Abstract
Chloroplasts are known to sustain life on earth by providing food, fuel and oxygen through the process of photosynthesis. However, the chloroplast genome has also been smartly engineered to confer valuable agronomic traits and/or serve as bioreactors for production of industrial enzymes, biopharmaceuticals, bio-products or vaccines. The recent breakthrough in hyper-expression of biopharmaceuticals in edible leaves has facilitated the advancement to clinical studies by major pharmaceutical companies. This review critically evaluates progress in developing new tools to enhance or simplify expression of targeted genes in chloroplasts. These tools hold the promise to further the development of novel fuels and products, enhance the photosynthetic process, and increase our understanding of retrograde signaling and cellular processes.
Keywords: biofuels, biopharmaceuticals, molecular farming, metabolic engineering, biotic/abiotic stress tolerance, retrograde signaling
Two decades of chloroplast genetic engineering
Almost two decades ago, the tobacco (Nicotiana tabacum) chloroplast genome was engineered to confer herbicide and insect resistance, outperforming nuclear transgene expression by several hundred-fold [1, 2]. Another milestone was engineering salt tolerance in carrot (Daucus carota), a species requiring somatic embryogenesis [3]. This was followed by several other reports utilizing somatic embryogenesis including cotton (Gossypium hirsuturm) [4] and soybean (Glycine max) [5, 6]. Today, a number of edible crops have been transformed utilizing organogenesis, including lettuce (Lactuca sativa), cabbage (Brassica oleraceavar), potato (Solanum tuberosum), tomato (Lycopersicon esculentum), and sugar beet (Beta Vulgaris) [7–11]. However, plastid transformation of cereal crops remains elusive.
Current chloroplast genome engineering projects have led to stable integration and expression of transgenes from different kingdoms including bacterial, viral, fungal, animal and human genes to express biopharmaceutical proteins, antibiotics, vaccine antigens, industrial enzymes, biomaterials, and confer valuable agronomic traits. High levels of expression, multi-gene engineering in a single transformation event, transgene containment via maternal inheritance and minimal pleiotropic effects due to subcellular compartmentalization of toxic transgene products are typical advantages of transforming the chloroplast over the nuclear genome. In this review, we critically evaluate recent advances in this expanding field.
Biopharmaceuticals expressed in chloroplasts advanced to clinical studies by the pharmaceutical industry is a clear indication of the current maturity of this field and importance of this approach. Hyper-expression of biopharmaceuticals in healthy plants (making up to 70% of total leaf protein) and the ability to express in edible leaves enables their oral delivery, significantly reducing their production cost. Several metabolic and genetic diseases including Alzheimer’s, diabetes, hypertension, hemophilia, and retinal diseases were successfully treated by therapeutic proteins made in chloroplasts. Several new tools including Gateway/modular chloroplast vectors, splicing exons to facilitate expression of eukaryotic genes using overlapping PCR primers, multigene engineering concepts, and application of species specific vectors have significantly improved efficiency of chloroplast genome engineering. RNA interference concept has been explored for the first time via the chloroplast genome to silence genes in the insect gut. Single genes conferring diverse agronomic traits including enhanced biomass, resistance to biotic/abiotic stress have been explored. Most importantly, transgenes engineered via the chloroplast genome regulates nuclear gene expression, offering a valuable tool to understand retrograde signaling and other cellular processes
The art of chloroplast genome engineering – evolving new concepts
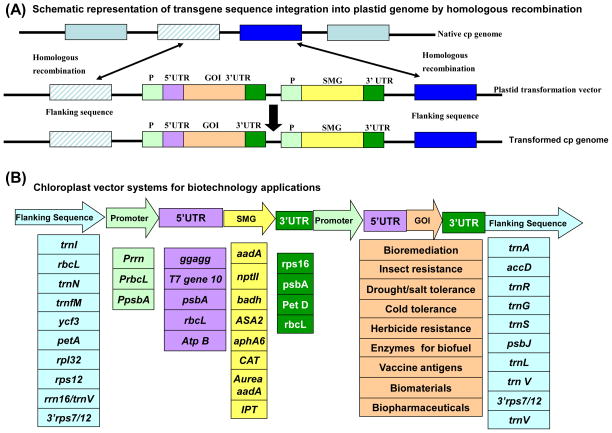
Chloroplast transformation requires double homologous recombination (Figure 1A) [12, 13]. Therefore, two chloroplast DNA segments are used as franking sequences in chloroplast vectors to insert transgene cassette into the intergenic spacer region, without disrupting any functional genes. So, the first debate that arose in this field was finding the ideal site for transgene integration. Two opposing theories emerged to insert transgenes into transcriptionally silent spacer regions (in which chloroplast genes are located in opposite DNA strands and orientations –the Maliga concept) or insertion into transcriptionally active spacer regions (within chloroplast operons – the Daniell concept). The advantage of each site was tested recently by insertion of the lux operon with identical expression cassette at both sites and transcriptionally active spacer region offered a 25-fold higher level of expression [14] and authors attributed this to higher read-through transcriptional activity. To date, one of the most commonly used site of transgene integration is the transcriptionally active intergenic region between the trnI-trnA genes (in the rrn operon), located within the IR regions of the chloroplast genome [12, 13, 15–17], although several other sites have been explored (Figure 1B). With insertion of seven transgenes at this site, up to thirteen genes could be driven by two endogenous 16S rrn and psbA promoters [18, 19]. This flanking sequence includes the chloroplast origin of replication (that provides more copies of templates for integration) and copy correction mechanism existing within the inverted repeat regions enhance homoplasmy (see Glossary) [20]; in addition, introns present within these genes facilitate efficient processing of transgene transcripts. Transgene expression levels inserted at this site are among the highest reported in the published literature [21, 22].
Figure 1.
The art of chloroplast genome engineering. (A) Schematic representation of the chloroplast vector. This includes at least two chloroplast DNA fragments as flanking sequences to facilitate double homologous recombination, a selectable marker gene and regulatory elements. (B) Examples of components commonly used in chloroplast vectors. Abbreviations: 3′ UTR to enhance transcript stability; 5′ UTR to enhance ribosome binding; cp: chloroplast; GOI: genes of interest for various biotechnology applications; P: promoters; SMG: selective marker genes.
It is important to use species specific endogenous regulatory sequences in transgene cassettes to achieve high levels of expression [23]. The endogenous psbA promoter and 5′ and 3′ untranslated regions [24] and heterologous T7gene 10[20, 25]continue to be widely used for transgene expression but more regulatory sequences are needed for multigene engineering. A chloroplast gene expression system driven by an inducible promoter would reduce pleiotropic effects of toxic foreign proteins expressed in transplastomic plants. Such an inducible plastid gene expression system was first developed using a nuclear inducible promoter. The phage T7 RNA polymerase was targeted to chloroplasts to drive transgenes integrated via the chloroplast genome [25] and was used to express the phb operon a decade later [26]. More recently, the riboswitch concept has been introduced to regulate transgene expression in transplastomic plants [27]. Although this concept represents a simple approach to turn on or off an introduced transgene, their switching efficiency is low and induction of transgene expression after ligand addition or removal is poor and requires further optimization for enhanced transgene expression.
Two new strategies for construction of chloroplast vectors have been developed recently. One method used the Gateway system for construction of chloroplast vectors to simplify vector construction and improve vector design [28]. Another group used modular design of genetic elements to construct chloroplast vectors to build transcriptional units as well as target any homologous recombination site of choice [29]. In addition, an intercistronic expression element (IEE) was introduced into the spacer region between cistrons to enhance processing of polycistrons into monocistrons to enhance translation [7]. However, this concept contradicts recent in depth ribosome profiling studies [30] that show similar translation efficiency in both spliced and unspliced native chloroplast polycistrons, as observed previously by high level expression of several heterologous polycistrons via the chloroplast genome without IEE [21, 31] or multigenes engineered recently via the chloroplast genome [18, 19]. New PCR methods using overlapping primers has been used to eliminate introns and splice exons facilitate expression of eukaryotic genes without the need for cDNA libraries; this concept was successfully employed to transform the chloroplast genome with fungal genes containing >10 introns [32]. Although codon optimization is desirable to enhance expression level of eukaryotic genes[33], the highest expressed genes are ironically encoded by native human genes [23], suggesting that codon usage in chloroplast is a lot more flexible than several other recombinant protein expression systems.
One major limitation is the availability of selectable markers that impact only chloroplast protein synthesis but not any other cellular compartment. The aadA gene, first successfully used for Chlamydomonas chloroplast transformation [34] and later in tobacco chloroplasts [35] on spectinomycin selection, is the only marker that has worked reproducibly to regenerate transplastomic events in a number of different plant species. In recent years, several antibiotic free selectable markers including D-amino acid oxidase [36], positive selectable marker isopentenyl transferase (ipt)[37], as well asanthranilate synthase [alpha]-subunit (ASA2) gene [38] have been developed (Figure1B). Removal of antibiotic selectable markers could be achieved using direct repeats or cre-lox recombination approaches [39]. Indeed, precise excision of the selectable marker gene (aadA) was accomplished recently from the most commonly used transgene integration site (trnA/trnI) by using Bxb1 recombinase and attP/attB recognition sites [40].
In the past two decades, chloroplast genetic engineering has been focused primarily on achieving hyper-expression of foreign proteins. Although chloroplast genome has the exceptional ability to produce abundant transcripts it has not been exploited to produce and deliver dsRNA. Application of RNAi technology through plant nuclear genome has several limitations. Likewise, delivery of small RNA prepared in other systems for human therapeutics is highly challenging [41]. In agriculture, there is a great need to down regulate harmful genes to protect plants from pests. Similarly, down regulation of dysfunctional genes that cause cancer or autoimmune diseases or immune disorders could help in their treatment. Due to the high level of chloroplast transcription, dsRNA could be synthesized in large quantities and orally delivered via bioencapsulation in plant cells to target harmful genes [42]. In two examples described below lepidopteran chitin synthase (Chi), cytochrome P450 monooxygenase (P450) and V-ATPase dsRNA made in chloroplasts were used to silence these target genes in the insect gut [10, 43].
