and the above adjuvants with bupivacaine (Figure 2). Upon histopathological examination, there were no changes detected in the sciatic nerve, the dorsal or ventral nerve roots, or the dorsal root ganglia. Experiments with multimodal infusions were similarly non-toxic and behaviorally reversible.
Figure 2.
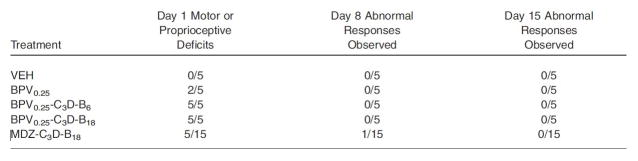
Behavioral response to single injection treatments.
B = buprenorphine, BPV = bupivacaine, C = clonidine, D = dexamethasone, MDZ = midazolam, VEH = vehicle (saline)
Source: Table 3, Williams BA, Butt MT, Zeller JR et al. Multimodal Perineural Analgesia with Combined Bupivacaine-Clonidine-Buprenorphine-Dexamethasone: Safe In Vivo and Chemically Compatible in Solution. Pain Med. 2015 Jan;16(1):186–198
In the chemical compatibility portion of the study, the authors employed concentration data from the simultaneous in vivo investigation. Using these concentrations, chemical compatibility for refrigerated storage of the described combinations was established. The combination of bupivacaine with C-B-D was shown to be stable for single injection. Although this was a small study underwritten by the Department of Defense (as opposed to a major pharmaceutical manufacturer), the results establish strong interval evidence for the safety of these drugs via perineural injection in vivo. Metabolic breakdown of drugs and lower bioavailability was avoided by local injection, enhancing the accuracy of the doses employed. Larger-scale animal models and subsequent human testing will be certainly useful, pending extramural funding for such study, to reinforce these findings.
Given the known safety of these medications in vitro and in vivo, their off-label adjuvant use in peripheral nerve blocks has also been described clinically13. In a large quality improvement report from the senior author’s institution (author BAW), data for various lower and upper extremity perineural single injections were reviewed. The drug combination entailed bupivacaine with C-B-D. Duration of blockade was 37.0 hours (interquartile range 30–49 hours). The incidence/severity of rebound pain was also reported in the context of adjuvant dose thresholds. The rebound pain results showed that for patients undergoing spinal anesthesia coupled with L2-4 and L4-S3 nerve/plexus blocks for total knee or hip arthroplasty, there was an increased incidence/severity of rebound pain with lower doses of both perineural buprenorphine (≤300μg associated with more rebound pain, vs >300μg) and higher doses of dexamethasone (4 mg with more rebound pain vs 2 mg). Clonidine (perineural dose range 50–100 μg) had no effect on rebound pain with any block method. When examining patients undergoing brachial plexus blocks for upper extremity surgeries, the addition of buprenorphine was also associated with prolonged duration of perineural analgesia. All results are described in Figure 3.
Figure 3.
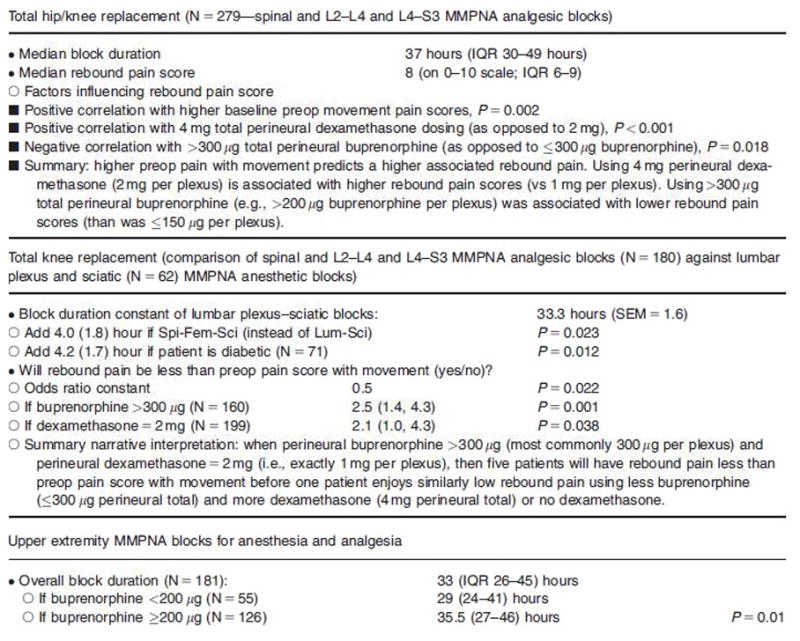
Data highlights regarding the described combined drugs used in MMPNA.
