Abstract
Introduction
Cancer stem cells (CSCs) possess characteristics associated with normal stem cells, specifically the abilities to renew themselves and to give rise to all cell types (differentiation). It is assumed that induction of differentiation in CSCs would reduce their ability to form tumors. What triggers CSC differentiation and the role of “differentiation” in tumorigenesis remain elusive.
Methods
Glioma stem cell (GSC) lines and subcutaneous as well as orthotopic xenografts established from fresh surgical specimens of glioblastoma multiforme were used.
Results
Exposure of GSCs to serum activates mitochondrial respiration and causes an increase in mitochondrial reactive oxygen species (ROS) as well as oxidative stress responses, leading to the appearance of differentiation morphology and a deceased expression of CSC markers. Chemical perturbation of the mitochondrial electron transport chain causes ROS increase and further downregulation of stem cell markers, while antioxidant N-acetyl-cysteine reduces ROS and suppresses the differentiation of GSCs. Surprisingly, the serum-induced differentiated GSCs exhibit greater ability to form tumor in both orthotopic and subcutaneous xenograft models, which can be suppressed by N-acetyl-cysteine. Mitochondrial ROS from the serum-stimulated cells triggered the activation of nuclear factor-kappa-B (NFκB) pathway, which is a potential mechanism for the promotion of tumorigenesis.
Conclusion
This study suggests that ROS generated from active mitochondrial respiration in the presence of serum is critical in CSCs activation, which promotes tumor development in vivo.
Electronic supplementary material
The online version of this article (doi:10.1186/s13287-015-0174-2) contains supplementary material, which is available to authorized users.
Introduction
Recent studies indicate the existence of cancer stem cells (CSCs) in various types of cancers, including leukemia and solid tumors [1, 2]. Similar to normal stem cells, CSCs are able to self-renew and to generate the downstream progeny. Although CSCs constitute a very small fraction of the total cancer cells in the tumor bulk, this special subpopulation of malignant cells is thought to play a major role in cancer initiation and development and may be a key cause of resistance to chemotherapy and radiotherapy, leading to persistence of residual disease and cancer recurrence [3]. This phenomenon is due in part to the unique biological properties of CSCs, including high capacity of DNA repair, high expression of certain ATP-dependent drug exporting pumps, high levels of glutathione synthesis, and high expression of cell survival factors [4–6]. A detailed understanding of factors that affect the ability of CSCs to maintain their self-renewal and promote disease progression is important for developing new strategies to effectively kill CSCs.
Mounting evidence suggests that the tissue microenvironment may profoundly affect the biological properties and the fates of stem cells and CSCs [7]. In vivo, normal stem cells or CSCs reside in special tissue locations known as stem cell niches, which are thought to provide the microenvironment important for the maintenance of their stemness [8]. Although the exact nature of the stem cell niches remains to be defined, it is known that low oxygen and proper levels of certain growth factors such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) are important to maintain the stemness of the cells [9]. Brain CSCs have been found in perivascular niches [8, 10]. Increasing the endothelial cells or blood vessels in orthotopic brain tumor xenografts enhances self-renewal of CSCs and accelerates the initiation and growth of tumors [10]. However, exposure of CSCs to serum in vitro usually induces differentiation and presumably may compromise their self-renewal ability [11, 12]. CSCs cultured in serum-free media seem to closely mimic the genotype and gene expression profiles of their primary tumors in vivo than do CSCs cultured in standard serum-containing medium [9]. Although the ability of serum to induce apparent differentiation of CSCs has been known for a long time, the underlying mechanisms remain largely unknown. It is also unclear whether exposure of CSCs to serum negatively or positively affects their ability to form tumor in vivo.
Reactive oxygen species (ROS) are known to play a role in affecting the fates of normal stem cells [13, 14]. Elevated ROS has been observed to induce differentiation of embryonic stem cells into cardiovascular and mesendodermal cells [7, 15]. The neural stem cells and hematopoietic stem cells contain lower levels of ROS than their mature progeny, whereas increased ROS levels are associated with lowered self-renewal capacity, increased cell cycling, and reduced viability [16–18]. Previous study showed that breast CSCs might have high ROS-scavenging capacity and contain lower cellular ROS compared with the corresponding non-tumorigenic cells [6]. A recent study suggests that ROS might affect the differentiation state of CSC by activation of p38 MAPK [19]. However, the role of ROS in serum-induce differentiation of CSCs and their physiological relevance in tumor development in vivo remain largely unclear. The present study was designed to investigate these important questions. We showed that serum could activate mitochondrial respiration and promote generation of mitochondrial ROS, leading to apparent loss of certain stem cell markers and lower ability to form neurospheres. However, despite these seemingly differentiation phenotypes in vitro, the serum-induced glioma stem cells exhibited greater capacity to form tumor in vivo. Our study revealed a novel role of mitochondrial ROS in serum activation of CSCs to produce the downstream progeny and promote tumor development in vivo. The regulation of this redox signaling mechanism has potential implications in developing new strategies to target CSCs.
Methods
Cell lines and cell culture
GSC11, GSC23, and GBM3752 cell lines were originally established from fresh surgical specimens of glioblastoma multiforme at the University of Texas MD Anderson Cancer Center [20, 21]. GSC11 and GSC23 were maintained in Dulbecco’s modified Eagle’s medium with nutrient mixture F-12 (DMEM/F12) (Mediatech Inc., Manassas, VA, USA) supplemented with B27 (Invitrogen, Carlsbad, CA, USA), 20 ng/ml epidermal growth factor (Miltenyi Biotec, Auburn, CA, USA), 20 ng/ml of basic fibroblast growth factor (Miltenyi Biotec), and 2 mM L-glutamine (Mediatech Inc.) without serum (designated as “stem cell medium”). Cells were cultured in a humidified incubator maintained at 37 °C with 5 % CO2. GBM3752 cells were obtained from GBM patients undergoing surgery at Texas Children’s Hospital and maintained in severe combined immunodeficiency (SCID) mice orthotopically [22]. The cells were freshly isolated from the tumors and cultured in stem cell medium for in vitro study within the first five passages. For serum treatment, cells were cultured in the stem cell medium with 5 % fetal bovine serum (FBS) with or without various concentrations of N-acetyl-cysteine (NAC) (Sigma-Aldrich, St. Louis, MO, USA).