Emerging new concepts for insect control via the chloroplast genome
Although major advances were made to hyper-express native biopesticide genes from Bacillus thuringiensis via the chloroplast genome to form Bt crystals within chloroplasts [21] and even kill 40,000 fold resistant insects [44], expression of Bt genes in important major crops [45], they did not yet reach commercial development, largely because the market is already saturated with Bt crops meeting farmer’s’ needs to eliminate the use of expensive chemical pesticides. However, a recent report of alarming Bt resistance that has led to new US EPA requirements on planting Bt corn (The Wall Street Journal, March 5, 2015), points out the need for high dose or multi-gene strategy. Thus, recent focus in this field is shifting to identify novel traits or methods currently not available or inadequate to facilitate commercial development.
Recently, the RNA interference (RNAi) concept was used for the first time to engineer the chloroplast genome (Figure 2E [43]). In that study the lepidopteran chitin synthase (Chi), cytochrome P450 monooxygenase (P450) and V-ATPase, β-actin genes were used to study RNAi of target genes. The abundance of cleaved dsRNA was greater than that of the highly expressed endogenous psbA transcript. Feeding off P450, Chi and V-ATPase siRNA leaves decreased transcription levels of the targeted gene to almost undetectable levels in the insect midgut, most likely after further processing of siRNA in the insect gut. Therefore, the net weight of larvae, growth and pupation rates were significantly reduced (Figure 2I [43]). In a parallel study, Bock’s group introduced dsRNA via the chloroplast genome to target β-actin gene against potato beetle and performed outstanding studies to demonstrate efficacy against this important pest in field studies [10]. Taken together, successful expression of dsRNAs via the chloroplast genome opens the door to study RNA interference via plastid genetic engineering and their utilization for gene inactivation to confer desired agronomic traits or for down regulation of dysfunctional genes for various biomedical applications in cancer or autoimmune disorders, after oral delivery of dsRNA bioencapsulated within plant cells.
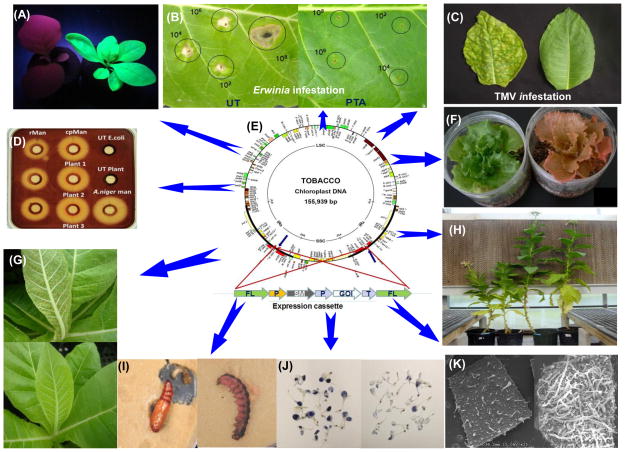
Figure 2.
Engineering chloroplast genome to confer biotic/abiotic stress tolerance or expression of high value products. (A) Antimicrobial peptide retrocyclin-101 fused with GFP expressed in chloroplasts display strong green florescence in contrast to untransformed leaf showing chlorophyll red fluorescence [49]. (B-C)) The transplastomic leaf expressingPinellia ternata agglutinin shows high level tolerance when challenged with bacterial (Erwinia) or viral (TMV) pathogens [47] (D) Gel diffusion assay shows the zone of clearance of chloroplast-derived endo-β-mannanase in crude plant extacts similar to purified recombinant commercial enzyme [51]. (E) Tobacco chloroplast genome and integration of the expression cassette. (F) Enhanced accumulation of astaxanthin, carotenoids in transplastomic lettuce [108]. (G) Transplastomic plants expressing the lectin gene shows broad spectrum resistance to Lepidopteran, homopteran (sap-sucking) insects as well as anti-bacterial (Erwinia) and anti-viral (TMV) activities [47]. (H, K) Chloroplast expression of β-glucosidase results in elevated phytohormone levels associated with significant increase in biomass and trichome density [46]. (I) Cotton bollworm larvae with normal pupation or dead larvae when fed with transplastomic tobacco leaves expressing dsRNAs [43]. (J) Overexpession of gamma-tocopherol methyl transferase chloroplasts conferrsabiotic stress tolerance and nutritional enhancement [55].
Broad spectrum agronomic traits conferred via the chloroplast genome
A novel mechanism to release active hormones (Gibberellin, indolyl-3-acetic acid and zeatin) from inactive ester conjugates was accomplished by expression of β-glucosidase in chloroplasts. Transplastomic lines showed increase in leaf area, height, biomass and internode length. Most importantly, the density of globular trichomes containing sugar esters on leaf surface increased dramatically conferring protection from whitefly and aphid infestations. These novel observations open new avenues to modify plants for enhanced biomass, confer novel traits, especially resistance to aphids and whiteflies (Figure 2H, 2K) [46].
Likewise, transplastomic plants expressing the Pinellia ternata agglutinin (pta) showed broad spectrum resistance to phloem -feeding pests as well as anti-viral and anti-bacterial activities, providing a new option to engineer protection against different types of biotic stress using a single protein that is naturally present in medicinal plants (Figure 2B, C, G) [47]. Likewise, gene stacking with protease inhibitors and chitinase expressed in transplastomic tobacco displayed broad-spectrum resistance against insects, pathogens and abiotic stress [48]. A list of recent reports on enhancing agronomic traits via the chloroplast genome is provided in Table 1. Other novel approaches used to engineer protection against viral, bacterial or fungal pathogens include expression of synthetic antimicrobial peptide genes [49, 50] (Figure 2A) or bacterial/fungal enzymes [51, 52] as described in sections below.
Table 1.
Recent reports on enhancement of agronomic traits engineered via the chloroplast genome.
| Protein/enzymes | Source of transgene | Plant species | Expression levels/activities | Function/phenotype | Refs |
|---|---|---|---|---|---|
| Retrocyclin-101 and Protegrin-1 | Synthetic | Tobacco | 32–38% and 17–26% total soluble protein in leaves (TSP) | Enhanced resistance to Erwinia soft rot and tobacco mosaic virus | [49] |
| β-glucosidase Bgl 1 | T. reesei | Tobacco | Up to 44.41 units/g leaf | Whitefly and aphid resistance | [46] |
| Metallothionein-1 | Mouse | Tobacco | 183000 transcripts/ng of RNA | Phytoremediation by Hg chelation | [92] |
| D-amino acid oxidase | Schizosaccharomy ces pombe | Tobacco | Not reported (NR) | D-alanine-based herbicides resistance | [36] |
| Agglutinin | Pinellia ternata | Tobacco | 7.1–9.2% TSP | Multiple resistances against aphid, whitefly, Lepidopteran insects, bacterial and viral pathogens | [47] |
| Thioredoxin f | Tobacco | Tobacco | NR | Enhanced starch accumulation in leaves | [93] |
| Toc cyclase and γ-tocopherol methyltransferase | Arabidopsis | Tobacco and lettuce | 3.05 nmol h−1 mg−1 protein | Enhanced vitamin E accumulation in tobacco and lettuce | [9] |
| Homogentisate phytyltransferase tocopherol cyclase, and γ-tocopherol methyltransferase | Synechocystis sp. PCC6803 | Tomato | NR | Enhanced vitamin E accumulation in fruits. Increased high light and cold stress tolerance | [7] |
| β,β-carotenoid-3,3′-hydroxylase, β, β-carotenoid 4,4′-ketolase (4,4′-oxygenase) | Brevundimonas sp. strain SD212 | Lettuce | NR | Increased astaxanthin fatty acid esters accumulation in lettuce plants | [54] |
| Thioredoxins m | Tobacco | Tobacco | NR | Enhanced resistance to oxidative stress in tobacco plants | [94] |
| γ-tocopherol methyltransferase | Arabidopsis | Tobacco | 7.7% of the total leaf protein | Enhanced accumulation of α-tocopherol in seeds. Increased salt and heavy tolerance | [55] |
| Protease inhibitors and chitinase | Paecilomyces javanicus | Tobacco | NR | broad-spectrum resistance against insects, pathogens and abiotic stresses | [48] |
| Chloroperoxidase (CPO) | Pseudomonas pyrrocinia | Tobacco | 15 μg CPO/ml extract | Enhanced resistance to fungal pathogens in vitro | [95] |
Metabolic engineering via the chloroplast genome
The first metabolic engineering via the chloroplast genome using bacterial chorismate pyruvate lyase gene accumulated p-hydroxybenzoic acid liquid crystal polymer up to 26.5% of dry weight with no pleiotropic effects [53]. Recently, the entire cytosolic mevalonate pathway coding for six enzymes and the selectable marker (total seven genes) were inserted into the tobacco chloroplast genome. Despite lack of enhanced regulatory sequences (promoters/UTRs), transplastomic plants accumulated significantly higher levels of mevalonate, carotenoids, squalene, sterols, and triacyglycerols than control plants, successfully redirecting metabolic fluxes for isoprenoid biosynthesis [18].
Astaxanthin, a natural pigment has attracted more attention recently for its antioxidant activity and color conferred to fish like salmon. Expression of CrtW (β-carotene ketolase) and CrtZ (β-carotene hydroxylase) and isopentenyl diphosphate isomerase via the lettuce chloroplast genome accumulated the astaxanthin fatty acid esters and other key carotenoids (arti cial ketocarotenoids corresponded to 95 % of total carotenoids ) [54]. Tocopherols (main forms of vitamin E) are lipid soluble antioxidants and play an important role in the plant antioxidant network by eliminating reactive oxygen species (ROS). Expression of γ-Tocopherol (γ-TMT)methyltransferase and tocopherol cyclase (TC) gene in chloroplasts resulted in α-Toc as a major isoform and increased total tocopherol levels [9]. Similarly, expression of HPT (homogentisate phytyltransferase), TC, and γ-TMT confirmed HPT as the limiting enzymatic step and increased total tocochromanol 10-fold [7]. More recently, γ-TMT gene expression resulted in massive proliferation of the inner chloroplast envelope membrane [55]. High level accumulation of α-tocopherol in transplastomic plants not only increased the nutritional value of plant but also enhanced the tolerance to abiotic stress by decreasing ROS (Figure 2J), lipid peroxidation and ion leakage [55]. These findings offer new insight into the regulation of vitamins or complex metabolite biosynthesis and potential of chloroplast genetic engineering for nutritional enhancement of edible plants (Figure 2F).
