Abstract
Key points
Exposure to microgravity induces postflight orthostatic intolerance on re‐exposure to 1 G gravity which is second to the structural and functional remodelling of arteries.
We found the maximal developed force (E max) of angiotensin II‐elicited vasoconstriction was decreased in abdominal aorta, unchanged in thoracic aorta and increased in carotid artery by simulated weightlessness. However, the sensitivity of the response (EC50) was decreased in all of the arteries as was the desensitization of angiotensin II type I receptor (AT1) upon angiotensin II stimulation.
We demonstrate that caveolae on vascular smooth muscle cells play a key role in the adaptation of EC50 and AT1 desensitization, but not E max of the response to simulated weightlessness.
This study gives insight into the mechanism underlying the arterial functional remodelling during weightlessness. Further, the findings might stimulate new ideas for research into countermeasures to postflight orthostatic intolerance upon astronauts returning to the earth.
Abstract
Weightlessness induces the functional remodelling of arteries, but the changes to angiotensin II (Ang II)‐elicited vasoconstriction and the underlying mechanism have never been reported. Caveolae are invaginations of the cell membrane crucial for the contraction of vascular smooth muscle cells, so we investigated the adaptation of Ang II‐elicited vasoconstriction to simulated weightlessness and the role of caveolae in it. The 4 week hindlimb unweighted (HU) rat was used to simulate the effects of weightlessness. Ang II‐elicited vasoconstriction was measured by isometric force recording. The morphology of caveolae was examined by transmission electron microscope. The binding of the angiotensin II type 1 receptor (AT1) and caveolin‐1 (cav‐1) was examined by coimmunoprecipitation and Western blot. We found that the maximal developing force (E max) of Ang II‐elicited vasoconstriction was decreased in abdominal aorta by 30.6%, unchanged in thoracic aorta and increased in carotid artery by 17.9% after HU, while EC50 of the response was increased in all three arteries (P < 0.05). AT1 desensitization upon activation was significantly reduced by HU in all three arteries, as was the number of caveolae (P < 0.05). Furthermore, Ang II promoted the binding of AT1 and cav‐1 significantly in control but not HU arteries. Both the number of caveolae and the binding of AT1 and cav‐1 in HU arteries were restored by cholesterol pretreatment which also reinstated the change in EC50 as well as the level of AT1 desensitization. These results indicate that modified caveolae in vascular smooth muscle cells could interfere with the binding of AT1 and cav‐1 mediating the adaptation of Ang II‐elicited vasoconstriction to HU.
Abbreviations
- AA
abdominal aorta
- Ang II
angiotensin II
- AT1
angiotensin II type 1 receptor
- CA
carotid artery
- cav‐1
caveolin‐1
- co‐IP
coimmunoprecipitation
- CON
control
- Emax
maximal effect
- HU
hindlimb unweighted
- TA
thoracic aorta
- TEM
transmission electron microscope
- VSMCs
vascular smooth muscle cells
Introduction
Exposure to microgravity induces postflight cardiovascular deconditioning exhibited as orthostatic intolerance and reduced exercise capacity on re‐exposure to 1 G gravity, which lowers the working ability of astronauts and menaces the flight safety (Buckey et al. 1996). The underlying mechanisms of postflight cardiovascular deconditioning are considered multifactorial and not completely understood. Reduced blood volume, cardiovascular dysfunction, diminished baroreflex sensitivity and altered vestibular–autonomic function have all been proved to play a role (Zhang, 2001).
A plethora of data has suggested that the arterial adaptation to microgravity was one of the most important causal factors for postflight cardiovascular deconditioning (Buckey et al. 1996; Hargens & Watenpaugh, 1996). For example, the vasoconstriction of both mesenteric artery and skeletal muscle resistance arterioles to potassium chloride (KCl) and noradrenaline (norepinephrine) was decreased after space flight which might contribute to the occurrence of postflight orthostatic intolerance (Stabley et al. 2012; Behnke et al. 2013). The tail‐suspended hindlimb unweighted (HU) rat is a widely used animal model to simulate weightlessness because it mimics head‐ward fluid shift, bone loss and load‐bearing skeletal muscle atrophy, the main features of body change during weightlessness (Morey‐Holton et al. 2005). Using this animal model, many studies have shown vascular adaptations to simulated weightlessness (Delp et al. 1993; Zhang, 2001; Ma et al. 2003). Our previous studies have revealed significant regional specific remodelling of the small resistance arteries by HU. Lin et al. (Lin et al. 2009; Zhang, 2013) have demonstrated that 28 day HU resulted in enhanced and decreased myogenic tone and vasoconstrictor responses, respectively, in middle cerebral arteries (MCAs) and mesenteric third‐order arterioles (MSAs). They also found that HU induced hypertrophic changes in MCAs but atrophic changes in MSAs. Taken together with other human studies (Gazenko et al. 1981; Levine et al. 2002; Arbeille et al. 2005, 2008), it could be deduced that the hypertrophic remodelling and enhanced vasoconstriction of the cerebral artery, together with the atrophic remodelling and reduced vasoconstriction of the splanchnic resistance arteries, contribute to the occurrence of postflight orthostatic intolerance (Zhang, 2001; Lin et al. 2009; Zhang, 2013). However, resistance artery remodelling cannot fully represent the overall vascular adaptation to simulated weightlessness and thus is not the only vascular factor accounting for postflight orthostatic intolerance. As reviewed by Padilla et al. (2014), resistant vessel tone could at least in part determine the conduit artery endothelial cell phenotype regulating its constriction and dilatation through changes in arterial blood pressure as well as shear stress patterns in conduit arteries, and the integrative effect of both types of artery contributes to the formation of resistant vessel tone. So in the study, we tried to elucidate the effect of HU on the function of conduit arteries and mechanisms underlying the remodelling.