RNA isolation, RNA microarray analyses, and reverse transcription-polymerase chain reaction
GSC11 and GSC23 cells were cultured in stem cell medium with or without serum for 1, 3, or 7 days in triplicate. Total RNA was isolated from the cells by using an RNeasy Mini kit (Qiagen Inc., Valencia, CA, USA). Sample labeling was performed with an RNA amplification kit in accordance with the conditions recommended by the manufacturer (Applied Biosystems, Foster City, CA, USA). Total RNA was reverse-transcribed by using a complementary DNA (cDNA) synthesis kit (Fermentas Inc., Glen Burnie, MD, USA). The quantitative polymerase chain reaction analyses were carried out in a 25-μl reaction mixture that contained 1 μl cDNA, 0.1 μg oligonucleotide primer pairs, 12.5 μl SYBR Green Mix (Invitrogen), and diethylpyrocarbonate-treated water. Human HT-12v3 expression beadchips containing 48,000 probes of 25,000 annotated genes were obtained from Illumina Inc. (San Diego, CA, USA). The gene expression microarray analysis was performed at the System Biology Department of the UT MD Anderson Cancer Center. Total RNA was extracted from GSC11 cells and used for labeling and hybridization to human expression beadchips in accordance with the protocols of the manufacturer. All experiments were performed in triplicate. Primary microarray data in this study are available in the National Cancer for Biotechnology Information Gene Expression Omnibus (GEO) database (GSE28220). The following primer sets were used for quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis: SOX2-sense, 5′-GCCTGGGCGCCGAGTGGA-3′; SOX2-antisense, 5′-GGGCGAGCCGTTCATGTAGGTCTG-3′); Olig2-sense, 5′-TGCGCAAGCTTTCCAAGA-3′; Olig2-antisense, 5′-CAGCGAGTTGGTGAGCATGA-3′.
Flow cytometric analyses
Cells were dissociated into single-cell suspension by using accutase reagents (Sigma-Aldrich), stained with allophycocyanin (APC)-conjugated CD133 antibody (clone AC133 from MACS) or the control APC-IgG2b antibody (MACS) by using the conditions recommended by the manufacturer. APC fluorescence was quantitated by flow cytometry analysis. To measure intracellular ROS, cells were collected and dissociated into single-cell suspension by accutase, washed with phosphate-buffered saline (PBS) once, and resuspended in pre-warmed PBS containing freshly prepared CM-H2DCFDA (1 μM) or MitoSOX-Red (5 μM; Molecular Probes, Eugene, OR, USA). After incubation at 37 °C for 30 min (H2DCFDA) or 15 min (MitoSOX-Red), the cells were washed with PBS twice and then subjected to flow cytometric analyses.
Immunoblots
Cultured cells were washed with cold PBS before homogenization in lysate buffer. Whole cell lysate (20 μg protein/sample) was used in Western blot analysis. Cell lysates were separated by electrophoresis on 10–12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After blocking with 5 % non-fat milk/PBS with Tween 20 for 1 h, the membranes were incubated at 4 °C overnight with primary antibodies, including mouse anti-human CD133 (Miltenyi Biotec), rabbit anti-human SOX2 (Cell Signaling Technology Inc., Danvers, MA, USA), rabbit anti-human Olig2 (Abcam, Cambridge, MA, USA), rabbit anti-human Catalase (EMD Chemicals, Gibbstown, NJ, USA), sheep anti-human SOD1 (EMD Chemicals), rabbit anti-human SOD2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and anti-mouse total OXPHOS (Abcam). The Western blot signals were detected with horseradish peroxidase-conjugated secondary antibodies. The membranes were developed by using a Pierce Supersignal West Pico Chemiluminescent Substrate (Fisher Scientific Inc., Pittsburgh, PA, USA).
Immunofluorescence staining
Cells were fixed in 4 % formaldehyde, washed in PBS, and permeabilized for the analysis of intracellular markers (20 min, 0.25 % Triton X-100; Sigma-Aldrich). The monolayers were then incubated with a blocking solution (PBS with 5 % FBS) (45 min, room temperature), followed by incubation (overnight at 4 °C) with the primary antibodies: anti-glial fibrillary acidic protein (anti-GFAP) (Miltenyi Biotec), anti-β-III tubulin (Abcam), anti-Nestin (Abcam), and anti-O4 (Miltenyi Biotec). After extensive washing in PBS, a second incubation (1 h; 37 °C) with Alexa Fluor-488- or Alexa Fluor-547-specific anti-mouse or anti-rabbit secondary antibodies (all from Invitrogen) was performed. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich). Florescence labeling was observed by using a fluorescent microscope (Olympus, Tokyo, Japan).
Oxygen consumption assay
Samples were dissociated into singles cells, washed with PBS once, and suspended at 4 to approximately 10 million cells per milliliter in stem cell medium. Oxygen consumption was measured in 1-ml medium by using Oxytherm equipped with a Clark-type electrode (Hansatech Instruments Ltd, Norfolk, UK) as described previously [23].
Mouse xenografts
Subcutaneous xenografts: GSC11 cells cultured under various conditions (stem cell medium without FBS, with 5 % FBS, or with FBS and 20 mM NAC for 7 days) were collected, treated with accutase to make single-cell suspension, and inoculated into the right flank of nude mice (2 × 106 cells per mouse). The mice were euthanized when the tumor diameter was greater than 1.5 cm. For orthotopic xenograft inoculation, GBM3752 cells were first cultured in stem cell medium with or without serum (5 % FBS). The cells were maintained in vitro under these two conditions for 60 passages. Cells were collected and inoculated intracranially into the brains of SCID mice (1 × 104 cells per mouse). The mice were euthanized when they developed signs of neurological deficit and became moribund. All experiments of the present study were performed in accordance with human protocols approved by the Institutional Review Board at UT MD Anderson Cancer Center and Baylor College of Medicine as well as animal protocols (ACUF 11-98-08136, AN-4548) approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Signed informed consent was obtained from all patients or their legal guardians prior to sample acquisition.
Results and Discussion
Induction of apparent differentiation of GSCs by serum and association with ROS stress responses
Both established glioma stem cell lines and primary glioma cells isolated from fresh tumor tissues were used in our study. GSC11 and GSC23 are two glioblastoma stem cell lines originally derived from glioblastoma multiforme (GBM) surgical specimens and exhibit the in vitro stem cell characteristics of extensive self-renewal, the ability to differentiate to neurons and astrocytes, and the ability to initiate tumor in vivo. GSC11 and GSC23 cells were maintained in serum-free stem cell culture medium as described previously (He, 2010 #274) [24]. An orthotopic xenograft model (GBM3752) that preserves glioblastoma stem cells was originally established by directly inoculating primary tumor cells from fresh GBM specimen into the right cerebellum of SCID mice brain [22]. The xenograft tumor cells preserve tumorigenicity, multi-lineage differentiation, and CD133+ expression after being subtransplanted in mice brain. GBM3752 cells were prepared freshly from tumors (maintained in SCID) mice for in vitro study. As shown in Fig. 1, GSC11 and GBM3752 cells grew well in stem cell medium containing EGF and bFGF without serum and exhibited the morphology of stem-like neurospheres (Fig. 1a) with high expression of CD133 (Fig. 1b, c). The addition of serum (% FBS) to the culture medium caused a significant change in cell morphology, manifested by a loss of neurosphere formation and the appearance of differentiated cells attaching to the culture dish (Fig. 1a). This was accompanied by a substantial decrease of CD133 expression in a time-dependent manner (Fig. 1b, c) and a decrease of Nestin (Additional file 1: Figure S1). Quantitative RT-PCR and Western blot analyses revealed a significant decrease in expression of Sox2 and Olig2, two transcription factors known to regulate neural stem cells and neural progenitor cells (Fig. 1d, e). The expression of Notch1, a molecule important for promoting neural stem cell function [25], was also downregulated (Fig. 1d). In contrast, the expression of differentiation markers, including GFAP, β-III tubulin, O4 (Additional file 1: Figure S1), and ANXA1, were increased after serum exposure (Additional file 1: Figure S1 and Fig. 1e). Similar results were observed in the third cell line GSC23 (Additional file 1: Figure S2). Surprisingly, this apparent differentiation induced by serum did not result in a decrease in tumorigenesis, and as will be described below, the glioma stem cells were activated by serum exposure (see in vivo study below).