Enhancing photosynthetic efficiency via the chloroplast genome
Rubisco, the key enzyme in the Calvin cycle has attracted mainly attention for genetic manipulation to enhance carbon fixation efficiency, increase catalytic activity and/or reduce photorespiration. Thus, early studies involved relocation of the small subunit gene to the chloroplast genome to assemble fully functional Rubisco within chloroplasts [56]. More recent studies focus on expression of heterologous Rubisco subunits in chloroplasts. Most recently, a breakthrough was made by introducing the CO2-concentrating mechanism (CCM) from cyanobacteria into transplastomic plants [57]. The native tobacco gene encoding the large subunit of Rubisco was knocked out by inserting the large and small subunit genes of Synechococcus elongates Se7942 enzyme. Se7942 Rubisco andCcmM35 (a β-Carboxysomal protein) hybrid assembly within chloroplasts resulted in higher rates of CO2 fixation efficiency but slows growth. This represents a key step towards improved photosynthesis by chloroplast genetic engineering. Whitney’s group also enhanced photosynthesis and growth by co-expressing RAF1 chaperone along with Rubisco, which improved recombinant Rubisco biogenesis [58]
Chloroplast bioreactors for biofuel enzymes
The demand for sustainable and renewable energy sources is an important global challenge because of dwindling fossil fuel reserves and increased demand with growing population [59]. Production of cellulosic-derived ethanol is currently limited by the lack of infrastructure, technology, and high cost of enzymes. For most bioethanol process, one ton of biomass requires 15–25 kg cellulase [60] or 11 million filter paper units (FPU) of cellulase (around 19 kg) to yield 84 gallons of ethanol. More importantly, it is necessary to produce different kinds of enzymes individually and prepare enzyme cocktails to hydrolyze different types of biomass based on their polymer compositions. Therefore, the first and foremost requirement for ligno-cellulosic ethanol production is to develop an efficient enzyme production system for economical and rapid biomass depolymerization. High levels of expression and compartmentalization of toxic proteins within chloroplasts protect transgenic plants from pleiotropic effects, making chloroplast an ideal bioreactor for industrial enzyme production.
Although single biofuel enzymes were expressed a decade ago [61] followed by other studies [15, 16], total biomass hydrolysis was not feasible because of the number of enzymes required. So, a major recent advancement is the development of chloroplast derived enzyme cocktails for production of fermentable sugars from different ligno-cellulosic biomass. Most notably, nine different genes from bacteria or fungi (acetyl xylan esterase, cutinase, endoglucanases, exoglucanase, pectate lyases,, xylanase, lipase, etc.) were expressed in Escherichia coli or/and tobacco chloroplasts. The cost of chloroplast-derived endoglucanase was estimated to be 1000–3000-fold lower than the same recombinant enzymes sold commercially [32]. This is the first report of using plant-derived enzyme cocktails for production of fermentable sugars from ligno-cellulosic biomass.
However, overexpression of the enzymes (5–40% of total leaf protein) resulted in pigment-deficient mutant phenotypes, especially those that destabilized membranes like swollenin or expansin [52]. Intertwined cotton fibers were irreversibly unwound and fully opened up when treated with chloroplast derived swollenin. Likewise, cutinase effectively hydrolyzed digalactosyldiacylglycerol (DGDG ) to monogalactosyldiacylglycerol (MGDG), showing alpha galactosidase activity, demonstrating DGDG as a novel substrate and function [52]. Mannan is the major backbone of woody biomass and β-mannanase could efficiently catalyze endo-hydrolysis of this constituent. So, addition of chloroplast-derived mannanase to other enzymes in the cocktail further enhanced biomass hydrolysis [51]. Gel diffusion assay for endo-β-mannanase confirmed the functionality of chloroplast-derived mannanase (Figure 2D). Another advancement is production of thermostable enzymes in chloroplasts that enabled biomass hydrolysis [62]. A list of recent chloroplast-derived biofuel enzymes is summarized in Table 2. These studies are promising and have advanced the use of chloroplast-derived enzyme cocktails for biofuel production.
Table 2.
Chloroplast bioreactors for proteins/enzymes for biofuel production
| Protein/enzymes | Source of transgene | Enzyme activity | Refs |
|---|---|---|---|
| Beta glucosidase | Trichoderma. reesei | Chloroplast-derived enzymes showed wider pH optima and higher temperature stability than enzymes expressed in E. coli. Chloroplast-derived crude-extract enzyme cocktails yielded more (up to 3625%) glucose from citrus peel, filter paper or pine wood than commercial cocktails. | [32] |
| Swollenin | T. reesei | ||
| Xylanase | T. reesei | ||
| Acetyl xylan esterase | T. reesei | ||
| Endoglucanase | T. reesei | ||
| Endoglucanase | C. thermocellum | ||
| Exoglucanase | C. thermocellum | ||
| Lipase | M. tuberculosis | ||
| Pectate lyase A | F. solani | ||
| Pectate lyase A | F. solani | ||
| Pectate lyase A | F. solani | ||
| Cutinase | F. solani | ||
| β-glucosidase BglC | T. fusca | Chloroplast-produced BglC was active against both cellobiose and lignocellulose. | [87] |
| β-glucosidase Bgl 1 | T. reesei | Chloroplast-produced | [46] |
| Bgl1 could digest pNPG substrate and release p-nitrophenol. | |||
| Xylanase Xyl10B | T. maritima | Catalytic activity of chloroplast derived Xyl10B in poplar, sweetgum and birchwood xylan and stable in dry leaves. | [62] |
| β-glucosidase Bgl1C | T. fusca | All four enzymes were highly active and hydrolyzed their synthetic test substrates in a dose-dependent manner. Also, the enzyme cocktail triggered efficient sugarrelease from straw. | [96] |
| Endoglucanase Cel 9A | T. fusca | ||
| Exoglucanase Cel 6B | T. fusca | ||
| Xyloglucanase Xeg74 | T. fusca | ||
| β-Mannanase | T. reesei | Chloroplast-derived mannanase showed 6–7 fold higher enzyme activity than E. coli extracts. The enzyme cocktail with chloroplast derived mannanse yielded 20% more glucose equivalents from pinewood than the cocktail without mannanase. | [51] |
| Cutinase or swollenin | Fusarium solani | Treatment of cotton fiber with chloroplast-derived swollenin showed enlarged segments and the intertwined inner fibers were irreversibly unwound due to expansin activity of swollenin. Chloroplast derived cutinase showed esterase and lipase activity. | [97] |
| T. reesei | |||
| β-1,4-endoglucanase | Pyrococcus horikoshii | Chloroplast-derived EGPh was recovered from dry leaves and digested carboxymethyl cellulose (CMC) substrate. | [98] |
| Xylanase | Bacillus sp. | Catalytic activity of chloroplast produced Xylanase was detected with birch wood xylan as substrate | [99] |
Chloroplast bioreactors for biopharmaceuticals
The first plant based expression of biopharmaceutical (a recombinant protein) is FDA approved and marketed by Pfizer [63]. Recombinant glucocerebrosidase made in carrot cells is now used as a replacement therapy to treat Gaucher’s disease, a rare lysosomal storage disorder. More recently, plant based production of the Ebola vaccine (three humanized monoclonal antibodies) has been used successfully to treat few infected individuals in the West African outbreak [63, 64]. These protein drugs lead the way for producing biopharmaceuticals in plants. Biopharmaceuticals produced in current fermentation systems are very expensive and not affordable for large majority of the global population. In the United States, the average annual cost of protein drugs is 25-fold more than small molecule drugs. This is because of their production in prohibitively expensive fermenters, purification, cold storage and sterile delivery methods (via injection). However, oral delivery of protein drugs in genetically modified plant cells is now emerging as a new platform for inducing tolerance against autoimmune disorders or to eliminate toxicity of injected protein drugs or deliver functional blood proteins [65–68]. Plant cells expressing high levels of therapeutic proteins can be lyophilized and stored at room temperature for several years [42]. These approaches should improve patient compliance in addition to lowering the cost of healthcare.
These studies point out the importance of oral delivery of protein drugs, which has been elusive for decades because of their degradation in the digestive system and inability to cross the gut epithelium. The first concern has been addressed by expression of protein drugs via the chloroplast genome in edible plant cells, taking advantage of several thousand genome copies present in each plant cell. Although early efforts to express therapeutic proteins in lettuce chloroplasts were unsuccessful [69], extensive optimization was undertaken to develop a reproducible expression system utilizing species-specific chloroplast vectors, endogenous regulatory sequences and optimal organogenesis/hormone concentrations to directly regenerate transplastomic lines without callus induction [23]. Today, lettuce serves as the only reproducible transplastomic system for oral delivery of vaccines and biopharmaceuticals (Table 3).
Table 3.