Our previous results have shown that vasoconstrictor responses induced by phenylephrine and KCl were significantly increased in carotid artery (CA) and thoracic aorta (TA) but were decreased in abdominal aorta (AA) and femoral artery by HU (Zhang, 2001; Zhang et al. 2009; Zhang, 2013). Moreover, we have also proved that the mRNA and protein expression of angiotensinogen and angiotensin II type 1 receptor (AT1) were both decreased in AA, but increased in CA by HU (Gao et al. 2009). Although it has been widely known that angiotensin II (Ang II) plays an important role in the physiological regulation and pathophysiological modification of the cardiovascular system as it could induce potent vasoconstriction as well as hypertrophy and inflammation of the arterial wall (Campbell, 2014), up to now, how HU affects Ang II‐elicited vasoconstriction has not yet been reported. We would firstly investigate this issue on AA, TA and CA.
Caveolae are membrane invaginations abundant in vascular smooth muscle cells (VSMCs). They are 50–100 nm in size and enriched with cholesterol and lipids with saturated acyl chains, such as sphingolipids and glycosphingolipids (Koleske et al. 1995; Parton & Simons, 2007). Caveolin‐1 (cav‐1) is the most crucial structural protein of the caveola, critical for the formation of its unique shape, which binds to numerous receptors or signal proteins via its scaffolding domain located at the carboxy‐terminus to form a signal platform (Parton & Simons, 2007). The role of caveolae in vesicular transportation, cholesterol homeostasis, signal transduction and mechanosensing has been recognized for a long time (Cohen et al. 2004). Recently, they were documented playing a crucial role in the regulation of Ang II‐elicited VSMC contraction because both the signal transductional cascade induced by Ang II (Dreja et al. 2002; Hardin & Vallejo, 2006) and the internalization or desensitization of AT1 (Linder et al. 2007) mainly depend on the AT1 translocation into caveolae and binding with cav‐1 upon activation.
Evidence has shown that caveolae on VSMC membrane could be modified by both acute and chronic mechanical stresses (Sinha et al. 2011). Since the changes in transmural pressure or blood flow among arteries during HU may influence the local mechanical stresses imposed on VSMCs, we hypothesized that the caveolae on VSMCs could be changed by HU, contributing to the adaptation of Ang II‐elicited vasoconstriction. Thus, the second aim of the present study was to examine how HU modified caveolae on VSMCs of AA, TA and CA and whether it contributed to the alteration of Ang II‐elicited vasoconstriction.
Methods
Ethical approval
The protocols and procedures employed in this study were approved by the University of Cambridge Ethics Review Committee. All experiments were performed in accordance with the UK Home Office guidance under the Animals (Scientific Procedures) Act 1986.
Tail‐suspended, hindlimb unweighted rat model and tissue preparation
Male Sprague–Dawley rats weighing 220–250 g were obtained from the Laboratory Animal Centre of the Fourth Military Medical University and individually caged within a 12 h:12 h light/dark cycle at 23 ± 1°C with standard lab chow and water available ad libitum. One week later, the rats were randomly assigned to HU or control (CON) groups. For the HU group, rats were subjected to tail suspension for 4 weeks. HU was achieved by partial elevation of the hindlimbs through a tail harness above the floor of the cage. The animals were maintained in ∼35 deg head‐down tilt position, with the hindlimbs elevated ∼0.5 cm above the floor when fully extended.
Four weeks later, rats were anaesthetized with pentobarbital sodium (40 mg kg−1 i.p.) and killed by exsanguination via the heart. Then AA, TA and CA were rapidly removed and placed in cold Krebs buffer solution consisting of (in mm): 118.3 NaCl, 4.7 KCl, 25 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 11.1 glucose and 0.026 EDTA (pH 7.4). Subsequently, the left wet weight of soleus and the relative muscle:body weight ratio were measured to confirm the efficacy of simulated weightlessness. Eventually, arteries were isolated carefully under a microscope and the artery rings were prepared for isometric force measurement. The remains were snap frozen in liquid nitrogen and stored at −80°C for Western blot and coimmunoprecipitation (co‐IP).
Isolation of arteries and functional examination
The four‐chamber organ bath (Radnoti, Monrovia, CA, USA) was used for the in vitro isometric force measurement as described in detail previously (Zhang et al. 2009). After being carefully isolated under a microscope, the endothelium of the artery rings (3 mm in length) was removed by inserting a stainless steel needle into the lumen rubbing the inner face slightly. Then the rings were mounted onto two gracile stainless steel hooks and suspended in each bath, with one hook attached to the tension transducer connected to a PowerLab system (ADInstruments, Bella Vista, NSW, Australia) for tension recording, and the other to the plastic holder in the chamber filled with Krebs buffer solution, bubbled continuously with 95% O2–5% CO2 and maintained at 37°C. Then the rings went through a period of equilibration for 30 min, followed by stimulation with 60 mm KCl and thorough washing four times until the optimal resting tension was achieved. Subsequently, the endothelium integrity of the rings was determined by contracting the artery with 10−6 m phenylephrine and then relaxing with 10−5 m acetylcholine. Rings were excluded for further study when this vasorelaxation was higher than 5%.
After preparations, dose‐dependent responses to Ang II were examined. The artery rings were contracted with accumulative addition of Ang II (10−9–10−6 m) with or without the pretreatment by losartan (100 μm, 1 h at 37°C) or cholesterol (10 mm, 1 h at 37°C). Then, the maximal effect (E max) and the concentration for 50% of maximal effect (EC50) were determined.
For AT1 desensitization analysis, artery rings were contracted with the accumulative addition of Ang II (10−9–10−6 m), twice, with a 1 h interval during which the artery was incubated in Krebs buffer solution. To quantify the desensitizing level, the decrease in E max between the first (R‐1) and second response (R‐2) was calculated as the percentage of E max R‐1, expressed as (E max(R‐1) − E max (R‐2))/E max(R‐1).