L2-L4: femoral nerve analgesic MMPNA blocks for knee replacement surgery, and lumbar plexus psoas compartment (“Lum”) analgesic MMPNA blocks for hip replacement surgery, coadministered with spinal (“Spi”) anesthesia. L4-S3: gluteal approach sciatic nerve (“Sci”) analgesic MMPNA blocks for knee replacement surgery, and parasacral plexus analgesic MMPNA blocks for hip replacement surgery coadministered with spinal anesthesia. IQR = interquartile range; SEM = standard error of the mean.
Source: Table 1, Williams BA, Ibinson JW, Mangione MP et al. Research Priorities Regarding Multimodal Peripheral Nerve Blocks for Postoperative Analgesia and Anesthesia Base on Hospital Quality Data Extracted from Over 1300 Cases (2011–2014). Pain Med. 2015 Jan;16(1):7–12
The data from this13 recent quality improvement observation report introduces the clinical use of local anesthetics combined with the various adjuvants discussed earlier. The mixture of drugs was injected simultaneously, resulting in nerve blocks of substantial duration (33 hours for lumbar plexus and sciatic anesthetic nerve blocks for total knee arthroplasty and for upper extremity blocks). The knee replacement analgesic duration was increased by addition of 2 mg (but not 4 mg) dexamethasone, while brachial plexus analgesic duration was extended by the use of buprenorphine ≥200 μg. Since the 2 mg total perineural dexamethasone appeared to be clinically sufficient after the described lower extremity blocks, and that higher doses yielded higher-associated rebound pain incidence and severity, there appears to be no need to increase perineural dexamethasone doses beyond 2 mg (i.e., 1 mg per blocked nerve/plexus in a dual nerve/plexus block). Buprenorphine dosing was not associated with longer block duration after lower extremity surgery, but doses above 300 μg were associated with less rebound pain. Based on these observations, future work could be directed toward finding an upper limit of dosing based on the aforementioned toxicology and chemical compatibility data. Since this was a retrospective review of quality improvement data, and in the absence of comparisons with local anesthetics sans adjuvants, a randomized control trial is the next appropriate step to fully elucidate the effects of these adjuvant medications.
Effects on Motor Blockade
The degree to which these adjuvants affect motor blockade of local anesthetics, an often undesirable side effect, is less well-defined. Dexmedetomidine and clonidine have both been found to prolong sensory but also motor blockade when combined individually with local anesthetics for use in supraclavicular brachial plexus blocks. When compared to one another, addition of dexmedetomidine (as opposed to clonidine) leads to a more pronounced motor blockade24. Addition of dexamethasone to 0.25% bupivacaine for brachial plexus blocks has also been shown to significantly increase duration of motor blockade, but a dose-response relationship with increasing concentrations of dexamethasone was not observed25. Buprenorphine was not observed to speed the onset of motor blockade in combination with levobupivacaine in interscalene blocks26; these authors were unaware of any trials that had specifically examined changes in motor block duration based on addition of buprenorphine to local anesthetics. No trials exist examining the prolongation of motor block when combining clonidine, buprenorphine, and dexamethasone with local anesthetics. Therefore, it is difficult to determine what effect if any these adjuvants may have on prolonging time to ambulation, effectiveness of physical therapy, or discharge from the hospital. Logic would indicate that when such 4-drug multimodal perineural blocks are combined with spinal (e.g., for lower extremity joint replacement surgery), that there is an effective dose (in 50 percent [i.e., ED50] or in 95 percent [i.e., ED95] where a fixed dose of adjuvants will lead to the smallest (higher than zero) duration of motor block, thus creating an optimal ratio of sensory-to-motor block duration. It will be important in future clinical research to emphasize that when the definitive lower extremity anesthetic is being provided by a spinal technique, that a “zero” motor block duration will most likely jeopardize a meaningful analgesic duration. Instead, the