Fig. 1.

Effect of serum on neurosphere formation and the expression of stem cell markers in glioblastoma stem cells. a Glioblastoma stem cells (GSC11 and GBM3752) formed neurospheres in serum-free medium supplemented with epidermal growth factor and basic fibroblast growth factor. Exposure of the cells to serum (5 % FBS) for 3 days led to a loss of neurosphere formation in both clones. b Western blot analysis of CD133 in GSC11 cells before and after exposure to serum for 1, 3, and 7 days. c Flow cytometry analysis of CD133 expression in GSC11 cells before and after exposure to serum for 7 days. The right panel shows quantitation of the percentage of CD133+ cells before and after GSC11 cells were exposed to serum for 1, 3, and 7 days; *P < 0.05. d Expression of SOX2, Olig2, and Notch1 mRNA in GSC11 cells before and after exposure to serum for 3 days. Expression of mRNA was measured by quantitative reverse transcription-polymerase chain reaction. **P < 0.001. e Effect of serum on protein expression of stem cell markers SOX2 and Olig2 and differentiation marker ANXA1. GSC11 cells were exposed to 5 % FBS for 1, 3, and 7 days as indicated. SOX2, Olig2, ANXA1 were detected by Western blot analysis. Cont control, D day, FBS fetal bovine serum, GBM glioblastoma multiforme, GSC glioma stem cell, PI
To investigate the molecular events and potential alterations in the signaling pathways of GSCs in response to serum induction, we treated GSC11 cells with 5 % FBS for 1, 3, and 7 days in triplicate cultures, and RNA was isolated from each sample for determination of gene expression profiles using microarray analyses. As shown in Additional file 1: Figure S3A, clustering analysis of gene expression profiles revealed that the serum-treated GSC11 cells exhibited gene expression profiles clearly distinct from that of the GSC11 cells cultured in serum-free medium. There was a further shift of gene expression profiles as the time of serum exposure prolonged. The fact that the three separated samples of the same time point (biological triplicate) displayed similar gene expression patterns and clustered in the same group demonstrated the high reproducibility of this experimental system. Using the Ingenuity pathway analysis, we found that the oxidative stress response pathway was induced by serum most significantly (P = 0.0005) at all time points tested. Additional file 1: Figure S3B shows the genes involved in oxidative stress response identified by this analysis in GSC11 cells. Among these genes, SOD2, catalase, NQO1, peroxiredoxin 1, thioredoxin reductase 1, and glutamate-cysteine ligase are involved in ROS scavenging. These results suggested that the homeostasis of reduction/oxidation (redox) balance might have been disrupted in the serum-induced GSCs.
Induction of mitochondrial ROS generation in glioma stem cells by serum through activation of electron transport chain
The observations that exposure of GSCs to serum caused consistent oxidative stress response in all tested time points prompted us to explore possible changes in cellular redox status. Since mitochondria are major sites of ROS production, we used MitoSOX-Red to detect mitochondrial superoxide (O2−) and 5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester (DCF-DA) to measure total cellular hydrogen peroxide (H2O2) and other ROS. The results showed that that serum induced a substantial increase of mitochondrial O2− in a time-dependent manner, with an increase of the median value from 46 units in control cells (serum-free) to 67, 188, and 268 units on days 1, 3, and 7 after serum exposure, respectively (Fig. 2a). Interestingly, total cellular ROS (as measured by DCF-DA) also showed a moderate increase, from 84 units in the control to 112, 126, and 159 units on days 1, 3, and 7, respectively (Fig. 2a). Similar results were observed in another glioblastoma stem cell line GSC23 (Additional file 1: Figure S4). Exposure of GSC23 cells to serum led to a 5- and 11-fold increase of mitochondrial O2− on days 3 and 7, respectively, and the total cellular ROS detected by DCF-DA also moderately increased (Additional file 1: Figure S4A).
Fig. 2.
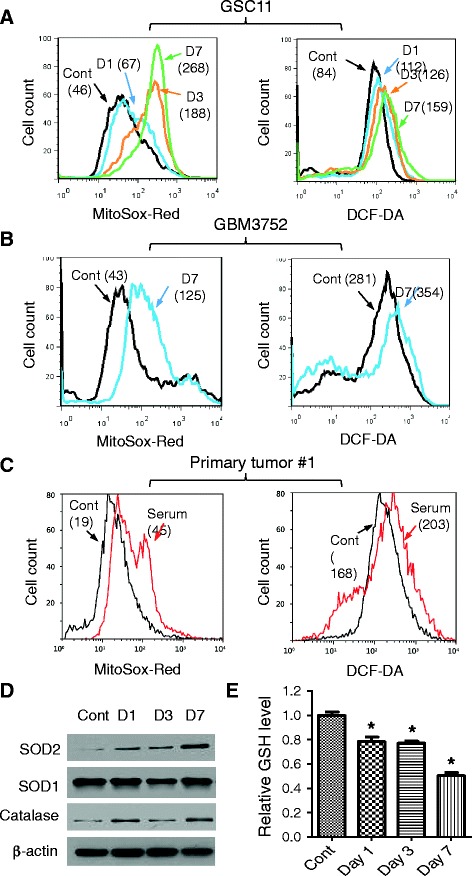
Induction of mitochondrial O2 − generation and oxidative stress response in glioblastoma stem cells. a Effect of serum on mitochondrial O2 − and total cellular ROS in GSC11 cells. Cells were incubated without or with 5 % FBS for 1, 3, or 7 days (D1, D3, D7). Mitochondrial O2 − was measured by flow cytometry analysis after cells were stained with MitoSOX-Red, and total cellular ROS were detected by using CM-H2-DCFDA staining followed by flow cytometry analysis. The numbers in parentheses indicate the median fluorescent intensity. b Effect of serum exposure (1–7 days) on mitochondrial O2 − and total cellular ROS in GBM3752 cells. c Effect of serum on mitochondrial O2 − and total cellular ROS in primary tumor cells isolated from fresh GBM tumor tissue. The freshly isolated tumor cells were divided into two portions for incubation in stem cell medium without or with serum (5 % FBS) for 7 days, and mitochondrial O2 − and total cellular ROS were then measured. The shaded curve shows the background (Bkg) fluorescent without dye. d Western blot analysis of SOD1, SOD2, and catalase in GSC11 cells before and after exposure to serum for 1, 3, and 7 days. e Cellular glutathione in GSC11 cells cultured in stem cell medium without or with serum for 1, 3, and 7 days. *P < 0.05. Cont control, DCF-DA 5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester, FBS fetal bovine serum, GBM glioblastoma multiforme, GSH glutathione, GSC glioma stem cell, O 2 − superoxide, ROS reactive oxygen species, SOD cytosolic superoxide dismutase
We then used two types of fresh glioma cells to further confirm the above observations. First, the stem-like GBM3752 cells [22] were obtained freshly from tumor xenografts and divided into two portions; one portion was cultured in serum-free stem cell medium and the other portion was cultured in serum-containing medium. After 7 days, the mitochondrial ROS and total cellular ROS in each culture condition were measured by MitoSOX and DC-FDA. As shown in Fig. 2b, a 3-fold increase in mitochondrial O2− and a moderate increase (26 %) in total cellular ROS were observed, consistent with that seen in GSC11 and GSC23 cells. Furthermore, this pattern of redox alterations was consistently observed in primary glioma cells isolated from fresh GBM tumor tissues (Fig. 2c and Additional file 1: Figure S4B), suggesting that the induction of mitochondrial O2− generation might be a highly consistent event in serum-induced changes in GSCs.