Transmission electron microscope (TEM)
After deep anaesthetization, rats were perfused with PBS (pH 7.4) and then with precooled PBS containing 4% formaldehyde and 2.5% glutaraldehyde. Arteries were rapidly removed and carefully cleaned as described above. Subsequently, with or without pretreatment with cholesterol (10 mm, 1 h at 37°C), artery rings (3 mm in length) were prepared and kept in 2.5% glutaraldehyde in 0.1 m PBS (pH 7.4). Then tissues were further fixed in 1% osmium acid for 1 h and rinsed twice with PBS. Afterwards, tissues were dehydrated in graded ethanol, followed by dry acetone to a critical point, and finally embedded in epoxy resin. Ultrathin sections (100 nm) were obtained and viewed under a JEM‐2000EX TEM (JEOL, Tokyo, Japan). Caveolae were distinguished by their characteristic size (50–100 nm), flask‐like shape, and location on or in juxtaposition to the plasma membrane (Koleske et al. 1995; Parton & Simons, 2007). Two observers who were blind about the objectives of the experiment counted the caveolae number in 20 randomly selected fields separately and then calculated the average to obtain the caveolae number in each field of the artery.
Coimmunoprecipitation
After arteries were incubated with Ang II (100 nm) or cholesterol (10 mm) for 1 h, co‐IP was performed according to the manufacturer's instructions for a Pierce Classic IP kit (Pierce, Rockford, IL, USA). As mentioned previously, tissues were homogenized and the supernatants were collected. According to the results of protein quantification determined by a bicinchoninic acid assay kit (Pierce), equal amounts of proteins were applied and combined with 2 μg rabbit polyclonal antibody against cav‐1 in a microcentrifuge tube. Then the mixture was diluted to 300–600 μl with IP Lysis/Wash Buffer and incubated overnight at 4°C to form an immune complex. Afterwards, a spin column was made with 20 μl of the resin slurry and incubated with the immune complex with gentle end‐over‐end mixing for 1 h. Subsequently, the spin column was centrifuged to exclude the flow‐through and the immune complex was left and conglutinated to the column. Finally, the Non‐Reducing Lane Marker Sample Buffer was prepared and mixed with the spin column. After incubation at 100°C for 5–10 min, the spin column was centrifuged again and the eluate was collected. Then the eluate sample was allowed to cool to room temperature and subsequently applied to Western blot analysis. The density of immunoreactive bands of AT1 was expressed as a percentage relative to correspondingly captured cav‐1 in the precipitate.
Western blot
The cav‐1 protein content was measured by Western blot as described in detail previously (Zhang et al. 2009). In brief, with the aid of a glass tissue grinder, artery samples were minced into small pieces and homogenized on ice in tissue protein extraction reagent (Pierce) containing protease inhibitor (Pierce). Afterwards, homogenates were centrifuged at 12,000 g for 10 min at 4°C and the supernatants were collected. The total protein concentration was determined and then equivalent amounts of proteins from both groups were loaded onto 4–12% Bis‐Tris PAGE gels under denaturing conditions within the NuPAGE Bis‐Tris Pre‐cast Gel System (Invitrogen Life Technologies, Carlsbad, CA, USA). After gel electrophoresis, the fractionated proteins were electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) in an XCell Blot Module transfer system (Invitrogen Life Technologies). Then the membrane was blocked for 6 h at room temperature with 5% non‐fat milk powder in PBS containing 0.1% (w/v) Tween 20 (PBS‐T). Subsequently, the membrane was incubated with rabbit polyclonal antibody against cav‐1 (1:1000; Abcam, Cambridge, MA, USA) or mouse monoclonal antibody against β‐actin (1:1500; Proteintech, Chicago, IL, USA), respectively, in blocking buffer at 4°C overnight. Finally, the membrane was incubated with horseradish peroxidase conjugated goat anti‐mouse or anti‐rabbit IgG (1:12,000; Proteintech) in PBS‐T for 90 min at room temperature. Enhanced chemiluminescence (ECL) detection reagents (Millipore) in a Gel Image Analyzing System (Tanon‐4200; Tanon Science & Technology, Shanghai, China) were applied to detect the bound antibodies. The density of immunoreactive bands of cav‐1 was expressed as a percentage relative to β‐actin density.
Data analysis
Data are expressed as means ± SEM. Student's t test was used to determine the significance of differences in body weight, soleus wet weight, muscle:body mass ratio, protein expression and caveolae numbers between groups. Results of isometric force measurement were analysed by repeated‐measures two‐way ANOVA. Statistical analysis was performed by SPSS 16.0 (SPSS Inc.) and Prism 5.0 (Graphpad). A value of P < 0.05 was considered to be statistically significant.
Results
Body weight and soleus wet weight
The data are summarized in Table 1. There were no significant differences in either the initial or final body weight between CON and HU group, suggesting that normal growth was not interfered with by tail suspension. The wet weight of soleus in HU rats was 53% less than that in CON rats (P < 0.05), which was evidence of the prerequisite of simulated weightlessness.
Table 1.
Body weight and wet weight of left soleus of CON and HU rats
| Group | Body weight (g) | Wet weight of left soleus | ||
|---|---|---|---|---|
| Initial | Final | Absolute (mg) | Relative (mg (g body wt)−1) | |
| CON | 225.4 ± 4.3 | 384.5 ± 5.1 | 145.3 ± 3.4 | 0.38 ± 0.03 |
| HU | 244.6 ± 4.5 | 372.4 ± 4.6 | 68.7 ± 2.7* | 0.18 ± 0.02* |
Values are means ± SEM; n = 10 rats in each group. CON, control group; HU, 4 week tail suspended hindlimb unweighting group. *P < 0.05 vs. CON.
Vasoconstrictive response to Ang II
As shown in Fig. 1 A, Ang II produced a dose‐dependent bi‐phasic force development, including an initial rise and a following fall. In AA, the force development of HU was lower than that of CON in both phases. In TA of HU, it was lower in the rise phase but higher in the fall phase while in CA of HU, it was higher in both phases as compared to that of CON. Pretreatment with losartan, an AT1 inhibitor, significantly attenuated the response to Ang II in all the CON and HU arteries.