optimal motor block duration would roughly equate the duration of motor block from the residual spinal, and block-specific multimodal sensory analgesia would incorporate true motor-sensory separation for ideally more than 12–24 hours after the spinal (and perineural local anesthetic) motor effects have dissipated, such that focused immediate postoperative physical therapy can maximize the remaining perineural analgesia provided by the adjuvants. This dose-finding challenge will likely mark the ultimate research goal for fast-track joint replacement (i.e., same-day or next-day discharge home) as well as for moderately invasive outpatient orthopedic surgery (such as hip arthroscopy or anterior cruciate ligament reconstruction of the knee). Clinicians over the foreseeable future should carefully titrate via a single-injection technique adequate pain control with minimal (but likely greater than zero) motor blockade in this patients, especially in a busy outpatient or fast-tracking surgical setting. The senior author predicts that combined midazolam-C-B-D may prove to be a worthwhile analgesic block for cases such as (i) hip arthroscopy with minimal procedures beyond debridement (e.g., L2-L4 psoas compartment block with or without an L4-S3 parasacral plexus block), or (ii) anterior cruciate ligament reconstruction (e.g., with an L2-L4 femoral block with or without and L4-S3 gluteal/subgluteal sciatic block). We do not forecast that the midazolam-C-B-D combination will be sufficiently potent for major L2-L4 analgesia in arthroplasty or other resurfacing procedures of the hip or knee; it seems more likely that at least a low concentration local anesthetic will be required.
Conclusion
This review provides a reasonable foundation for establishing the safety and rational clinical administration and dosing of local anesthetics (e.g., bupivacaine, ropivacaine) combined with the adjuvants clonidine, dexamethasone, and buprenorphine. We now know that midazolam should never be combined with local anesthetics, with basic science reports coming after two clinical reports27,28 before in vitro testing of this combination demonstrating significantly-worsened neuronal cytotoxicity. C-B-D in combination was found to be safe at clinical concentrations combined with local anesthetics in vitro and in vivo; midazolam, however, with local anesthetics was not. Since these studies were not industry-sponsored, they were relatively small, and further large animal studies and subsequent human trials are still needed (pending extramural funding research priority) before the physiologic analgesic mechanisms and/or neurotoxicity risk of these adjuvant medications is fully understood. Once understood, the subsequent clinical utility and efficacy of these drugs can be optimally utilized in the future, en route toward prolonged analgesia with a single-injection nerve block in cases where perineural catheters may prove to be unnecessary (i.e., when an approximate 36-hour analgesic-duration block is reliably available for clinical use).
Key Points.
Local anesthetics are by nature neurotoxic, this neurotoxicity is worsened when combined with midazolam. However, there is a reasonable clinical concentration threshold of combined clonidine-buprenorphine-dexamethasone that is (i) non-toxic when combined with midazolam (33 mcg/mL), and (ii) not contributory to worsening baseline neurotoxicity when combined with local anesthetics.
High (supraclinical) concentrations of some adjuvants combined with local anesthetics, or in some cases as single drugs, can be harmful in vitro.
Clinically relevant concentrations of bupivacaine-C-B-D are chemically compatible and stable in solution, and also show no evidence of in vivo neurotoxicity in animal models in the described doses
Larger studies are necessary for better mechanistic, safety, and clinical understanding.
Acknowledgments
Financial Support and Sponsorship
This work was supported by the Department of Anesthesiology, University of Pittsburgh Medical Center (Drs. Knight, Schott, and Kentor), and by the VA Pittsburgh Health System (Dr. Williams), Pittsburgh, Pennsylvania
Footnotes
Conflicts of Interest
There are no conflicts of interest present. The contents do not reflect the views of the Department of Veterans Affairs or the United States Government.