To test whether the increase in mitochondrial O2− and cellular ROS induced by serum in GSCs might cause stress response, we used Western blotting to analyze the expression of antioxidant molecules before and after serum exposure. As shown in Fig. 2d, there was a time-dependent increase in expression of SOD2, a mitochondrial superoxide dismutase that converts O2− to H2O2. Interestingly, the cytosolic superoxide dismutase (SOD1) did not exhibit significant change after GSC11 and GSC23 were exposed to serum (Fig. 2d, Additional file 1: Figure S4C), suggesting that the main source of ROS stress might be mainly from mitochondria and was consistent with the increase in mitochondrial O2− shown in Fig. 2a-c. The expression of catalase, an enzyme that converts cellular H2O2 to water and oxygen, increased after serum incubation (Fig. 2d, Additional file 1: Figure S4C). Cellular glutathione (GSH), a major endogenous antioxidant, decreased after serum exposure in GSC11 cells (Fig. 2e) and G23 cells (Additional file 1: Figure S4D), reflecting a consumption of this antioxidant. These data together suggest that the increase in SOD2 expression might be a stress response to elevated mitochondrial O2− generation induced by serum. SOD2 converted O2− to H2O2, which was then able to pass the mitochondrial membranes to cytosol, where it was converted to O2 and H2O by catalase or neutralized by GSH, resulting in only a moderate increase of overall cellular ROS and a decrease in GSH.
Since mitochondrial O2− is generated mainly during respiration because of the release of electrons from complexes I and III of the electron transport chain, we speculated that the increased mitochondrial O2− might be a result of active mitochondrial respiration induced by serum and not a consequence of a slower O2− elimination since SOD2 expression was increased. To test this possibility, we measured oxygen consumption in GSCs as an indicator of mitochondrial respiration. As shown in Fig. 3a and b, exposure of GSC11 cells to serum led to a time-dependent increase of oxygen consumption, with approximately a 100 % increase by day 3. Interestingly, measurement of mitochondrial mass by using MitoTracker Green as well as mitochondria electron transport chain (ETC.) and the ATP synthase complex antibodies showed that serum did not cause any significant change in mitochondrial mass and complexes (Fig. 3c and Additional file 1: Figure S5), suggesting that the increase in respiration was mainly a functional activation of the pre-existing mitochondria. GBM3752 cells from freshly dissected orthotopic tumor xenografts were cultured in either serum-free medium or serum-containing medium for 7 days. A significantly higher oxygen consumption was observed in GBM3752 cells cultured with serum (Additional file 1: Figure S6A) without any significant changes in mitochondrial mass (Additional file 1: Figure S6B). The increase in respiration without increase of mitochondrial mass was also consistently observed in GSC23 cells (Additional file 1: Figure S6C). Despite the increase of mitochondrial respiration, cells at G0/G1 phase were decreased only on day 1 compared with cells cultured in serum-free medium (Additional file 1: Figure S7). Cells at G2/M phase were not changed.
Fig. 3.
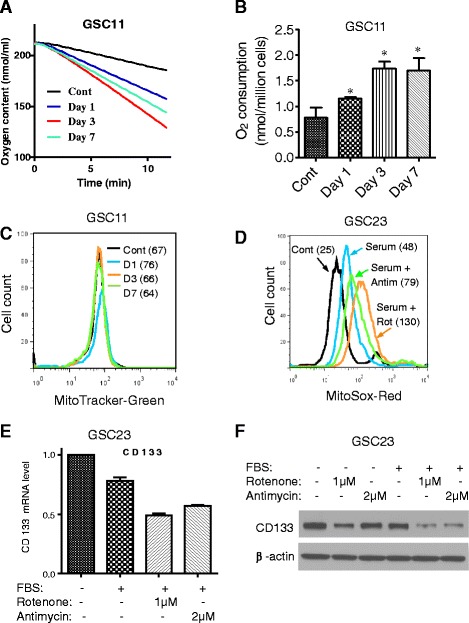
Novel role of mitochondrial activation and ROS generation in activation of glioblastoma stem cells. a Comparison of oxygen consumption in GSC11 cells before and after exposure to serum for 1, 3, and 7 days. Oxygen consumption was measured by using an Oxytherm system as described in Methods. b Quantitative analysis of oxygen consumption in GSC11 cells exposed to serum for 1, 3, and 7 days. *P < 0.01. c Serum did not induce increase of mitochondrial mass in GSC11 cells, measured by MitoTracker-Green. d GSC23 cells were cultured in stem cell medium without or with serum (5 % FBS) in the presence or absence of 1 μM rotenone (ETC. complex I inhibitor) or 2 μM antimycin (complex III inhibitor), and mitochondrial O2 − was measured by flow cytometric analysis after cells were stained with MitoSOX-Red. e Effect of serum and ETC. inhibitors on CD133 expression. GSC23 cells were cultured in stem cell medium in the presence or absence of serum (5 % FBS), rotenone (1 μM), or antimycin (2 μM). CD133 mRNA expression levels were measured by quantitative reverse transcription-polymerase chain reaction. f GSC23 cells were cultured in stem cell medium in the presence or absence of serum (5 % FBS), rotenone (1 μM), or antimycin (2 μM). CD133 protein expression was measured by Western blot. Cont control, ETC. electron transport chain, FBS fetal bovine serum, GSC glioma stem cell, O 2 − superoxide
Important role of mitochondrial ROS in mediating the serum effect on glioma stem cells
To evaluate the role of activation of mitochondrial respiration and ROS generation in serum-induced apparent differentiation of GSCs, we first used ETC. complex I inhibitor rotenone and complex III inhibitor antimycin to inhibit mitochondrial respiration in glioma stem cells and then tested whether this affected the ability of serum to induce apparent differentiation in GSCs. The results showed that both ETC. inhibitors disrupted mitochondrial respiration and caused a further increase of mitochondrial O2− in the presence of serum (Fig. 3d), but neither of them prevented the serum-induced GSCs from attaching to the flasks and exhibiting apparent differentiation morphology (data not shown). In fact, adding rotenone or antimycin even caused a further decrease of CD133 at both mRNA (Fig. 3e) and protein levels (Fig. 3f).