Figure 1. Angiotensin II (Ang II, 10−9–10−6 m)‐elicited vasoconstriction with or without losartan pretreatment in abdominal aorta (AA), thoracic aorta (TA) and carotid artery (CA) of control (CON) and hindlimb unweighted (HU) rats .

Concentration–response curves (A), maximal effect (E max, B) and concentration for 50% of E max (EC50, C). Values are means ± SEM. n = 8, *P < 0.05 vs. CON, # P < 0.05 vs. HU.
E max and EC50 values of the vasoconstrictive response were analysed as shown in Fig. 1 B and C. E max was significantly decreased in AA by 30.6% (P < 0.05), unaffected in TA, and significantly increased in CA by 17.9% (P < 0.05) after HU. Losartan pretreatment significantly reduced E max of all arteries in both groups (P < 0.05). Moreover, EC50 was significantly increased by HU in all three arteries (P < 0.05).
AT1 desensitization
Besides vasoconstriction, we also examined the process of AT1 desensitization by comparing E max of two consecutive vasoconstrictive responses. As shown by the original force recording in Fig. 2 A, E max of R‐2 was much lower than that of R‐1, indicating remarkable AT1 desensitization upon the first stimulation by Ang II. The ratio of the decrease in E max between R‐1 and R‐2 to E max of R‐1 was calculated to represent the extent of AT1 desensitization. As summarized in Fig. 2 B, the level of AT1 desensitization was significantly decreased by HU in all three arteries (P < 0.05), 47.7 vs. 35.0% in AA, 67.5 vs. 45.7% in TA and 50.9 vs. 37.0% in CA.
Figure 2. Effect of HU on AT1 desensitization in AA, TA and AA .

A, representative original force recording of AT1 desensitization examination, including two consecutive vasoconstrictive responses elicited by Ang II (10−9–10−6 m; R‐1 and R‐2). B, summarized data of AT1 desensitization expressed as (E max (R‐1) − E max (R‐2))/E max (R‐1). Values are means ± SEM. n = 8, *P < 0.05 vs. CON.
Caveolae number and protein expression of cav‐1
Caveolae can be readily visualized by TEM. Normally, caveolae are distinguished by their characteristic size (50–100 nm), flask‐like shape, and location on or in juxtaposition to the plasma membrane (Fig. 3 A). The summarized data in Fig. 3 B show that HU significantly reduced caveolae number on VSMCs of AA, TA and CA by 60.4, 48.2 and 37.3%, respectively (P < 0.05).
Figure 3. Caveolae on vascular smooth muscle cells (VSMCs) of CON and HU arteries .

A, micrograph of caveolae structure detected by TEM. Magnification ×80,000; scale bars, 100 nm; black arrows indicate flask‐shaped invaginations of caveolae. B, summarized data of the average number of caveolae in each field of CON and HU arteries. Values are means ± SEM. n = 5, *P < 0.05 vs. CON.
As shown in Fig. 4 A, antibody against cav‐1 detected a band at 23 kDa in accordance with the molecular mass of cav‐1. The summarized data indicated there was no significant difference in protein expression of cav‐1 between CON and HU arteries (Fig. 4 B).
Figure 4. Effects of HU on protein expression of caveolin‐1 (cav‐1) .

A, representative band of cav‐1 protein in AA, TA and CA. B, summarized data for cav‐1 protein expression normalized by β‐actin. Values are means ± SEM. n = 5.
Binding of AT1 and cav‐1
Upon activation by Ang II, AT1 would move into caveolae and bind to cav‐1 which is critical for both boosting the following signal transduction and mediating AT1 desensitization. Thus, co‐IP and Western blot were conducted in this study to examine the binding of AT1 and cav‐1. As shown in Fig. 5, Ang II stimulation (100 nm, 1 h) significantly increased the protein content of AT1 in the precipitate captured with antibody to cav‐1 in all CON arteries (2.31‐fold increase in AA, 2.15‐fold increase in TA and 1.97‐fold increase in CA), meaning the binding of AT1 and cav‐1 was markedly enhanced (P < 0.01). HU did not affect the basic binding of the two proteins, but significantly decreased the extent of Ang II‐stimulated AT1–cav‐1 binding in AA (P < 0.01), TA (P < 0.05) and CA (P < 0.05).
Figure 5. Basic and Ang II‐stimulated binding of AT1 and cav‐1 in CON and HU arteries .

A, representative bands. B, summarized data of AT1 expression in coimmunoprecipitation (co‐IP) captured by antibody against cav‐1. Values are means ± SEM. n = 5, **P < 0.01 vs. CON; # P < 0.05 vs. HU; ## P < 0.01 vs. HU.
Effect of cholesterol pretreatment on caveolae formation as well as binding of AT1 and cav‐1
Cholesterol is one of the major components of caveolae which is always used to enhance the caveolae formation on cell membrane. In this study, the number of caveolae on VSMCs was not changed in CON but significantly increased in HU arteries (P < 0.05) by 1 h of cholesterol incubation (Fig. 6). As caveolae number is one of the causal factors for the binding of AT1 and cav‐1, we subsequently examined the effect of cholesterol on AT1–cav‐1 binding. As shown in Fig. 7, in HU arteries, cholesterol did not affect the basic binding of AT1 and cav‐1, but significantly enhanced that stimulated by Ang II (P < 0.05).
Figure 6. Effect of cholesterol (chol) pretreatment on caveolae formation in CON and HU arteries .

A, micrograph of caveolae structure with vehicle (vehl) or chol pretreatment. Magnification ×80,000; scale bars, 500 nm; black arrows indicate flask‐shaped invaginations of caveolae. B, summarized data of the average number of caveolae. Values are means ± SEM. n = 5, *P < 0.05 vs. HU.
Figure 7. Effect of cholesterol (chol) pretreatment on the basic and Ang II‐stimulated binding of AT1 and cav‐1 in HU arteries .