Chloroplast bioreactors for functional biopharmaceuticals and vaccine antigens
| Biophamarceuticals Vaccine antigens |
Expression system | Expression level | Functional evaluation | Refs |
|---|---|---|---|---|
| CTB-AMA1 (Malarial vaccine antigens apical membrane antigen-1 ) | Lettuce | 7.3% TSP | Dual immunity against two major infectious diseases -cholera and malaria for long-term | [78] |
| Tobacco | 13.2 % TSP | |||
| CTB-MSP1(Malarial vaccine antigens merozoite surface protein-1) | Lettuce | 6.1% TSP | Dual immunity against two major infectious diseases-cholera and malaria for long-term | [78] |
| Tobacco | 10.1 TSP | |||
| EDA (Extra domain A-fibronectin) | Tobacco | 2.0 % TSP | Retains the proinfammatory properties of the EDA produced in E. coli | [100] |
| 2 L21-TD | Tobacco | 6% TSP | Immunogenic response in mice. | [101] |
| The immunogenic fusion protein F1-V from Y. pestis | Lettuce | 0.08% TSP | Immunogenic response in mice. | [79] |
| Coagulation factor IX | Tobacco | 3.8 % TSP | Prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice | [75] |
| BACE ( human β-site APP cleaving enzyme) | Tobacco | 2.0% TSP | Immunogenic response against the BACE antigen in mice. | [102] |
| Human papillomavirus L1 protein | Tobacco | 21.5 % TSP | Confirmed the formation of capsomeres | [103] |
| Proinsulin | Tobacco | 47% TSP | Oral delivery of proinsulin in plant cells or injectable delivery into mice showed reduced blood glucose levels | [70] |
| PA(dIV) (Domain IV of Bacillus anthracis protective antigen) | Tobacco | 5.3% TSP | Demonstrates protective immunity in mice against anthrax | [104] |
| Human thioredoxin 1 protein | Lettuce | 1% TSP | Protected mouse insulinoma line 6 cells from hydrogen peroxide | [105] |
| Thioredoxins–human serum albumin fusions | Tobacco | 26% TSP | The in vitro chaperone activity of Trx m and f was demonstrated | [106] |
| HPV-16 L1 antigen fused with LTB | Tobacco | 2% TSP | Proper folding and display of conformational epitopes | [107] |
| Exendin-4 (EX4) fused to CTB | Tobacco | 14.3 % TSP | CTBEX4 showed increased insulin secretion similar to the commercial EX4 in beta-TC6. | [66] |
| CTB-ESAT-6 (6kDa early secretory antigenic target) | Tobacco | up to 7.5%; | Hemolysis assay and GM1-binding assay confirmed functionality and structure of the ESAT-6 antigen | [80] |
| Lettuce | 0.75% | |||
| CTB-Mtb72F (a fusion polyprotein from two Tuberculosis antigens, Mtb32 and 39) | Tobacco | up to 1.2 % | Not reported | |
| CTB fused with MBP (myelin basic protein) | Amyloid loads were reduced in vivo in brain regions of 3xTgAD mice fed with bioencapsulated CTB-MBP. Also reduced A beta(42) accumulation in retinae and prevented loss of retinal ganglion cells were observed in 3xTgAD mice treated with CTB-MBP. | [65] | ||
| Coagulation factor VIII (FVIII) antigens: heavy chain (HC) and C2 fused with CTB | Tobacco | 80 or 370 μg/g in fresh leaves | Feeding of HC/C2 mixture substantially suppressed T helper cell responses and inhibitor formation against FVIII in hemophilia A mice. | [77] |
Upon oral delivery, plant cell wall protects expressed protein drugs from acids and enzymes in the stomach via bio-encapsulation. But when intact plant cells containing protein drugs reach the gut, commensal microbes are able to digest plant cell wall and release protein drugs. When tags (eg. CTB: cholera toxin B) are fused to protein drugs, they efficiently cross intestinal epithelium and are delivered to the circulatory or immune system. Tags that bind to the GM1 receptor present in gut epithelium deliver drugs to the circulatory system. When tags are not cleaved, protein drugs cross the blood brain barrier (BBB) or retinal barrier to facilitate their delivery to the brain and retina, crossing the blood brain or retinal barriers [68]. These steps are explained in detail in figure 3. Recently, more than 40 biopharmaceuticals and vaccine antigens have been expressed via the chloroplast genome (Table 3, Figure 3).
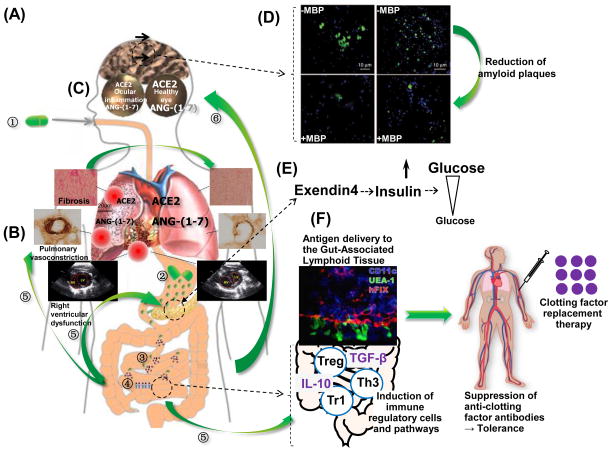
Figure 3.
Oral drug delivery for treatment of metabolic disorders or induction of tolerance (A) Therapeutic proteins expressed in chloroplasts and bioencapsulated in plant cells when orally delivered (step 1) go through the following process. (2) Protein drugs are protected from acids/enzymes in the digestive system because human enzymes do not break down glycosidic bonds of plant cell wall components. (3) Microbes colonizing the gut break down the plant cell wall, releasing therapeutic proteins into the gut. (4) Transmucosal carriers (cholera toxin B: CTB fused to protein drugs facilitate their delivery to the sera by binding to gut epithelial receptors (GM1). (5) Protein drugs from sera are delivered to different organs including heart, lung, pancreas or directly to immune modulatory cells. (6) Proteins fused with CTB also cross the blood brain or retinal barrier through GM1 receptors present in these barriers. (B) Metabolic disorders caused by unbalanced Renin Angiotensin System are prevented or delayed by oral delivery of bioencapsulated angiotensin converting enzyme 2 (ACE2) and angiotensin-(1-7) [Ang-(1-7)]. Delivery of ACE2 and Ang-(1-7) to circulatory system reversed or prevented pulmonary hypertension by shifting RAS axis to protective axis, resulting in decrease of fibrosis, improvement of cardiopulmonary structure and functions, and restoration of right heart function. Oral protein drug delivery across blood brain or retinal barriers: (C) Ocular inflammation caused by decreased activity of protective axis of RAS was improved by oral delivery of bioencapsulated ACE2 and Ang-(1-7) across BRB entered the retina and reduced endotoxin-induced uveitis and experimental autoimmune uveoretinitis. (D) Likewise, oral delivery of myelin basic protein fused with CTB (CTB-MBP) entered the brain by crossing BRB and reduced abeta plaques in advanced Alzheimer’s brain (E) Oral delivery of exendin expressed in chloroplasts increased insulin secretion and regulated blood sugar levels. (F) Oral tolerance induction: Blood coagulation factor expressed in chloroplasts delivered into gut-associated lymphoid tissue (GALT) of hemophilia A mice induces immune regulatory cells and pathways. Upregulation of Treg markers (CD25, FoxP3, CTLA-4) and suppressive cytokines IL-10, and transforming growth factor β (TGF-β) are observed in plant-FVIII-fed hemophilia A mice. After induction of oral tolerance, injection of recombinant FVIII into hemophilia A mice suppressed anti-clotting factor antibodies.
Type 2 diabetes is more prevalent than Type 1 and affects a vast majority of the global population and an economic treatment is needed to deal with this global pandemic. Oral delivery of human proinsulin, expressed in chloroplasts and bioencapsulated in plant cells or injectable delivery (purified from chloroplasts) into mice decreased blood glucose levels with similar efficiency as commercial insulin treatment [70].
Glucagon-like peptide (GLP-1) increases insulin secretion, but this peptide has a very short half-life (only 2 min in the circulatory system). The exenatide (an analogue of GLP-1) has a much longer half-life (3.3–4 h) and strong insulinotropic effects but requires cold storage, daily abdominal injections with short shelf life [66]. Oral delivery of chloroplast-derived CTB-EX4(CTB fused to Exendin-4) increased insulin secretion similar to the commercial EX4 (Figure 3A, E), which could eliminate injections, increase patient compliance/ convenience and significantly lower their cost [66].
Antimicrobial peptides (AMP) have the advantage over current antibiotics because they are effective against drug resistance microbes. However, the high cost of producingantimicrobial peptides is a major barrier for their clinical development and commercialization. Therefore, two important antimicrobial peptides: Retrocyclin 101 (RC-101) and protegrin 1 (PG1) were expressed in chloroplasts [49] for clinical development. Despite requirement of complex post-translational modifications including cyclization, both AMPs were active against bacterial and viral pathogens. Transplastomic plants showed normal growth. Likewise, a phage lytic protein was also expressed in the transplastomic tobacco plants and accumulated to high levels (>70% of TSP) but transplastomic plants showed retarded growth [71]. In a follow up study, toxin shuttle strategy was used to address this concern [72].
Delivering neuro-therapeutics to target brain-associated diseases is a major challenge. Alzheimer’s disease (AD) is the most common neurodegenerative genetic disorder and the sixth leading cause of death in the United States, affecting ~5.4 million Americans and 36 million patients globally. Oral delivery of CTB (cholera toxin B) fused with myelin basic protein (MBP) in healthy and Alzheimer’s mouse model increased MBP levels in different regions of the brain, effectively crossing the BBB. When sections of human and mice Alzheimer’s brain were incubated with CTB-MBP ex vivo, the intensity of amyloid plaque was reduced up to 60%. Moreover, bioencapsulated CTB-MBP treatment in vivo decreased amyloid loads by 70% in the cortex and hippocampus regions of Alzheimer’s mice brains. CTB-MBP oral delivery also reduced accumulation of plaque in retinae. Thus, low-cost oral delivery of therapeutic proteins across the blood brain and retinal barriers was first demonstrated (Figure 3A–D) [65].
Retinal inflammation is the main cause of visual impairment and is responsible for several retinal diseases. In the United States, 5–15% of total blindness is caused by Uveitis, an intraocular inflammatory disorder. The protective axis of the RAS (Renin-angiotensin system) was activated by oral delivery of chloroplast-derived ACE2 and Ang-(1-7) and this conferred protection against ocular inflammation (Figure 3A). With this treatment retinal vasculitis, cellular infiltration and damages were dramatically decreased in experimental autoimmune uveoretinitis (EAU, Figure 3 A, C). Thus CTB facilitates delivery of protein drugs across the blood retinal barrier [73].
Pulmonary arterial hypertension (PAH) is afatal disease characterized by increased blood pressure in the pulmonary arteries. Oral delivery of plant cells expressing ACE2 or Ang-(1-7) significantly improved cardiopulmonary structure and functions in rats with monocrotaline (MCT)-induced PAH in both prevention and reversal protocols. Not only was the elevated right ventricular systolic blood pressure decreased but the pulmonary blood flow was also improved [Figure 3 A, B; 68].
Chloroplast bioreactor for induction of oral tolerance
Several protein drugs delivered via injections for longer duration develop unintended consequences. One such complication is development of antibodies to injected proteins, neutralizing the effect of injected drug or in some cases develop toxic antibodies (like IgE) causing allergies, anaphylaxis or even death. Treatment of the genetic disease hemophilia, A or B is severely hampered by antibody (“inhibitor”) formation against the infused therapeutic clotting factors [74].