Considering the observations that exposure of GSCs to serum caused an increase in mitochondrial respiration and O2− generation but inhibition of mitochondria respiration by ETC. inhibitors (rotenone and antimycin) did not prevent serum to induce changes in GSCs, we speculate it was the increase in mitochondrial ROS generation, not the respiration per se, that plays a key role in mediating the serum effect on GSCs. To test this possibility, we used exogenous H2O2 to cause a level of increase in mitochondrial ROS comparable to that caused by serum in GSC11 and GSC23 cells (Fig. 4a, b). Interestingly, a short-term treatment of GSCs with such exogenous H2O2 for 6 h led to a significant decrease of SOX2, Olig2, and CD133 mRNA expression in GSC11 cells (Fig. 4c) and GSC23 cells (Fig. 4d), similar to those observed in serum-induced cells.
Fig. 4.

Exogenous H2O2 suppressed the expression of Olig2, SOX2, and CD133 in GSCs. a Exogenous H2O2 caused an increase in mitochondrial O2 − in GSC11 cells. Cells were treated with 200 μM or 1 mM of H2O2 for 1 h, and mitochondrial O2 − was measured by flow cytometric analysis. b Exogenous H2O2 caused an increase in mitochondrial O2 − in GSC23 cells. Cells were treated with 500 μM or 1 mM of H2O2 for 1 h, and mitochondrial O2 − was then measured by flow cytometric analysis. c GSC11 cells were treated with 200 μM or 1 mM H2O2 for 6 h with addition of H2O2 at 1-h intervals. Expression of SOX2, Olig2, and CD133 mRNA in GSC11 cells was measured by quantitative reverse transcription-polymerase chain reaction. *P < 0.05. d Effect of exogenous H2O2 on the expression of SOX2, Olig2, and CD133 in GSC23 cells under the same conditions as in (c). *P < 0.05. Cont control, GSC glioma stem cell, H 2 O 2 hydrogen peroxide, O 2 − superoxide
To further validate this novel role of ROS, we used NAC, a precursor for glutathione synthesis with potent antioxidant property to reduce ROS stress, to test whether it could prevent the effect of serum on GSCs. As shown in Fig. 5a, incubation of GSC11 cells with serum for 7 days caused a significant increase of mitochondrial O2−, and the presence of NAC effectively suppressed such ROS increase. Importantly, NAC also prevented serum-induced loss of ability to form stem-like neurospheres (Fig. 5b) and partially preserved the expression of CD133 in serum-induced cells (Fig. 5c). These results suggest that the increase in mitochondrial ROS generation might play an important role in mediating the serum effect on GSCs. Since there was a significant decrease in expression of SOX2, Olig2, and Notch1 in serum-induced GSCs, we tested whether NAC might also suppress this serum effect. As shown in Fig. 5d, quantitative RT-PCR revealed that the addition of NAC to the serum-treated GSC11 cells largely blocked the decrease of SOX2 and Olig2 expression, suggesting that the expression of these two molecules might be redox-sensitive. Similar results were observed in GSC23 (Additional file 1: Figure S8). Furthermore, gene expression analysis of molecules involved in the Notch pathway revealed that serum caused a significant decrease in the expression of Notch1, MFNG, LFNG, HESs, DTX3, and DLL1 (Fig. 5e). Consistently, the presence of antioxidant NAC largely prevented the downregulation of the Notch-related genes (Fig. 5e), again suggesting the important role of ROS and redox signaling in regulation of GSCs.
Fig. 5.
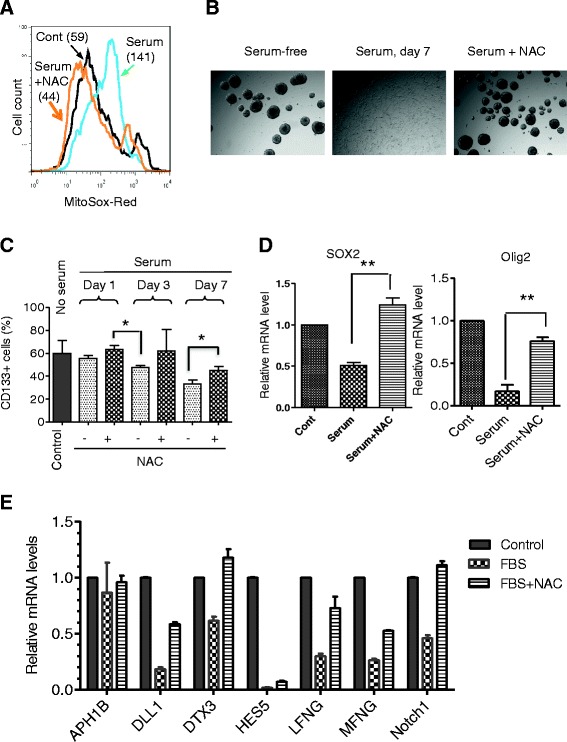
Effect of antioxidant NAC on serum-induced ROS generation and the expression of stem cell markers in GSCs. a Effect of NAC on mitochondrial O2 − levels in GSC11 cells exposed to serum. Cells were cultured in stem cell medium with or without serum (5 % FBS) in the presence or absence of 20 mM NAC for 7 days. Mitochondrial O2 − was measured by using MitoSOX-Red. b Comparison of neurosphere formation in GSC11 cells cultured in serum-free or serum-containing medium in the presence and absence of 20 mM NAC for 7 days. c NAC suppressed serum-induced loss of CD133 expression. GSC23 cells were exposed to serum for 1, 3, or 7 days in the presence or absence of 20 mM NAC as indicated, and CD133-positive cells were quantitated by flow cytometry analysis. *P < 0.05. d GSC11 cells were exposed to serum (5 % FBS) for 3 days in the presence and absence of 20 mM NAC. The expression of SOX2 and Olig2 mRNA was measured by quantitative RT-PCR. **P < 0.001. e NAC suppressed serum-induced downregulation of the Notch pathway. GSC11 cells were incubated without or with serum (5 % FBS) for 3 days in the presence or absence of 20 mM NAC. RNA was isolated from each sample, and the expression of molecules involved in Notch signaling was measure by quantitative RT-PCR. FBS fetal bovine serum, GSC glioma stem cell, NAC N-acetyl-cysteine, O 2 − superoxide, ROS reactive oxygen species, RT-PCR reverse transcription-polymerase chain reaction
Serum induction of GSCs promotes tumorigenesis in vivo
Because exposure of GSCs to serum caused the cells to exhibit apparent differentiation morphology and a decrease in neurosphere formation, we used two in vivo models to test whether serum induction of such changes might alter the ability of GSCs to form tumors in vivo. First, GSC11 cells were incubated with or without serum for 7 days, and the same numbers (2 × 106) of the control cells or serum-induced cells were inoculated subcutaneously into the right flank of nude mice. Under these subcutaneous inoculation conditions, only two out of seven of the mice inoculated with the control GSC11 cells (serum-free) form tumors, and surprisingly seven out of seven mice inoculated with the serum-induced GSC11 cells developed tumor (Fig. 6a), suggesting that exposure of GSC11 cells to serum promotes their tumorigenesis. Interestingly, five out of seven mice inoculated with GSC11 cells treated with serum in the presence of the antioxidant NAC formed tumors (Fig. 6a). The overall survival of the mice inoculated with serum-induced GSC11 cells was significantly shorter than that of the control mice inoculated serum-free GSC11 cells (P = 0.0019, Fig. 6b). No significant difference (P = 0.064) in overall survival was found between the control group (serum-free) and the group of mice inoculated with GSC11 cells exposed to serum in the presence of NAC (20 mM).