Contributor Information
Joshua B. Knight, University of Pittsburgh Medical Center, Department of Anesthesiology
Nicholas J. Schott, University of Pittsburgh Medical Center, Department of Anesthesiology
Michael L. Kentor, University of Pittsburgh Medical Center, Associate Professor, Department of Anesthesiology
Brian A. Williams, VA Pittsburgh Health System, and Professor of Anesthesiology, University of Pittsburgh School of Medicine.
References
- 1.Lavand’homme PM, Eisenach JC. Perioperative administration of the α2-adrenoceptor agonist clonidine at the site of nerve injury reduces the development of mechanical hypersensitivity and modulates local cytokine expression. Pain. 2003;105(1–2):247–254. doi: 10.1016/s0304-3959(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 2.Kroin JS, Buvanendran A, Beck DR, et al. Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by α-adrenoreceptors. Anesthesiology. 2004 Aug;101(2):488–494. doi: 10.1097/00000542-200408000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Brummett CM, Hong EK, Janda AM, et al. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011 Oct;115(4):836–843. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain. 2005 Nov;118(1–2):15–22. doi: 10.1016/j.pain.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Leffler A, Frank G, Kristner K, et al. Local anesthetic-like inhibition of voltage gated Na+ channels by the partial μ-opioid receptor agonist buprenorphine. Anesthesiology. 2012 Jun;116(6):1335–1346. doi: 10.1097/ALN.0b013e3182557917. [DOI] [PubMed] [Google Scholar]
- 6.Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anesthesiol Scand. 1990 Jul;34(5):335–338. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 7**.Yilmaz E, Hough KA, Gebhart GF, et al. Mechanisms underlying Midazolam-induced Peripheral Nerve Block and Neurotoxicity. Reg Anesth Pain Med. 2014 Nov-Dec;39(6):525–533. doi: 10.1097/AAP.0000000000000176. Discusses peripheral actions of midazolam and that selective inhibition of TPSO receptors may achieve analgesia normally caused by benzodiazepines while circumventing their neurotoxic effects. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Norat MA, Palmisano JM, et al. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008 Sep;109(3):502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritsch G, Danninger T, Allerberger K, et al. Dexemedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014 Jan-Feb;39(1):37–47. doi: 10.1097/AAP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 10.Williams BA, Murinson BB, Grable BR, Orebaugh SL. Future Considerations for Pharmacologic Adjuvants in Single-Injection Peripheral Nerve Blocks in Patients with Diabetes Mellitus. Reg Anesth Pain Med. 2009 Sep-Oct;34(5):445–457. doi: 10.1097/AAP.0b013e3181ac9e42. [DOI] [PubMed] [Google Scholar]
- 11.Williams BA, Hough KA, Tsui BY, et al. Neurotoxicity of Adjuvants Used in Perineural Anesthesia and Analgesia in Comparison with Ropivacaine. Reg Anesth Pain Med. 2011 May-Jun;36(3):225–230. doi: 10.1097/AAP.0b013e3182176f70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Williams BA, Butt MT, Zeller JR, et al. Multimodal Perineural Analgesia with Combined Bupivacaine-Clonidine-Buprenorphine-Dexamethasone: Safe In Vivo and Chemically Compatible in Solution. Pain Med. 2015 Jan;16(1):186–198. doi: 10.1111/pme.12592. One of the primary subjects of this review, this article outlines the safety of the adjuvants clonidine, buprenorphine, and dexamethasone when employed in nerve blocks with bupivacaine, as well as the chemical stability of mixtures of these drugs. [DOI] [PubMed] [Google Scholar]