Oral tolerance induced by coagulation factor antigens bioencapsulated in plant cells is emerging as an alternative cost-effective and promising strategy to eliminate this problem while avoiding the side effects of immune suppressive drugs. In a murine model of hemophilia B that mimics the human inhibitor and anaphylactic responses to FIX replacement therapy, repeated oral delivery of plant cells expressing human FIX fused with CTB effectively prevented this pathogenic antibody formation against FIX and this treatment eliminated fatal anaphylactic reactions that occurred after four to six exposures to intravenous FIX [75, 76]. More recently, this approach was tested for hemophilia A, the prevalent form of the disease with the high incidence of inhibitor formation. Oral delivery of a mixture of plant cells expressing either the entire heavy chain or the C2 domain of human factor VIII (FVIII), suppressed inhibitor formation against FVIII in two different strains of hemophilia A mice. This study also contained first evidence that the plant-based oral tolerance protocol could reverse pre-existing responses and provided data on the underlying tolerance mechanisms [77] (Figure 3A, F). Delivery of antigen to dendritic cells (in the lamina propria and Peyer’s patches) throughout the small intestine, results in a complex immune regulatory mechanism. Adoptive transfer studies show that in addition to CD4+CD25+FoxP3+ Treg, CD4+CD25−LAP+ Treg are induced that potently suppress antibody formation. These studies for the first time reveal the ability of LAP+ Treg to suppress inhibitor formation. These cells are also the primary source of T cells expressing the immune suppressive cytokines TGF-β and IL-10 in response to coagulation factor antigen during oral tolerance. Because similar results were obtained for FVIII and FIX antigen delivery, the same tolerance mechanism appears to generally apply to the plant cell-based protocol [76, 77].
Chloroplast bioreactors for infectious disease vaccines
Although plant-made vaccines field started two decades ago with the promise of developing low cost vaccines to prevent infectious disease outbreaks and epidemics around the globe, this goal has not yet been realized. There are a number of major technical hurdles to achieve this goal including inadequate levels of expression in edible plant systems and inability to succeed in oral priming to induce adequate immunity against pathogens. Currently, there is no method available to induce oral priming and the only reproducible priming is done by injections of antigens bound to adjuvants. The major advantage of the oral vaccination system is the stimulation of both mucosal (IgA) and systemic (IgG1) immunity but this is now achieved by injectable priming of antigens with vaccine antigens followed by oral boosters with antigens bioencapsulated in plant cells. In addition, oral antigen delivery requires fusion of transmucosal carriers to cross the gut epithelium and delivery to the immune system [78]. Very few vaccine candidates listed in Table 3 meet all these requirements and therefore their efficacy has not been tested in suitable animal models or they failed such tests. In contrast, candidates that meet these criteria performed successful studies and showed efficacy of oral vaccines to boost the immune system and confer greater/prolonged protection against pathogen challenge. However, all these studies require priming by injections and therefore are not truly free of cold chain requirement. Furthermore, only a few vaccine antigens are expressed in edible crops (lettuce) [78–80] and those expressed in tobacco would face challenge in FDA approval process because of concerns of nicotine in orally delivered drugs.
Chloroplast genome engineering enables understanding of complex cellular processes
Studies on the native chloroplast genome and endogenous regulatory sequences contribute greatly to our understanding of molecular biology, physiology and biochemistry of chloroplasts. Transplastomic lines contribute in resolving complex processes that are difficult to study in native systems or when such results are inconclusive. For example, it was challenging to determine the precise site of cleavage of transit peptides after import of precursor proteins into chloroplasts but could be clearly resolved by expressing precursor proteins via the chloroplast genome and demonstrated that this step takes place in the stroma and not in the chloroplast envelope [2]. Most importantly the role of nuclear encoded cytosolic proteins that bind to regulatory sequences and their species specificity could be clearly studied using transgene expression [23]. For example, lettuce psbA regulatory sequence decreased transgene expression >90% in tobacco chloroplasts or vice versa, underscoring the importance of species specificity of chloroplast regulatory sequences[23]. Such studies are not possible using native genes.. This may explain failure of several laboratories in transforming unrelated crop species using tobacco chloroplast vectors. Likewise, details of homologous recombination process, and the deletion of mismatched nucleotides were evident using heterologous flanking sequences [23]. Translation of polycistrons without need for processing to monocistrons has been studied using ribosome profiling [30] but similarity of this process using heterologous polycistrons engineered via the chloroplast genome offered even more direct evidence for this process [21, 31]. Insertion of replication origins into chloroplast vectors offered further insight into minimal sequences required to study this process [24].
Plastid and nuclear genome require frequent and accurate signaling to coordinate the assembly of multi-subunit complexes or enzymes involved in biosynthetic pathways. The nuclear encoded plastid protein subunits are regulated by anterograde signaling pathways, which have been studied in depth. Interestingly, plastid derived signals can also coordinate expression of nuclear genes encoding plastid-localized proteins via retrograde signaling [81, 82]. A number of recent publications reveal that transcript or proteins could be exported from plastids. For example, expression of Tic40 (a protein in the import complex localized in the inner plastid envelope) via the chloroplast genome resulted in a massive proliferation of the inner membrane (up to 19 layers in electron micrographs of transformed chloroplasts) without any impact on plant growth or reproduction. Consistent with IM proliferation, expression of several other IM proteins (IEP 37, PPT, Tic 110) were upregulated but none of the outer membrane (Toc 159), stromal (hsp 93, cpn 60)or thylakoid (LHCP, OE23) proteins were increased, suggesting specific retrograde signal(s) [83]. This phenomenon is highly reproducible and happens in the absence of any environmental stress. Expression of gamma TMT (inserted into the inner membrane) via the chloroplast genome again resulted in massive proliferation of the inner envelope membrane (up to eight layers, Figure 4A–B) [55]. When lectin or antimicrobial peptide genes were expressed via the chloroplast genome, they conferred a broad protection against bacterial or viral pathogens [47, 49]. Release of antimicrobial proteins from chloroplasts can be simply explained by the lysis of plastids but retention of antimicrobial peptides within intact plastids do not support this hypothesis because invading pathogens are in the cytosol [49]. NRIP1 (a chloroplast localized receptor interacting protein) interacted with a cytoplasm localized enzyme P50 helicase when infected by tobacco mosaic virus [84]. In fact, NRIP1 is not the only receptor since several nucleotide-binding receptors are localized within the chloroplasts. Caplan and his colleague’s showed that most secreted proteins of Pseudomonas syringae contain chloroplast targeting signal sequences, which need retrograde signaling to the nucleus for eliciting defense responses [85].
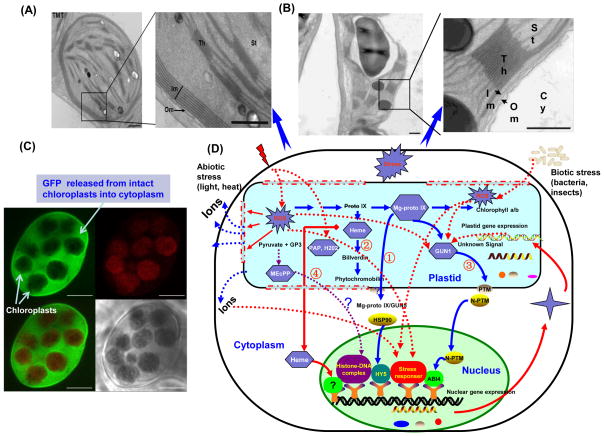
Figure 4.
Chloroplast genome engineering facilitates study of retrograde signaling (A) Expression of gamma tocopherol methyl transferase via the chloroplast genome results in massive proliferation of the inner envelope membrane (up to eight layers) [55], very similar to 19 layers observed when inner membrane protein Tic40 was overexpressed [83]. While several inner membrane proteins encoded by the nuclear genome are upregulated, none of the outer membrane, stromal or thylakoid proteins are affected, suggesting specificity of retrograde signaling. (B) Note a single inner envelope membrane in control chloroplasts [55]. (C) Green florescent protein fused with an antimicrobial peptide (AMP) is released from intact chloroplasts when the leaf discs were infected with Erwinia carotovora and plant cells were imaged by confocal microscopy[for time lapse images of GFP-AMP, see reference 50]. Transient release of GFP-AMP triggered by Erwinia infection stops soon after conferring protection against the invading pathogen, further confirming protein export from chloroplasts. Light and paraquat stress modifies the structure of chloroplast envelope resulting in increased ion leakage and facilitates AMP release [50]. These results suggest novel retrograde signaling mechanisms, triggered by foreign proteins expressed in chloroplasts and offer new opportunities to study pathways outside chloroplasts (D) Proposed signaling mechanisms between plastid and nuclear genome are shown. ① Mg-protoporphyrin IX (Mg-Proto IX) pathway: Mg-Proto IX is an intermediate of tetrapyrrole pathway, which is suggested to be exported from chloroplasts to bind HSP90 in the cytoplasm, resulting in a HY5-dependent activated repression and/or inhibited activation of nuclear expression [86]; ② Heme, specifically produced by the plastid ferrochelatase (FC1) has been suggested to coordinate photosynthesis-associated nuclear gene expression with chloroplast development [109]; ③ Tetrapyrrole or other signals may work on Genome Uncoupled 1, which could either generate or transmit a second signal, thereby activating N-PTM protein. The processed PTM may modulate nuclear gene expression by inducing the ABA insensitive 4 transcription factor [86]; ④ MEcPP (methylerythritol cyclodiphosphate), a precursor of isoprenoids produced by the plastid methylerythritol phosphate (MEP) pathway, accumulates during stress. MEcPP destabilizes histone-like-DNA complexes in bacteria, suggesting a possible model for gene regulation [81]. The redox state of photosynthetic electron transport components of the plastids and the levels of reactive oxygen species (ROS) in plastids may be yet another candidate for retrograde signaling. ROS accumulated promptly when plants cells are exposed to stress. ROS alters the membrane structure and increases membrane penetrability, which is helpful for the transmission of signal molecules from plastids. More importantly, ROS may be directly involved in other retrograde signaling pathway such as MEcPP pathway as well as plastid gene expression pathway [50, 81].