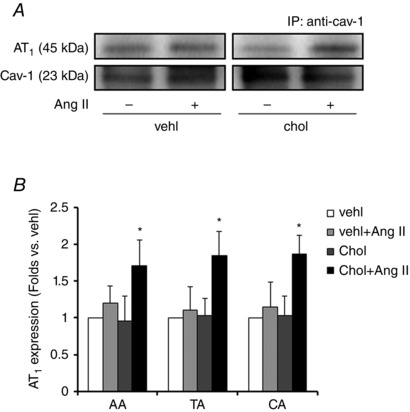
A, representative bands. B, summarized data of AT1 expression in co‐IP captured by antibody against cav‐1. Values are means ± SEM. n = 5, *P < 0.05 vs. vehl.
Effect of cholesterol pretreatment on Ang II‐elicited vasoconstriction and AT1 desensitization
As caveolae on VSMCs are involved in both Ang II‐elicited vasoconstriction and AT1 desensitization, we further tested whether cholesterol pretreatment also restored the change in Ang II‐elicited vasoconstriction and AT1 desensitization in HU arteries. As shown in Fig. 8 A and B, cholesterol pretreatment did not change E max but significantly reduced the EC50 of the Ang II‐elicited vasoconstriction in all HU arteries (P < 0.05). Moreover, we found cholesterol pretreatment did not affect AT1 desensitization in CON, but significantly improved it in all the HU arteries (P < 0.05, Fig. 8 C).
Figure 8. Effect of cholesterol (chol) pretreatment on Emax (A), EC50 (B) and AT1 desensitization (C) in CON and HU arteries .
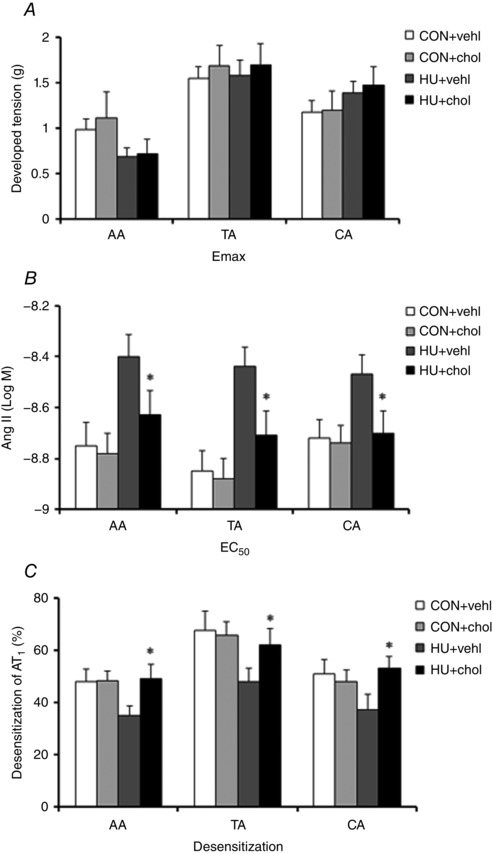
Values are means ± SEM. n = 5, *P < 0.05 vs. HU.
Discussion
In this study, we investigated how Ang II‐elicited vasoconstriction in conduit arteries was adapted to simulated weightlessness and whether caveolae played a role in the adaptation. We have demonstrated that the E max of the Ang II‐elicited vasoconstrictive response exhibited regional‐specific changes as it was decreased in AA, unchanged in TA, but increased in CA. Interestingly, EC50 of the vasoconstriction was increased and AT1 desensitization upon activation was abrogated in all three arteries, which showed no regional specification. Moreover, we found the amount of caveolae on VSMCs was reduced and the extent of Ang II‐stimulated binding of cav‐1 and AT1 was also decreased in HU, which were both restored by cholesterol treatment. Finally, we demonstrated that EC50 and AT1 desensitization of Ang II‐elicited vasoconstrictive responses in HU were also recovered when caveolae formation was regained by cholesterol treatment.
Prolonged exposure to weightlessness causes orthostatic intolerance when an astronaut returns to the earth, the underlying mechanism being multifactorial including at least blood volume reduction, cardiovascular dysfunction, diminished baroreflex sensitivity and altered vestibular–autonomic function (Buckey et al. 1996; Hargens & Watenpaugh, 1996; Zhang, 2001). The structural and functional adaptation of arteries to weightlessness has also been reported to be crucial in this process (Zhang, 2001). For example, in rodents undergoing space flight, the vasoconstriction elicited by KCl, phenylephrine and caffeine was depressed in mesenteric artery or skeletal muscle resistance arteries but enhanced in cerebral artery (Buckey et al. 1996; Hargens & Watenpaugh, 1996; Zhang, 2001; Stabley et al. 2012; Behnke et al. 2013). Simulated weightlessness experienced by HU rats or bed‐resting humans induces regional‐specific arterial structural and functional remodelling, with cerebral and carotid arteries showing hypertrophy and up‐regulated vasoconstriction, but abdominal and femoral arteries exhibiting atrophy and down‐regulated vasoconstriction (Zhang, 2001; Ma et al. 2003; Hwang et al. 2007; Zhang et al. 2009). The mechanisms underlying the functional remodelling of arteries involves an impact of altered haemodynamic stimuli on the arterial wall, inactivity‐related metabolic adjustment of tissue, as well as the characteristics of the different vascular beds (Zhang, 2001; Palombo et al. 2015).