- 13**.Williams BA, Ibinson JW, Mangione MP, et al. Research Priorities Regarding Multimodal Peripheral Nerve Blocks for Postoperative Analgesia and Anesthesia Base on Hospital Quality Data Extracted from Over 1300 Cases (2011–2014) Pain Med. 2015 Jan;16(1):7–12. doi: 10.1111/pme.12609. One of the primary subjects of this review, this article discusses the overall clinical performance of local anesthetic nerve blocks containing combinations of clonidine, buprenorphine, and dexamethasone in a large hospital setting. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Zhao F, Li C, et al. Molecular Mechanisms Underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: an in vivo and in vitro experimental study. PLoS One. 2013;8(2):e55556. doi: 10.1371/journal.pone.0055556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006 Feb;50(2):222–227. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Bogra J, Verma R, et al. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011 Jul-Aug;55(4):347–351. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Huang Y, Lu Y, Zang L, et al. Perineural Dexmedotomidine Attenuates Inflammation in Rat Sciatic Nerve via the NF-kB pathway. Int J Mol Sci. 2014 Mar;45(3):4049–4059. doi: 10.3390/ijms15034049. Discusses molecular mechanisms of another α-2 agonist at the site of injection and shows how this could protective in the setting of regional nerve blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackinnon SE, Hudson AR, Gentili F, et al. Peripheral nerve injection injury with steroid agents. Plast Reconstr Surg. 1982 Mar;69(3):482–490. doi: 10.1097/00006534-198203000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Cairns BE, Sessle BJ, Hu JW. Activation of peripheral GABAA receptors inhibits temporomandibular joint-evoked jaw muscle activity. J Neurophysiol. 1999 Apr;81(4):1966–1969. doi: 10.1152/jn.1999.81.4.1966. [DOI] [PubMed] [Google Scholar]
- 20.Kugawa F, Arae K, Ueno A, et al. Buprenorphine hydrochloride induces apoptosis in NG108-15 nerve cells. Eur J Pharmacol. 1998 Apr 17;347(1):105–112. doi: 10.1016/s0014-2999(98)00080-6. [DOI] [PubMed] [Google Scholar]
- 21.Johansen MJ, Gradert TL, Satterfield WC, et al. Safety of continuous intrathecal midazolam infusion in the sheep model. Anesth Analg. 2004 Jun;98(6):1528–1535. doi: 10.1213/01.ANE.0000120086.35289.9D. [DOI] [PubMed] [Google Scholar]
- 22.Tucker AP, Lai C, Nadeson R, et al. Intrathecal midazolam I: a cohort study investigating safety. Anesth Analg. 2004 Jun;98(6):1512–1520. doi: 10.1213/01.ANE.0000087075.14589.F5. [DOI] [PubMed] [Google Scholar]
- 23*.Schott NJ, Williams BA. Intravenous and perineural dexamethasone in peripheral nerve block: Are they truly equivalent? Anesth Analg. 2015 doi: 10.1213/ANE.0000000000000762. Forthcoming. This reply to a letter to the editor, currently in press, discusses the performance of dexamethasone administered intravenously versus perineurally in controlling postoperative pain. [DOI] [PubMed] [Google Scholar]
- 24.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anesthesia in supraclavicular brachial plexus block: A randomized double blind prospective study. Indian J Anaesth. 2012 May;56(3):243–249. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Richman KA, Grodofsky SR, et al. Is there a dose response of dexamethasone as adjuvant for supraclavicular brachial plexus nerve? A prospective randomized double-blinded clinical study. J Clin Anesth. 2015 May;27(3):237–242. doi: 10.1016/j.jclinane.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Behr A, Freo U, Ori C, et al. Buprenorphine added to levobupivacaine enhances post-operative analgesia of middle interscalene brachial plexus block. J Anesth. 2012 Oct;26(5):746–751. doi: 10.1007/s00540-012-1416-4. [DOI] [PubMed] [Google Scholar]
- 27.Jarbo K, Batra YK, Panda NB, et al. Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth. 2005 Oct;52(8):822–826. doi: 10.1007/BF03021776. [DOI] [PubMed] [Google Scholar]
- 28.Laiq N, Khan MN, Arif M, Khan S. Midazolam with bupivacaine for improving analgesia quality in brachial plexus block for upper limb surgeries. J Coll Physcians Surg Pak. 2008 Nov;18(11):674–678. [PubMed] [Google Scholar]