To date, the mechanism of retrograde-signaling pathway has remained elusive. There are several proposed retrograde signaling pathways [81, 86]. Recently, a novel operational retrograde signaling pathway has been described by Xiao et al., [82]. Methylerythritol cyclodiphosphate (MEcPP) is the precursor of isoprenoids generated in plastid methylerythritol phosphate (MEP) pathway of plastids and this accumulates in plastids to activate nuclear stress-responsive genes. The redox state of photosynthetic electron transport components and the levels of ROS in plastids may be another important retrograde signal. Unfortunately, none of these proposed candidates to date have been unequivocally tested. To investigate the potential role of ROS in the retrograde signal pathway and transmembrane transport, transplastomic plants expressing GFP fused with antimicrobial peptide (AMP) were developed. These results showed chloroplast GFP was gradually released from intact chloroplasts into the cytoplasm after the abiotic and biotic stress (Figure 4C). Light and paraquat stress modified the structure of chloroplast envelope resulting in increased ion leakage and facilitated protein release. Release of an antimicrobial peptide (GFP-RC101) triggered by Erwinia infection could be blocked after conferring protection, further confirming the export of protein from chloroplast phenomenon. These results suggest novel retrograde signaling mechanisms, triggered by chloroplast proteins and offer new opportunities to study pathways outside chloroplasts (Figure 4D) [50]. Thus chloroplast genetic engineering offers an ideal new tool to study interaction with other cellular compartments or within chloroplasts.
Concluding remarks and future perspectives
Although hundreds of foreign proteins have been expressed in chloroplasts and achieved much higher levels of expression than nuclear expression systems, in a few cases it failed to achieve the desired levels of expression. N-terminal degradation of proteins in heterologous systems is a well-known phenomenon. Indeed, the oldest and best known human blood protein, recombinant insulin has never been expressed in any expression system without N-terminal fusion proteins. Therefore, many human therapeutic proteins have been successfully expressed in chloroplasts by fusion with GFP [49] to confer stability or CTB to facilitate stability and oral delivery [65–68]. Several upstream and downstream transcriptional and translational regulatory sequences have been used recently to enhance transgene expression via the chloroplast genome [15–17, 87]. In addition, reduced expression could be due to misfolding of proteins. Indeed, this was clearly evident with human blood clotting factor IX fused with CTB with or without a furin cleavage site; when the furin cleavage site was eliminated, expression level was decreased 50-fold and homoplasmy couldn’t be achieved [75]. For expression of toxic proteins, inducible expression system would be ideal to synthesize foreign proteins when needed, conserving cellular resources for normal growth and development. However, further research is needed to develop highly efficient inducible systems in chloroplasts. The only reproducible system currently used for oral delivery of therapeutic proteins is transforming the genome of lettuce chloroplasts. Further studies are needed to develop chloroplast transformation in other leafy edible systems that could be orally delivered with minimal processing. Most importantly, further studies are needed to understand post-translational modifications of proteins within chloroplasts. Recent studies have shown that human blood proteins with disulfide bonds (like insulin, interferon, etc) are properly folded and are fully functional in the chloroplast [70, 88]. Chloroplasts are also capable of assembling multimeric structures (like CTB) with disulfide bonds that bind to GM1 receptors [65–68, 73, 75, 76]. Likewise, assembly of virus like particles has been observed in chloroplasts [89, 90]. Protein disulfide isomerase/thioredoxin expression has been shown to enhance folding and assembly of human serum albumin within chloroplasts [91]. Cyclization with disulfide bonds is required for antimicrobial activity of retrocyclin and chloroplasts synthesize and fold such cyclic proteins [49]. However, a number of complex post-translational modifications take place within chloroplasts. Human blood proteins correctly expressed in chloroplasts should facilitate understanding of hitherto unknown post-translational modifications that take place within chloroplasts.
There is enormous potential for synergistic utilization of chloroplast genome engineering with synthetic biology, opening ways to introduce entire genomes. While current approaches facilitate engineering pathways, introducing synthetic genomes would be revolutionary. Therefore, further research could advance chloroplast engineering towards clinical products, develop agronomic traits, metabolic engineering to produce novel fuels, enhance nutrition and advance our understanding of basic cellular signaling and metabolic processes.
Outstanding questions box.
-
What are the limitations in transforming the chloroplast genome of cereals?
While regeneration via somatic embryogenesis is feasible in dicots, homoplasmic transplastomic plants have not yet been created after two decades of research. Because proplastids or non-green plastids in carrot or soybean have been transformed successfully, it is unlikely that gene delivery or regeneration process will provide a hurdle, but there is need for identifying suitable selectable markers.
-
What are the limitations in transforming chloroplast genome of any new crop species?
There is a great need to transform edible leafy crops, especially for oral drug delivery or enhancing nutrition. Edible non-green parts of plants (e.g. tomato fruits) have very low levels of foreign protein accumulation compared to transplastomic leaves. With the exception of lettuce chloroplasts, no other edible system has yielded reproducible results so far. Lessons learned from optimization of lettuce plastid transformation, especially species specific chloroplast vectors and endogenous regulatory sequences should offer some guidance.
-
Is it possible to achieve inducible expression?
Although the T7 RNA polymerase system was introduced in 1994, it is still challenging to regulate transgene expression and synthesize products on demand. Constitutive expression of certain proteins is problematic.
-
Can chloroplast serve as bioreactor for RNA synthesis and delivery?
While chloroplasts are ideal for protein expression and delivery, could this system be used for RNA silencing, a major need in agriculture and medicine? Indeed chloroplasts are capable of producing more abundant transcripts than proteins but heterologous RNA processing is poorly understood.
-
Can synthetic plastome be engineered?
Foreign operons or new pathways have been engineered via the chloroplast genome with great success, including observing protein crystals. But several challenges need to be overcome to use chloroplasts for synthetic biology applications, including the introduction of large DNA fragments.
TRENDS BOX.
Hyper-expression of biopharmaceuticals in edible leaf chloroplasts documents a recent breakthrough in low cost oral delivery of biopharmaceuticals that are bio-encapsulated in plant cells. This will enable treating human metabolic or genetic diseases, such as Alzheimer’s, diabetes, hypertension, hemophilia, and retinal diseases.
New tools for smart chloroplast genome engineering are now available, including Gateway/modular vectors, RNAi interference, species specific vectors for efficient transformation of new crop chloroplast genomes and enhanced transgene expression.
Multi-gene metabolic engineering of chloroplasts can be used to produce high value bio-products.
Single chloroplast transgenes are used to confer biotic/abiotic stress tolerance or enhance biomass.
Regulation of nuclear genome is enabled by genes expressed in chloroplasts via retrograde signaling
Acknowledgments
Research in the Daniell laboratory discussed in this review was supported by NIH grants R01 GM 63879, R01 HL107904 and R01 HL109442. The authors are thankful to Kwang-chul Kwon for help with illustrations in figure 3.
Glossary
- Blood brain barrier (BBB)
BBB is a highly selective permeability barrier that separates the circulating blood from the brain extracellular fluid in the central nervous system, which only allows the passage of small molecules (water, gas, glucose) and lipid-soluble molecules by passive diffusion.
- CCM
CO2 concentrating mechanism (CCM), is an effective adaptation that increases the CO2 concentration around the primary photosynthetic enzyme Ribulose-1,5-bisphosphate Carboxylase/Oxygenase (RuBisCO).
- Cpn 60 and Hsp 93
Heat shock proteins of different molecular size (0, 93 kDa) are generally responsible for preventing damage to proteins in response to high levels of heat.
- CTB
cholera toxin B binds to GM1 ganglioside receptors on the surface of target cells. Once bound, the entire toxin complex and fused proteins are endocytosed by that cell. So it is used as a fusion tag to facilitate protein drug delivery.
- Gama-TMT
γ-tocopherol methyltransferase catalyzes the last step of α-tocopherol biosynthesis and transfers γ-tocopherol to α-tocopherol.
- Homoplasmy
is a term used to describe presence of only one type of chloroplast genome in a genetically modified plant cell.
- IEE element
Optional intercistronic expression element in plastid intended to splice monocistronic mRNAs from polycistrons
- IEP 37
The 37 kD inner envelope protein (IEP 37) of chloroplast that is part of the protein import machinery in TIC-TOC system..
- IgA, IgG1
These are antibodies developed after immunization and offer protection against invading pathogens by binding to their surface proteins.
- IL 10
Interleukin 10, also known as human cytokine synthesis inhibitory factor (CSIF), is an anti-inflammatory cytokine.
- IM
Inner chloroplast envelope membrane. Chloroplasts have a double membranes system: inner membrane and outer membrane.
- LHCP
the light-harvesting chlorophyll a/b protein is an integral membrane protein.
- OE23
The 23-kDa subunit of the oxygen-evolving complex is located deeply inside chloroplasts within the thylakoid lumen.
- PPT
Phosphoenolpyruvate translocatoris a part of protein import machinerylocated in the inner chloroplast envelope membrane.
- RAS axis
Renin-angiotensin system plays an important role in cardiovascular homeostasis, pathogenesis of inflammation and autoimmune dysfunction in which angiotensin II functions as the proinflammatory effector via angiotensin type 1 receptor. RAS imbalance results in development of pulmonary hypertension, retinal diseases and muscular dystrophy.
- Retrograde signaling
Signal between different subcellular organelles and the nucleus; in this context chloroplast protein expression is shown to regulate nuclear gene expression.
- Riboswitch
a regulatory fragment of mRNA that binds to its effectors, resulting in changes in its own activity.
- TGF-β
Immune suppressive cytokine involved in induction of tolerance.
- Tic 110, Tic 40, Toc 159
Two successive protein translocons allocated at the inner and outer chloroplast envelope membranes.
- Treg
Regulatory T cells expressing immune suppressive cytokines.