Ang II is one of the potent vasoconstrictors playing a very important role in the physiological regulation of arterial structure and function, as well as progress of cardiovascular diseases (Campbell, 2014). The effect of Ang II was mainly mediated by two types of receptors. AT1 is the most studied type and expressed broadly in the cardiovascular system through which Ang II elicits vasoconstriction and hypertrophy of the vascular wall (de Gasparo et al. 2000; Castrop, 2015). AT2 was mainly found in the fetus and neonate through which Ang II could elicit vasodilatation and atrophy of the vessel wall (de Gasparo et al. 2000; Sumners et al. 2015). Although in our former studies AT1 protein content has been proved to be increased in CA and decreased in AA by HU (Gao et al. 2009), Ang II‐elicited vasoconstriction, which we first elucidated in the present work, has never been reported. Our results showed Ang II elicited a bi‐phasic vascular force development similar to trace recordings in other studies (Linder et al. 2007). Through the force development measurement, we found E max of the response exhibited a clearly regional‐specific adaptation to HU, which was decreased in AA, unchanged in TA, but increased in CA. This E max alteration was consistent with the changes in AT1 protein expression in corresponding HU arteries; this meant that the AT1 density would determine the potency of the vasoconstriction elicited by Ang II. Since changes in AT1 protein content indicate the alterations of local renin–angiotensin system activity by HU, which might result from the regional‐specific change in haemodynamics among arteries during tail suspension (Gao et al. 2009), the different changes in E max of Ang II‐elicited vasoconstriction found in AA, TA and CA might also stem from the altered local haemodynamics during simulated weightlessness. However, the EC50 of the response was increased in all three arteries, showing no regional specification, indicating that the sensitivity to AT1 was compromised through arteries. As Ang II has been well known to be the most critical responding endocrine and paracrine factor of the body in an emergency to increase blood pressure (Campbell, 2014), we may infer from the results that the depressed sensitivity of arteries to Ang II in simulated/real weightlessness would cause a tardy arterial vasoconstrictive response which could contribute to the occurrence of postflight orthostatic intolerance upon returning to 1 G gravity.
As documented, desensitization or tachyphylaxis upon activation is a key characteristic of AT1 which is considered to be a protective device of the artery and mainly attributable to the internalization of the receptor (Linder et al. 2007). To determine the extent of AT1 desensitization, as performed in other studies (Linder et al. 2007), we conducted two consecutive Ang II‐elicited vasoconstrictive responses with a 1 h interval and then compared the E max of the two responses. We found that the E max of the second response was much lower than that of the first one indicating that some of the receptors had been desensitized after the first time of Ang II stimulation. Our results also showed that the difference in E max between the two responses was significantly smaller in HU than that in CON suggesting AT1 desensitization was prevented by HU. Similar to EC50, the change in AT1 desensitization showed no regional specification among HU arteries, which in combination with the above results indicates that the change of E max in HU might result from the remodelling of AT1 abundance, but that of EC50 or AT1 desensitization might be regulated through other mechanisms.
Caveolae are membrane invaginations 50–100 nm in size and abundantly expressed in VSMCs. They are enriched with cholesterol and lipids with saturated acyl chains, such as sphingolipids and glycosphingolipids (Koleske et al. 1995; Parton & Simons, 2007). The critical roles of caveolae in vesicular transportation, cholesterol homeostasis, signal transduction and mechanosensing have been intensively investigated in previous studies (Schroeder et al. 2001; Cohen et al. 2004). Recently, another possible role of caveolae has also been reported that they could regulate the contraction of VSMCs, as a number of receptors are located in or could be translocated into or out of caveolae upon stimulation with agonists (Dreja et al. 2002; Ushio‐Fukai & Alexander, 2006; Yu et al. 2006; Cristofaro et al. 2007). Moreover, caveolae could act as a cellular structure promoting the endocytosis and recycling of receptors similar to the effects of clathrin‐coated pits/vesicles (Linder et al. 2007; Mayor et al. 2014). Cav‐1 is the most crucial structural protein of the caveola, critical for the formation of its unique shape, which could be linked to numerous receptors or signal proteins via the scaffolding domain located at the carboxy‐terminus to form a signal platform (Drab et al. 2001; Hardin & Vallejo, 2006; Parton & Simons, 2007). It has been reported that the provoking effects of Ang II stimulation on AT1 translocation into caveolae and binding with cav‐1 depends on the intactness of the caveolae structure which mediates the Ang II‐elicited signal transductional cascade (Hardin & Vallejo, 2006; Ushio‐Fukai & Alexander, 2006) as well as the internalization or desensitization of AT1 (Linder et al. 2007; Mayor et al. 2014). As HU induced the changes of both Ang II‐elicited vasoconstriction and AT1 desensitization, we subsequently investigated how caveolae were modulated and whether they played a role in the arterial adaptation.
We found that HU significantly reduced caveolae number on VSMCs of AA, TA and CA, but did not change the protein content of cav‐1, indicating that cav‐1 might not be the reason for the alteration of caveolae. According to the studies on lipid rafts, caveolae could be remodelled by various factors, including cav‐1 protein expression (Sedding & Braun‐Dullaeus, 2006), mechanical stress (Boyd et al. 2003; Rizzo et al. 2003; Sedding et al. 2005; Yu et al. 2006; Sinha et al. 2011), cholesterol content (Bastiani & Parton, 2010) and humoral regulators (Stralfors, 2012). It has been documented that transmural pressure across arteries could be changed by HU, resulting in the alteration of mechanical stress posed on VSMCs (Buckey et al. 1996; Hargens & Watenpaugh, 1996; Zhang, 2001). At the same time, smooth muscle atrophy and bone loss in the HU rat would change the level of regulatory factors and ions such as TNF‐α, interleukin‐6 and magnesium in blood flow which might influence caveolae formation on VSMCs (Ray, 2008). As shown in a previous study, caveolae act as a reservoir for the cell membrane to respond to the immediate mechanical stimulus, such as hyposmotic and stretch stress, through flattening the invagination structures leading to tension buffering on the cell membrane (Sinha et al. 2011). Thus, a decreased number of caveolae in the cell membrane may weaken the cellular ability to tolerate acute mechanical stresses (Sinha et al. 2011). One of the supporting cases is that, in a form of human muscular dystrophy in which the caveolin gene was mutated and caveolae number was reduced dramatically, the myotubes were more fragile than wild‐type myotubes when exposed to acute osmotic shock (Sinha et al. 2011). So decreased caveolae on the VSMCs of HU arteries suggests the cells are more prone to be damaged when encountering acute mechanical stresses.