Fig. 6.
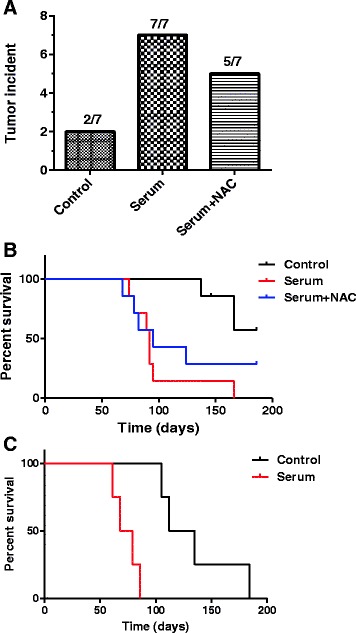
Serum promotes in vivo tumorigenesis of glioblastoma stem cells. a GSC11 cells were cultured in serum-free medium or serum-containing medium in the presence or absence of 20 mM NAC for 7 days. Equal numbers of cells (2 × 106) from each sample were inoculated subcutaneously into the right flank of nude mice (seven mice per group). The numbers of mice that developed tumors in each group are shown. b Survival curves for the mice in the three groups of mice described in (a). c GBM3752 cells were isolated from orthotopic tumor xenografts cultured in stem cell medium with or without serum as described in Methods. Equal numbers of cells (10,000 per injection) were inoculated into non-obese diabetic/severe combined immunodeficiency mice intracranially. The mice were observed for survival and euthanized when they developed signs of neurological deficit and became moribund. NAC N-acetyl-cysteine
We also used another mouse model, orthotopic inoculation of GBM3752 cells into the SCID mice (Shu et al. [22]), to further evaluate the role of serum exposure on tumorigenesis. The same numbers (1 × 104) of GBM3752 cells with or without serum exposure were inoculated into SCID mice intracranially, and the mice were observed for tumor development and survival. As shown in Fig. 6c, the overall survival of mice inoculated with serum-induced GBM3752 cells was significantly shorter than that of the mice bearing the control GBM3752 cells cultured in stem cell medium without serum (P = 0.0067). These results were consistent with those of the subcutaneous tumor model and suggest that serum exposure activated glioma stem cells and promoted tumor formation.
Activation of the NFĸB survival pathway in serum-induced glioma stem cells
To explore the possible mechanisms by which serum induction of mitochondrial ROS generation could lead to activation of GSCs and promote tumorigenesis, we further analyzed the gene expression microarray data from GSC11 cells exposed to serum for various times (Additional file 1: Figure S2) and found that the expression of multiple genes downstream of nuclear factor-kappa-B (NFκB) (including CD44, IL-8, IL-11, CCND1, TFPI2, and PLAUR) consistently increased after incubation with serum (Fig. 7a–g). Since oxidative stress is known to activate NFκB [26, 27], it is possible that in vivo. Indeed, incubation of GSC23 cells with serum caused a substantial increase in IκBα phosphorylation and p65 phosphorylation (Fig. 7h), two molecular events indicative of NFκB activation. Importantly, the addition of the antioxidant NAC partially suppressed the serum-induced phosphorylation of IκBα and p65, suggesting the role of ROS in mediating serum-induced NFκB activation. Similar results were observed in GSC11 cells (Fig. 7i). Serum exposure caused a significant increase in phosphorylated IκBα and p65 (Fig. 7i, lanes 1 and 2). The addition of NAC suppressed these phosphorylations (Fig. 7i, lane 7). These data together with the known function of IKK in phosphorylating IκBα and p65 suggest a possibility that serum might activate NFκB in GSCs through ROS-induced activation of IKK, a redox-sensitive molecule known to be activated by ROS [28]. To exam this possibility, we used a specific IKK inhibitor, BMS-345541, to test whether inhibition of IKK would prevent serum-induced phosphorylation of IκBα and p65. As shown in Fig. 7i, BMS-345541 at concentrations of 10–20 μM effectively suppressed serum-induced phosphorylation of IκBα and p65, associated with a preservation of CD133 expression of suppression of ANXA1. These data suggest that IKK might play an important role in mediating serum-induced activation of NFκB through a redox-sensitive mechanism.
Fig. 7.