- TSP
total soluble protein in leaf extracts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McBride KE, et al. Amplification of a Chimeric Bacillus Gene in Chloroplasts Leads to an Extraordinary Level of an Insecticidal Protein in Tobacco. Nat Biotech. 1995;13:362–365. doi: 10.1038/nbt0495-362. [DOI] [PubMed] [Google Scholar]
- 2.Daniell H, et al. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotech. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, et al. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–2854. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, et al. Stable transformation of the cotton plastid genome and maternal inheritance of transgenes. Plant Mol Biol. 2004;56:203–216. doi: 10.1007/s11103-004-2907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufourmantel N, et al. Generation of fertile transplastomic soybean. Plant Mol Biol. 2004;55:479–489. doi: 10.1007/s11103-004-0192-4. [DOI] [PubMed] [Google Scholar]
- 6.Dufourmantel N, et al. Generation and characterization of soybean and marker-free tobacco plastid transformants over-expressing a bacterial 4-hydroxyphenylpyruvate dioxygenase which provides strong herbicide tolerance. Plant Biotechnology Journal. 2007;5:118–133. doi: 10.1111/j.1467-7652.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu YH, et al. Efficient metabolic pathway engineering in transgenic tobacco and tomato plastids with synthetic multigene operons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E623–E632. doi: 10.1073/pnas.1216898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CW, et al. Expression of a Bacillus thuringiensis toxin (cry1Ab) gene in cabbage (Brassica oleracea L. var. capitata L.) chloroplasts confers high insecticidal efficacy against Plutella xylostella. Theor Appl Genet. 2008;117:75–88. doi: 10.1007/s00122-008-0754-y. [DOI] [PubMed] [Google Scholar]
- 9.Yabuta Y, et al. Improvement of vitamin E quality and quantity in tobacco and lettuce by chloroplast genetic engineering. Transgenic Res. 2013;22:391–402. doi: 10.1007/s11248-012-9656-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, et al. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science. 2015;347:991–994. doi: 10.1126/science.1261680. [DOI] [PubMed] [Google Scholar]
- 11.De Marchis F, et al. Genetic transformation of the sugar beet plastome. Transgenic Res. 2009;18:17–30. doi: 10.1007/s11248-008-9193-4. [DOI] [PubMed] [Google Scholar]
- 12.Verma D, Daniell H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007;145:1129–1143. doi: 10.1104/pp.107.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma D, et al. A protocol for expression of foreign genes in chloroplasts. Nature Protocols. 2008;3:739–758. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- 14.Krichevsky A, et al. Autoluminescent Plants. Plos One. 2010:5. doi: 10.1371/journal.pone.0015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray BN, et al. High-Level Bacterial Cellulase Accumulation in Chloroplast-Transformed Tobacco Mediated by Downstream Box Fusions. Biotechnology and Bioengineering. 2009;102:1045–1054. doi: 10.1002/bit.22156. [DOI] [PubMed] [Google Scholar]
- 16.Yu LX, et al. Expression of thermostable microbial cellulases in the chloroplasts of nicotine-free tobacco. J Biotechnol. 2007;131:362–369. doi: 10.1016/j.jbiotec.2007.07.942. [DOI] [PubMed] [Google Scholar]
- 17.Hanson MR, et al. Chloroplast transformation for engineering of photosynthesis. Journal of Experimental Botany. 2013;64:731–742. doi: 10.1093/jxb/ers325. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, et al. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metabolic Engineering. 2012;14:19–28. doi: 10.1016/j.ymben.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena B, et al. Metabolic engineering of chloroplasts for artemisinic acid biosynthesis and impact on plant growth. J Biosciences. 2014;39:33–41. doi: 10.1007/s12038-013-9402-z. [DOI] [PubMed] [Google Scholar]
- 20.Guda C, et al. Stable expression of a biodegradable protein-based polymer in tobacco chloroplasts. Plant Cell Reports. 2000;19:257–262. doi: 10.1007/s002990050008. [DOI] [PubMed] [Google Scholar]
- 21.De Cosa B, et al. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh ND, et al. Chloroplast-derived vaccine antigens and biopharmaceuticals: protocols for expression, purification, or oral delivery and functional evaluation. Methods Mol Biol. 2009;483:163–192. doi: 10.1007/978-1-59745-407-0_10. [DOI] [PubMed] [Google Scholar]
- 23.Ruhlman T, et al. The Role of Heterologous Chloroplast Sequence Elements in Transgene Integration and Expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniell H, et al. Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:88–92. doi: 10.1073/pnas.87.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride KE, et al. Controlled expression of plastid transgenes in plants based on a nuclear DNA-encoded and plastid-targeted T7 RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7301–7305. doi: 10.1073/pnas.91.15.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lossl A, et al. Inducible trans-activation of plastid transgenes: expression of the R. eutropha phb operon in transplastomic tobacco. Plant Cell Physiol. 2005;46:1462–1471. doi: 10.1093/pcp/pci157. [DOI] [PubMed] [Google Scholar]
- 27.Verhounig A, et al. Inducible gene expression from the plastid genome by a synthetic riboswitch. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6204–6209. doi: 10.1073/pnas.0914423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottschamel J, et al. A novel chloroplast transformation vector compatible with the Gateway(A (R)) recombination cloning technology. Transgenic Res. 2013;22:1273–1278. doi: 10.1007/s11248-013-9726-3. [DOI] [PubMed] [Google Scholar]
- 29.Vafaee Y, et al. A modular cloning toolbox for the generation of chloroplast transformation vectors. Plos One. 2014;9:e110222. doi: 10.1371/journal.pone.0110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoschke R, et al. A Rapid Ribosome Profiling Method Elucidates Chloroplast Ribosome Behavior in Vivo. The Plant cell. 2013;25:2265–2275. doi: 10.1105/tpc.113.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quesada-Vargas T, et al. Characterization of heterologous multigene operons in transgenic chloroplasts. Transcription, processing, and translation. Plant Physiol. 2005;138:1746–1762. doi: 10.1104/pp.105.063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma D, et al. Chloroplast-derived enzyme cocktails hydrolyse lignocellulosic biomass and release fermentable sugars. Plant Biotechnol J. 2010;8:332–350. doi: 10.1111/j.1467-7652.2009.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gisby MF, et al. A synthetic gene increases TGF beta 3 accumulation by 75-fold in tobacco chloroplasts enabling rapid purification and folding into a biologically active molecule. Plant biotechnology Journal. 2011;9:618–628. doi: 10.1111/j.1467-7652.2011.00619.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of chlamydomonas. Nucleic Acids Res. 1991;19:4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gisby MF, et al. Growth of Transplastomic Cells Expressing D-Amino Acid Oxidase in Chloroplasts Is Tolerant to D-Alanine and Inhibited by D-Valine. Plant Physiol. 2012;160:2219–2226. doi: 10.1104/pp.112.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunne A, et al. Modifying fatty acid profiles through a new cytokinin-based plastid transformation system. Plant J. 2014;80:1131–1138. doi: 10.1111/tpj.12684. [DOI] [PubMed] [Google Scholar]
- 38.Barone P, et al. Tobacco plastid transformation using the feedback-insensitive anthranilate synthase [alpha]-subunit of tobacco (ASA2) as a new selectable marker. Journal of Experimental Botany. 2009;60:3195–3202. doi: 10.1093/jxb/erp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day A, Goldschmidt-Clermont M. The chloroplast transformation toolbox: selectable markers and marker removal. Plant Biotechnology Journal. 2011;9:540–553. doi: 10.1111/j.1467-7652.2011.00604.x. [DOI] [PubMed] [Google Scholar]
- 40.Shao M, et al. Precise excision of plastid DNA by large serine recombinase Bxb1. Plant Biotechnology Journal. 2014;12:322–329. doi: 10.1111/pbi.12139. [DOI] [PubMed] [Google Scholar]
- 41.Rothschild SI. microRNA therapies in cancer. Molecular and Cellular Therapies. 2014;2:1–8. doi: 10.1186/2052-8426-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon KC, et al. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev. 2013;65:782–799. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin S, et al. Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa armigera larval development and pupation. Plant Biotechnol J. 2015;13:435–446. doi: 10.1111/pbi.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kota M, et al. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1840–1845. doi: 10.1073/pnas.96.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dufourmantel N, et al. Generation and analysis of soybean plastid transformants expressing Bacillus thuringiensis Cry1Ab protoxin. Plant Mol Biol. 2005;58:659–668. doi: 10.1007/s11103-005-7405-3. [DOI] [PubMed] [Google Scholar]
- 46.Jin S, et al. Release of hormones from conjugates: chloroplast expression of beta-glucosidase results in elevated phytohormone levels associated with significant increase in biomass and protection from aphids or whiteflies conferred by sucrose esters. Plant Physiol. 2011;155:222–235. doi: 10.1104/pp.110.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin S, et al. Pinellia ternata agglutinin expression in chloroplasts confers broad spectrum resistance against aphid, whitefly, Lepidopteran insects, bacterial and viral pathogens. Plant Biotechnol J. 2012;10:313–327. doi: 10.1111/j.1467-7652.2011.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen PJ, et al. Transplastomic Nicotiana benthamiana plants expressing multiple defence genes encoding protease inhibitors and chitinase display broad-spectrum resistance against insects, pathogens and abiotic stresses. Plant Biotechnology Journal. 2014;12:503–515. doi: 10.1111/pbi.12157. [DOI] [PubMed] [Google Scholar]
- 49.Lee SB, et al. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J. 2011;9:100–115. doi: 10.1111/j.1467-7652.2010.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon KC, et al. Release of proteins from intact chloroplasts induced by reactive oxygen species during biotic and abiotic stress. Plos One. 2013;8:e67106. doi: 10.1371/journal.pone.0067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agrawal P, et al. Expression of Trichoderma reesei beta-Mannanase in Tobacco Chloroplasts and Its Utilization in Lignocellulosic Woody Biomass Hydrolysis. Plos One. 2011:6. doi: 10.1371/journal.pone.0029302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma D, et al. Expression of Fungal Cutinase and Swollenin in Tobacco Chloroplasts Reveals Novel Enzyme Functions and/or Substrates. Plos One. 2013:8. doi: 10.1371/journal.pone.0057187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viitanen PV, et al. Metabolic engineering of the chloroplast genome using the Echerichia coli ubiC gene reveals that chorismate - Is a readily abundant plant precursor for p-hydroxybenzoic acid biosynthesis. Plant Physiol. 2004;136:4048–4060. doi: 10.1104/pp.104.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harada H, et al. Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res. 2014;23:303–315. doi: 10.1007/s11248-013-9750-3. [DOI] [PubMed] [Google Scholar]