Next, we examined the effect of HU on the binding of AT1 and cav‐1. Consistent with other studies, we proved Ang II stimulation significantly increased AT1–cav‐1 binding in CON arteries (Linder et al. 2007). Yet, although HU did not affect the basic AT1–cav‐1 binding, it significantly abolished the promoting effects of Ang II stimulation. Since we did not find any changes in cav‐1 or AT1 protein expression induced by Ang II treatment in either CON or HU (data not shown), it was most possibly that the mechanism underlying dysregulation of AT1–cav‐1 binding was the defect of caveolae structure.
Previous studies have shown that cholesterol is enriched 10‐fold in caveolae relative to the bulk plasma membrane and the integrity of caveolae mainly depends on the cholesterol content (Schroeder et al. 2001; Cohen et al. 2004). So cholesterol has always been the choice for adjusting caveolae formation. For example, methyl‐β‐cyclodextrin, which selectively entraps cholesterol rather than other membrane lipids, could effectively remove cholesterol from membranes and has been employed extensively to reduce the amount of membrane caveolae (Dreja et al. 2002). Inversely, cholesterol supplementation is always undertaken to increase the caveolae formation (Cohen et al. 2004). In the present work, we pretreated the arteries with cholesterol and found the number of caveolae on VSMCs of HU but not CON arteries was obviously increased. Moreover, we found that the binding of AT1 and cav‐1 was also recovered with cholesterol pretreatment, further testifying to the importance of caveolae structure with regard to the interaction of the two proteins upon Ang II stimulation. The following functional study showed that after caveolae formation and AT1–cav‐1 binding were recovered, both the decreased sensitivity to Ang II stimulation and compromised AT1 desensitization in HU arteries were restored, supporting the importance of caveolae in functional adaptation of arteries to HU.
Previous data from space flight or ground based studies have suggested that testosterone levels might be reduced during flight or on landing (Smith et al. 2012). In studies carried out on HU rats, a significant decrease in testosterone levels and testis weight has also been observed (Mendis‐Handagama et al. 1998; Zhou et al. 2006). As reported, testosterone is a vasoactive hormone predominantly imposing vasodilatory actions on several vascular beds (Kelly & Jones, 2013). It is also a critical physiological regulator associated with cholesterol metabolism, endothelial dysfunction and inflammation (Kelly & Jones, 2013). The total cholesterol level has been reported to be significantly increased by 15% 2 days after a single dose injection of testosterone (Gårevik et al. 2012). As we know, arteries are under the influence of local haemodynamic stress and systemic regulators. The former is accountable for the regional specific structural and functional remodelling of arteries and the latter would impose unified effect on the whole vascular beds. In the present study, we found consistent defective modification of caveolae in AA, TA and CA of HU rats indicating that systemic regulators might play a role. Given that the caveolae formation is closely linked to the cholesterol level of the local environment (Schroeder et al. 2001; Cohen et al. 2004), there is a great possibility that the deregulated caveolae in the arteries result from the lower cholesterol level which is induced by compromised testosterone in HU rats.
In summary, we have demonstrated that both the sensitivity of Ang II‐elicited vasoconstriction and AT1 desensitization were downregulated in AA, TA and CA by HU, which might result from the decreased number of caveolae and lowered binding of cav‐1 and AT1 on VSMCs. We have proposed a new mechanism for the vascular functional adjustment during HU in this work, which may stimulate new ideas for research into countermeasures to cardiovascular deconditioning induced by real/simulated weightlessness.
Additional information
Competing interests
None declared.
Author contributions
The experiments in this study were performed in the Department of Aerospace Physiology, Fourth Military Medical University, P. R. China. J.M. and J.B. conceived and designed the experiments. Z.W., Y.B., J.Y., H.L. and Y.C. conducted experiments. Z.W., Y.L., X.X. and J.B. collected, analysed, and interpreted the experimental data. Z.W. and J.B. drafted the manuscript. Z.W., J.M. and J.B. revised the manuscript critically for important intellectual content. All authors have given their approval of the final version of the paper to be published.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31071045, 81401550).
Acknowledgements
We would like to thank all the other staff from the Department of Aerospace Physiology, and Department of Aerospace Hygiene, Fourth Military Medical University for their help with the animal and equipment maintenance.
Z. Wang, Y. Bai and J. Yu contributed equally to this work.
References
- Arbeille P, Kerbeci P, Mattar L, Shoemaker JK & Hughson R (2008). Insufficient flow reduction during LBNP in both splanchnic and lower limb areas is associated with orthostatic intolerance after bedrest. Am J Physiol Heart Circ Physiol 295, H1846–H1854. [DOI] [PubMed] [Google Scholar]
- Arbeille PP, Besnard SS, Kerbeci PP & Mohty DM (2005). Portal vein cross‐sectional area and flow and orthostatic tolerance: a 90‐day bed rest study. J Appl Physiol (1985) 99, 1853–1857. [DOI] [PubMed] [Google Scholar]
- Bastiani M & Parton RG (2010). Caveolae at a glance. J Cell Sci 123, 3831–3836. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Stabley JN, McCullough DJ, Davis RT 3rd, Dominguez JM 2nd, Muller‐Delp JM & Delp MD (2013). Effects of spaceflight and ground recovery on mesenteric artery and vein constrictor properties in mice. FASEB J 27, 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M & Jo H (2003). Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 285, H1113–H1122. [DOI] [PubMed] [Google Scholar]
- Buckey JJ, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA & Blomqvist CG (1996). Orthostatic intolerance after spaceflight. J Appl Physiol (1985) 81, 7–18. [DOI] [PubMed] [Google Scholar]