Activation of the NFĸB pathway by serum in glioblastoma stem cells is associated with ROS stress. a–g Induction of expression of genes which are downstream of NFĸB by serum exposure. GSC11 cells were cultured in serum-free medium or serum-containing medium for 1, 3, and 7 days. The relative mRNA expression levels of the target genes were analyzed by microarray assays in triplicate for each time point. h Antioxidant NAC suppressed serum-induced NFĸB pathway activation in GSC23 cells. Protein samples were obtained from GSC23 cells cultured in serum-free or serum-containing medium in the presence or absence of 20 mM NAC for 3 days. The expression of phospho-IKBα, phosphor-p65, total p65, and β-actin was assayed by Western blot. i IKK inhibitor BMS-345541 and antioxidant NAC suppressed serum-induced NFĸB activation in GSC11 cells. GSC11 cells were cultured in serum-free or serum-containing medium with the indicated concentrations of BMS345541 or 20 mM of NAC for 3 days. The expression of phospho-IKBα, phosphor-p65, CD133, ANXA1, and β-actin was measured by Western blot analysis. CCND1 cyclin D1, CD44-4 CD44 transcript variant 4, CD44-5 CD44 transcript variant 5, FBS fetal bovine serum, GSC glioma stem cell, IL-8 interleukin 8, IL-11 interleukin 11, NAC N-acetyl-cysteine, NFĸB nuclear factor-kappa-B, PLAUR plasminogen activator urokinase receptor, ROS reactive oxygen species, TFP1-2 tissue factor pathway inhibitor 2
It has been known for some time that CSCs, similar to normal stem cells, require a certain tissue microenvironment to maintain their stemness [29, 30] and that exposure of stem cells to serum in vitro usually induces differentiation phenotype [9]. However, the underlying mechanisms remain unclear. Although apparent differentiation phenotypes are also observed when CSCs are exposed to serum, it is unclear whether this would cause a decrease in tumorigenesis. Our study revealed a ROS-mediated mechanism by which serum induces apparent differentiation in glioma stem cells. We found that exposure of GSCs to serum resulted in activation of mitochondrial respiration, leading to an increase of oxygen consumption and high generation of mitochondrial O2−, which induced the expression of SOD2 to convert O2− to H2O2. Owing to its relatively long half-life and ability to cross biological membranes, H2O2 has been considered a second messenger that mediates redox-sensitive signaling in cellular response to growth factors [31, 32]. The ability of H2O2 to cause oxidation of protein thiol via catalytic cysteine can alter the function of the target proteins and thus provides a mechanism for redox signaling [32]. Since O2− has an extremely short half-life and cannot pass the mitochondrial membranes, the increased O2− within the mitochondria could not directly function as a second messenger to affect the nuclear gene expression. As such, conversion of the mitochondrial O2− to H2O2 by SOD2 seems important to relay the redox signal from mitochondria to cytosol and nucleus during serum-induced differentiation of GSCs. The upregulation of SOD2 in serum-treated GSCs would facilitate the conversion of O2− to H2O2.
Although the ability of ROS to induce normal stem cell differentiation has been noticed in some experimental systems [33, 34], the exact mechanisms remain unclear. Our study found that downregulation of SOX2, Olig2, and the Notch-related molecules by ROS might be a potential mechanism. The ability of exogenous antioxidant NAC to prevent the decreased expression of these genes and to block the serum-induced differentiation phenotype supports this notion. It is possible that the expression of SOX2, Olig2, and the Notch-related molecules is regulated via a redox-sensitive mechanism, with ROS being a negative regulator. Since SOX2, Olig2, and the Notch pathway are involved in regulation of neural stem cells [35, 36], downregulation of these genes could promote apparent differentiation of GSCs and drive them to enter a process in which GSCs progress to become the downstream progeny cancer cells.
Surprisingly, we found that although incubation of GSCs with serum induced apparent differentiation morphology and caused a downregulation of certain stem cell markers, including CD133, SOX2, and Olig2, this led to an increase of tumorigenicity and a reduction of survival in mice in two different mouse models (subcutaneous and orthotopic). These new findings seem to challenge the traditional view that CSCs are responsible for cancer development and their ability to form tumor would decrease once they are induced to undergo differentiation. Interestingly, a recent study showed that tumor cell self-renewal capacity did not predict tumor growth potential in vivo [37]. The study by Barrett et al. showed that glioma cells with low self-renewal capacity were more tumorigenic and generate tumor more rapidly than cells with high self-renewal capacity. These observations are consistent with our findings. Furthermore, our study revealed that activation of the nuclear factor-kappa-B (NFĸB) survival pathway by ROS might be a mechanism that promotes GSC survival and enhances tumorigenesis. The role of the NFĸB pathway in normal stem cell proliferation has been implicated previously. For instance, NFĸB is activated during human embryonic stem cell (hESC) differentiation, and inhibition of NFĸB leads to a reduction of hESC proliferation and suppression of their progression toward primitive extraembryonic and embryonic lineages [38]. As such, inhibition of NFĸB may potentially prevent activation of CSCs and thus suppress tumor development. Interestingly, recent studies have shown that targeting the NFĸB pathway may be effective against CSCs [39–41]. Their studies showed that inhibition of NFĸB by using compounds such as niclosamide and disulfiram suppressed acute myeloid leukemia stem cells and breast CSCs.
The NFĸB signaling pathway plays a crucial role in cancer development and progression [42]. It can either promote or inhibit carcinogenesis, depending on the cell types and experimental conditions. The NFĸB signaling pathway is often activated by ROS through IKK phosphorylation of IĸB [43] and p65 [44]. Our study found that serum could cause the activation of NFκB, which could be blocked by the antioxidant NAC. These results suggest that the activation of NFĸB in the serum-induced cells is probably mediated by a redox-regulatory mechanism due to ROS generation in the serum-activated mitochondria. It is worth noting that deletion of NFĸBIA which encodes the NFĸB inhibitor IĸBα seems to be an oncogenic event in GBM [45]. Interestingly, a recent study showed that the NFĸB pathway was activated in glioblastoma-initiating cells (GICs) after induction of differentiation and that blockade of the NFĸB pathway could drive differentiating GSCs into senescence [40], again suggesting that activation of NFĸB is important in maintaining the proliferation of differentiating GSCs. This effect was partly mediated by reduced levels of the NFκB target gene cyclin D1. Furthermore, a novel small-molecule inhibitor of the NFκB pathway induced senescence of tumor cells in a mouse model bearing human GIC-derived tumors. These findings reveal that activation of NFκB may keep differentiating GICs from acquiring a mature postmitotic phenotype, thus allowing cell proliferation. Our study showed that, in the case of GSC exposure to serum, activation of NFĸB is likely through ROS stimulation of IKK, which promotes phosphorylation of IĸBα and p65. We found that antioxidant NAC or inhibition of IKK by BMS-345541 could effectively prevent the serum-induced NFĸB activation and loss of CD133, suggesting a novel role of ROS in driving the progression of GSCs toward downstream progeny cells to promote tumor development.
Conclusions
In summary, our study showed that exposure of glioma stem cells to serum could stimulate mitochondrial respiration leading to increased generation of mitochondrial ROS, which activated NFκB to promote cancer cell survival and tumorigenesis. A key underlying mechanism is likely through a redox-mediated activation of IKK to phosphorylate IκBα and p65. Although the serum-induced elevation of ROS in GSCs also caused a decrease in neurosphere formation in vitro and a reduced expression of stem cell markers such as CD133, this apparent differentiation did not reduce the ability of the glioma stem cells to form tumor in vivo. Instead, the serum-induced GSCs exhibited greater tumorigenesis in both subcutaneous and orthotopic xenograft models. These new findings suggest that serum may activate glioma stem cells to progress toward the downstream cancer progenitor cells and promote tumor formation and that activation of mitochondrial respiration and ROS generation may play a key role in redox signaling during this tumorigenesis process. It is also important to note that the apparent differentiation phenotype such as neurosphere formation and CD133 expression in vitro observed might not necessarily predict tumorigenesis in vivo.