- 55.Jin SX, Daniell H. Expression of gamma-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant biotechnology Journal. 2014;12:1274–1285. doi: 10.1111/pbi.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhingra A, et al. Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6315–6320. doi: 10.1073/pnas.0400981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin MT, et al. A faster Rubisco with potential to increase photosynthesis in crops. Nature. 2014;513:547–550. doi: 10.1038/nature13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitney SM, et al. Improving recombinant Rubisco biogenesis, plant photosynthesis and growth by coexpressing its ancillary RAF1 chaperone. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3564–3569. doi: 10.1073/pnas.1420536112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ndimba BK, et al. Biofuels as a sustainable energy source: an update of the applications of proteomics in bioenergy crops and algae. J Proteomics. 2013;93:234–244. doi: 10.1016/j.jprot.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 60.Carroll A, Somerville C. Cellulosic biofuels. Annu Rev Plant Biol. 2009;60:165–182. doi: 10.1146/annurev.arplant.043008.092125. [DOI] [PubMed] [Google Scholar]
- 61.Leelavathi S, et al. Overproduction of an alkali- and thermo-stable xylanase in tobacco chloroplasts and efficient recovery of the enzyme. Molecular Breeding. 2003;11:59–67. [Google Scholar]
- 62.Kim JY, et al. Production of hyperthermostable GH10 xylanase Xyl10B from Thermotoga maritima in transplastomic plants enables complete hydrolysis of methylglucuronoxylan to fermentable sugars for biofuel production. Plant Mol Biol. 2011;76:357–369. doi: 10.1007/s11103-010-9712-6. [DOI] [PubMed] [Google Scholar]
- 63.Walsh G. Biopharmaceutical benchmarks 2014. Nature Biotechnology. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 64.Qiu XG, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohli N, et al. Oral Delivery of Bioencapsulated Proteins Across Blood-Brain and Blood-Retinal Barriers. Mol Ther. 2014;22:535–546. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwon KC, et al. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mager I, et al. From Gut to Brain: Bioencapsulated Therapeutic Protein Reduces Amyloid Load Upon Oral Delivery. Mol Ther. 2014;22:485–486. doi: 10.1038/mt.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shenoy V, et al. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248–1259. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanamoto H, et al. Efficient and stable transformation of Lactuca sativa L. cv. Cisco (lettuce) plastids. Transgenic Res. 2006;15:205–217. doi: 10.1007/s11248-005-3997-2. [DOI] [PubMed] [Google Scholar]
- 70.Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J. 2011;9:585–598. doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oey M, et al. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009;57:436–445. doi: 10.1111/j.1365-313X.2008.03702.x. [DOI] [PubMed] [Google Scholar]
- 72.Oey M, et al. Plastid production of protein antibiotics against pneumonia via a new strategy for high-level expression of antimicrobial proteins. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6579–6584. doi: 10.1073/pnas.0813146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shil PK, et al. Oral Delivery of ACE2/Ang-(1-7) Bioencapsulated in Plant Cells Protects against Experimental Uveitis and Autoimmune Uveoretinitis. Mol Ther. 2014;22:2069–2082. doi: 10.1038/mt.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.High KA. Gene transfer as an approach to treating hemophilia. Semin Thromb Hemost. 2003;29:107–119. doi: 10.1055/s-2003-37945. [DOI] [PubMed] [Google Scholar]
- 75.Verma D, et al. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang XM, et al. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP(+)CD4(+) T cells. Blood. 2015;125:2418–2427. doi: 10.1182/blood-2014-08-597070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sherman A, et al. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–1668. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davoodi-Semiromi A, et al. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J. 2010;8:223–242. doi: 10.1111/j.1467-7652.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosales-Mendoza S, et al. Expression of an immunogenic F1-V fusion protein in lettuce as a plant-based vaccine against plague. Planta. 2010;232:409–416. doi: 10.1007/s00425-010-1176-z. [DOI] [PubMed] [Google Scholar]
- 80.Lakshmi PS, et al. Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. Plos One. 2013;8:e54708. doi: 10.1371/journal.pone.0054708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woodson JD, Chory J. Organelle Signaling: How Stressed Chloroplasts Communicate with the Nucleus. Current Biology. 2012;22:R690–R692. doi: 10.1016/j.cub.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao Y, et al. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149:1525–1535. doi: 10.1016/j.cell.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 83.Singh ND, et al. Arabidopsis Tic40 Expression in Tobacco Chloroplasts Results in Massive Proliferation of the Inner Envelope Membrane and Upregulation of Associated Proteins. The Plant cell. 2008;20:3405–3417. doi: 10.1105/tpc.108.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caplan JL, et al. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell. 2008;132:449–462. doi: 10.1016/j.cell.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caplan J, et al. Plant NB-LRR immune receptors: from recognition to transcriptional reprogramming. Cell Host Microbe. 2008;3:126–135. doi: 10.1016/j.chom.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 86.Chi W, et al. Intracellular signaling from plastid to nucleus. Annu Rev Plant Biol. 2013;64:559–582. doi: 10.1146/annurev-arplant-050312-120147. [DOI] [PubMed] [Google Scholar]
- 87.Gray BN, et al. An efficient downstream box fusion allows high-level accumulation of active bacterial beta-glucosidase in tobacco chloroplasts. Plant Mol Biol. 2011;76:345–355. doi: 10.1007/s11103-011-9743-7. [DOI] [PubMed] [Google Scholar]
- 88.Arlen PA, et al. Field production and functional evaluation of chloroplast-derived interferon-alpha 2b. Plant biotechnology Journal. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Millan AFS, et al. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant biotechnology Journal. 2008;6:427–441. doi: 10.1111/j.1467-7652.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 90.Kanagaraj AP, et al. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol. 2011;76:323–333. doi: 10.1007/s11103-011-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanz-Barrio R, et al. Tobacco plastidial thioredoxins as modulators of recombinant protein production in transgenic chloroplasts. Plant biotechnology Journal. 2011;9:639–650. doi: 10.1111/j.1467-7652.2011.00608.x. [DOI] [PubMed] [Google Scholar]
- 92.Ruiz ON, et al. Metallothionein expression in chloroplasts enhances mercury accumulation and phytoremediation capability. Plant Biotechnol J. 2011;9:609–617. doi: 10.1111/j.1467-7652.2011.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanz-Barrio R, et al. Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnol J. 2013;11:618–627. doi: 10.1111/pbi.12052. [DOI] [PubMed] [Google Scholar]
- 94.Rey P, et al. Overexpression of plastidial thioredoxins f and m differentially alters photosynthetic activity and response to oxidative stress in tobacco plants. Front Plant Sci. 2013;4:article 390. doi: 10.3389/fpls.2013.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruhlman TA, et al. Expression of chloroperoxidase from Pseudomonas pyrrocinia in tobacco plastids for fungal resistance. Plant Sci. 2014;228:98–106. doi: 10.1016/j.plantsci.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Petersen K, Bock R. High-level expression of a suite of thermostable cell wall-degrading enzymes from the chloroplast genome. Plant Mol Biol. 2011;76:311–321. doi: 10.1007/s11103-011-9742-8. [DOI] [PubMed] [Google Scholar]
- 97.Xue XY, et al. New Approaches to Agricultural Insect Pest Control Based on RNA Interference. Adv Insect Physiol. 2012;42:73–117. [Google Scholar]
- 98.Nakahira Y, et al. Overproduction of hyperthermostable beta-1,4-endoglucanase from the archaeon Pyrococcus horikoshii by tobacco chloroplast engineering. Biosci Biotechnol Biochem. 2013;77:2140–2143. doi: 10.1271/bbb.130413. [DOI] [PubMed] [Google Scholar]
- 99.Pantaleoni L, et al. Chloroplast molecular farming: efficient production of a thermostable xylanase by Nicotiana tabacum plants and long-term conservation of the recombinant enzyme. Protoplasma. 2014;251:639–648. doi: 10.1007/s00709-013-0564-1. [DOI] [PubMed] [Google Scholar]
- 100.Farran I, et al. The vaccine adjuvant extra domain A from fibronectin retains its proinflammatory properties when expressed in tobacco chloroplasts. Planta. 2010;231:977–990. doi: 10.1007/s00425-010-1102-4. [DOI] [PubMed] [Google Scholar]
- 101.Ortigosa SM, et al. Stable production of peptide antigens in transgenic tobacco chloroplasts by fusion to the p53 tetramerisation domain. Transgenic Res. 2010;19:703–709. doi: 10.1007/s11248-009-9348-y. [DOI] [PubMed] [Google Scholar]
- 102.Youm JW, et al. High-level expression of a human beta-site APP cleaving enzyme in transgenic tobacco chloroplasts and its immunogenicity in mice. Transgenic Res. 2010;19:1099–1108. doi: 10.1007/s11248-010-9383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waheed MT, et al. Transplastomic expression of a modified human papillomavirus L1 protein leading to the assembly of capsomeres in tobacco: a step towards cost-effective second-generation vaccines. Transgenic Res. 2011;20:271–282. doi: 10.1007/s11248-010-9415-4. [DOI] [PubMed] [Google Scholar]
- 104.Gorantala J, et al. A plant based protective antigen [PA(dIV)] vaccine expressed in chloroplasts demonstrates protective immunity in mice against anthrax. Vaccine. 2011;29:4521–4533. doi: 10.1016/j.vaccine.2011.03.082. [DOI] [PubMed] [Google Scholar]
- 105.Lim S, et al. Production of biologically active human thioredoxin 1 protein in lettuce chloroplasts. Plant Mol Biol. 2011;76:335–344. doi: 10.1007/s11103-011-9745-5. [DOI] [PubMed] [Google Scholar]
- 106.Sanz-Barrio R, et al. Tobacco plastidial thioredoxins as modulators of recombinant protein production in transgenic chloroplasts. Plant Biotechnol J. 2011;9:639–650. doi: 10.1111/j.1467-7652.2011.00608.x. [DOI] [PubMed] [Google Scholar]
- 107.Waheed MT, et al. Plastid expression of a double-pentameric vaccine candidate containing human papillomavirus-16 L1 antigen fused with LTB as adjuvant: transplastomic plants show pleiotropic phenotypes. Plant Biotechnol J. 2011;9:651–660. doi: 10.1111/j.1467-7652.2011.00612.x. [DOI] [PubMed] [Google Scholar]
- 108.Harada H, et al. Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res. 2014;23:303–315. doi: 10.1007/s11248-013-9750-3. [DOI] [PubMed] [Google Scholar]
- 109.Woodson JD, et al. Heme Synthesis by Plastid Ferrochelatase I Regulates Nuclear Gene Expression in Plants. Current Biology. 2011;21:897–903. doi: 10.1016/j.cub.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]