Accession numbers
The Gene Expression Omnibus (GEO) accession number for the RNA microarray data in this study is GSE28220.
Acknowledgments
The authors thank Zahid H. Siddik, Anthony Lucci, and Hector Martinez-Valdez for helpful discussion. This work was supported in part by a grant from the major science and technology project of the National Basic Research (973) Program of China (2012CB967004), grants from National Natural Science Foundation of China (81302194 and 81302144), a Specialized Research Fund for the Doctoral Program of Higher Education (20130171120047), a grant from the National Institutes of Health (CA100428), and the Fundamental Research Funds for the Central Universities (14ykpy40). FW is a recipient of the Rosalie B. Hite Fellowship from UT MD Anderson Cancer Center and the Outstanding Young Talents of Sun Yat-sen University Cancer Center.
Abbreviations
- APC
Allophycocyanin
- bFGF
Basic fibroblast growth factor
- CSC
Cancer stem cell
- DCF-DA
5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate acetyl ester
- EGF
Epidermal growth factor
- ETC.
Electron transport chain
- FBS
Fetal bovine serum
- GBM
Glioblastoma multiforme
- GIC
Glioblastoma-initiating cell
- GSC
Glioma stem cell
- GSH
Glutathione
- H2O2
Hydrogen peroxide
- hESC
Human embryonic stem cell
- NAC
N-acetyl-cysteine
- NFκB
Nuclear factor-kappa-B
- O2−
Superoxide
- PBS
Phosphate-buffered saline
- ROS
Reactive oxygen species
- RT-PCR
Reverse transcription-polymerase chain reaction
- SCID
Severe combined immunodeficiency
- SOD1
Cytosolic superoxide dismutase
- UT
University of Texas
Additional file
Supplementary Figures. (PDF 628 kb)
Footnotes
SY and YL are co-first authors.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SY and YL contributed to conception and design, collection/assembly of data, data analysis/interpretation, manuscript writing, and final approval and contributed equally to this article. JY, MO, HZ, JL, HC, and JL contributed to provision of study materials, manuscript writing, and final approval. GC and SK contributed to collection/assembly of data, manuscript writing, and final approval. LF, NH, WL, and XL contributed to design of animal study, data analysis/interpretation, manuscript writing, and final approval. RX, PH, and FW contributed to conception and design, provision of study materials, data analysis/interpretation, manuscript writing, and final approval. All authors read and approved the manuscript.
Contributor Information
Shuqiang Yuan, Email: yuanshq@sysucc.org.cn.
Yunxin Lu, Email: luyx@sysucc.org.cn.
Jing Yang, Email: yangjing@sysucc.org.cn.
Gang Chen, Email: gchen@mdanderson.org.
Sangbae Kim, Email: sangbae.kim@uth.tmc.edu.
Li Feng, Email: lfeng@mdanderson.org.
Marcia Ogasawara, Email: mogasawara@mdanderson.org.
Naima Hammoudi, Email: amian84@hotmail.com.
Weiqin Lu, Email: wqlu@mdanderson.org.
Hui Zhang, Email: huz012@eng.ucsd.edu.
Jinyun Liu, Email: jliu7@mdanderson.org.
Howard Colman, Email: howard.colman@hsc.utah.edu.
Ju-Seog Lee, Email: jlee@mdanderson.org.
Xiao-Nan Li, Email: xiaonan@bcm.edu.
Rui-hua Xu, Email: xurh@sysucc.org.cn.
Peng Huang, Email: phuang@mdanderson.org.
Feng Wang, Email: wangfeng@sysucc.org.cn.
References
- 1.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 3.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–45. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. 2011;2011:941876. doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells. 2010;28:639–48. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji AR, Ku SY, Cho MS, Kim YY, Kim YJ, Oh SK, et al. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp Mol Med. 2010;42:175–86. doi: 10.3858/emm.2010.42.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert CA, Ross AH. Cancer stem cells: cell culture, markers, and targets for new therapies. J Cell Biochem. 2009;108:1031–8. doi: 10.1002/jcb.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 13.Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal. 2009;11:2777–89. doi: 10.1089/ars.2009.2804. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer H, Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423–34. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 16.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–7. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–50. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Narita Y, et al. Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Res. 2013;12:119–31. doi: 10.1016/j.scr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 20.He H, Nilsson CL, Emmett MR, Marshall AG, Kroes RA, Moskal JR, et al. Glycomic and transcriptomic response of gsc11 glioblastoma stem cells to stat3 phosphorylation inhibition and serum-induced differentiation. J Proteome Res. 2010;9:2098–108. doi: 10.1021/pr900793a. [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–4. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 22.Shu Q, Wong KK, Su JM, Adesina AM, Yu LT, Tsang YT, et al. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26:1414–24. doi: 10.1634/stemcells.2007-1009. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, Keating MJ, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843–53. doi: 10.1074/jbc.M111.260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan S, Wang F, Chen G, Zhang H, Feng L, Wang L, et al. Effective elimination of cancer stem cells by a novel drug combination strategy. Stem Cells. 2013;31:23–34. doi: 10.1002/stem.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–90. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–43. [PubMed] [Google Scholar]
- 27.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 28.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.LaBarge MA. The difficulty of targeting cancer stem cell niches. Clin Cancer Res. 2010;16:3121–9. doi: 10.1158/1078-0432.CCR-09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–7. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 31.Cuddihy SL, Winterbourn CC, Hampton MB. Assessment of redox changes to hydrogen peroxide-sensitive proteins during EGF signaling. Antioxid Redox Signal. 2011;15:167–74. doi: 10.1089/ars.2010.3843. [DOI] [PubMed] [Google Scholar]
- 32.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–42. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez-Garcia D, Wood CD, Castro-Obregon S, Covarrubias L. Reactive oxygen species: a radical role in development? Free Radic Biol Med. 2010;49:130–43. doi: 10.1016/j.freeradbiomed.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–18. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–8. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 36.Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett LE, Granot Z, Coker C, Iavarone A, Hambardzumyan D, Holland EC, et al. Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell. 2012;21:11–24. doi: 10.1016/j.ccr.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Atkinson SP, Vilella F, Lloret M, Armstrong L, Mann DA, et al. Opposing putative roles for canonical and noncanonical NFkappaB signaling on the survival, proliferation, and differentiation potential of human embryonic stem cells. Stem Cells. 2010;28:1970–80. doi: 10.1002/stem.528. [DOI] [PubMed] [Google Scholar]
- 39.Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70:2516–27. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira L, Ruiz-Ontanon P, Vazquez-Barquero A, Lafarga M, Berciano MT, Aldaz B, et al. Blockade of the NFkappaB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene. 2011;30:3537–48. doi: 10.1038/onc.2011.74. [DOI] [PubMed] [Google Scholar]
- 41.Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, et al. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104:1564–74. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–61. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 43.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–15. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, et al. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–23. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 45.Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–37. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]