- Campbell DJ ( 2014). Clinical relevance of local renin angiotensin systems. Front Endocrinol (Lausanne) 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrop H (2015). A role for AT1 receptor‐associated proteins in blood pressure regulation. Curr Opin Pharmacol 21C, 43–47. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W & Lisanti MP (2004). Role of caveolae and caveolins in health and disease. Physiol Rev 84, 1341–1379. [DOI] [PubMed] [Google Scholar]
- Cristofaro V, Peters CA, Yalla SV & Sullivan MP (2007). Smooth muscle caveolae differentially regulate specific agonist induced bladder contractions. Neurourol Urodyn 26, 71–80. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW & Unger T (2000). International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52, 415–472. [PubMed] [Google Scholar]
- Delp MD, Holder‐Binkley T, Laughlin MH & Hasser EM (1993). Vasoconstrictor properties of rat aorta are diminished by hindlimb unweighting. J Appl Physiol (1985) 75, 2620–2628. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H & Kurzchalia TV (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin‐1 gene‐disrupted mice. Science 293, 2449–2452. [DOI] [PubMed] [Google Scholar]
- Dreja K, Voldstedlund M, Vinten J, Tranum‐Jensen J, Hellstrand P & Sward K (2002). Cholesterol depletion disrupts caveolae and differentially impairs agonist‐induced arterial contraction. Arterioscler Thromb Vasc Biol 22, 1267–1272. [DOI] [PubMed] [Google Scholar]
- Gao F, Bao JX, Xue JH, Huang J, Huang WQ, Wu SX & Zhang LF (2009). Regional specificity of adaptation change in large elastic arteries of simulated microgravity rats. Acta Physiol Hung 96, 167–187. [DOI] [PubMed] [Google Scholar]
- Gårevik N, Skogastierna C, Rane A & Ekström L (2012). Single dose testosterone increases total cholesterol levels and induces the expression of HMG CoA reductase. Subst Abuse Treat Prev Policy 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazenko OG, Genin AM & Egorov AD (1981). Summary of medical investigations in the U.S.S.R. manned space missions. Acta Astronaut 8, 907–917. [DOI] [PubMed] [Google Scholar]
- Hardin CD & Vallejo J (2006). Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res 69, 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargens AR & Watenpaugh DE (1996). Cardiovascular adaptation to spaceflight. Med Sci Sports Exerc 28, 977–982. [DOI] [PubMed] [Google Scholar]
- Hwang S, Shelkovnikov SA & Purdy RE (2007). Simulated microgravity effects on the rat carotid and femoral arteries: role of contractile protein expression and mechanical properties of the vessel wall. J Appl Physiol (1985) 102, 1595–1603. [DOI] [PubMed] [Google Scholar]
- Kelly DM & Jones TH (2013). Testosterone: a vascular hormone in health and disease. J Endocrinol 217, R47–R71. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D & Lisanti MP (1995). Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci U S A 92, 1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC Jr, Cooke WH, Baisch FJ, Eckberg DL & Blomqvist CG (2002). Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 538, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LJ, Gao F, Bai YG, Bao JX, Huang XF, Ma J & Zhang LF (2009). Contrasting effects of simulated microgravity with and without daily −Gx gravitation on structure and function of cerebral and mesenteric small arteries in rats. J Appl Physiol (1985) 107, 1710–1721. [DOI] [PubMed] [Google Scholar]
- Linder AE, Thakali KM, Thompson JM, Watts SW, Webb RC & Leite R (2007). Methyl‐β‐cyclodextrin prevents angiotensin II‐induced tachyphylactic contractile responses in rat aorta. J Pharmacol Exp Ther 323, 78–84. [DOI] [PubMed] [Google Scholar]
- Ma J, Kahwaji CI, Ni Z, Vaziri ND & Purdy RE (2003). Effects of simulated microgravity on arterial nitric oxide synthase and nitrate and nitrite content. J Appl Physiol (1985) 94, 83–92. [DOI] [PubMed] [Google Scholar]
- Mayor S, Parton RG & Donaldson JG (2014). Clathrin‐independent pathways of endocytosis. Cold Spring Harb Perspect Biol 6; DOI: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis‐Handagama SM, Watkins PA, Gelber SJ & Scallen TJ (1998). The effect of chronic luteinizing hormone treatment on adult rat Leydig cells. Tissue Cell 30, 64–73. [DOI] [PubMed] [Google Scholar]
- Morey‐Holton E, Globus RK, Kaplansky A & Durnova G (2005). The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 10, 7–40. [DOI] [PubMed] [Google Scholar]
- Padilla J, Jenkins NT, Laughlin MH & Fadel PJ (2014). Blood pressure regulation VIII: resistance vessel tone and implications for a pro‐atherogenic conduit artery endothelial cell phenotype. Eur J Appl Physiol 114, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo C, Morizzo C, Baluci M, Lucini D, Ricci S, Biolo G, Tortoli P & Kozakova M (2015). Large artery remodeling and dynamics following simulated microgravity by prolonged head‐down tilt bed rest in humans. Biomed Res Int 2015, 342565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG & Simons K (2007). The multiple faces of caveolae. Nat Rev Mol Cell Biol 8, 185–194. [DOI] [PubMed] [Google Scholar]
- Ray CA (2008). New insights into orthostatic hypotension. Am J Physiol Regul Integr Comp Physiol 294, R1575–R1576. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Morton C, DePaola N, Schnitzer JE & Davies PF (2003). Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285, H1720–H1729. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Gallegos AM, Atshaves BP, Storey SM, McIntosh AL, Petrescu AD, Huang H, Starodub O, Chao H, Yang H, Frolov A & Kier AB (2001). Recent advances in membrane microdomains: rafts, caveolae, and intracellular cholesterol trafficking. Exp Biol Med (Maywood) 226, 873–890. [DOI] [PubMed] [Google Scholar]
- Sedding DG & Braun‐Dullaeus RC (2006). Caveolin‐1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med 16, 50–55. [DOI] [PubMed] [Google Scholar]
- Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H & Braun‐Dullaeus RC (2005). Caveolin‐1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res 96, 635–642. [DOI] [PubMed] [Google Scholar]
- Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler‐Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C & Nassoy P (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Heer M, Wang Z, Huntoon CL & Zwart SR (2012). Long‐duration space flight and bed rest effects on testosterone and other steroids. J Clin Endocrinol Metab 97, 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabley JN, Dominguez JN, Dominguez CE, Mora SF, Ahlgren J, Behnke BJ, Muller‐Delp JM & Delp MD (2012). Spaceflight reduces vasoconstrictor responsiveness of skeletal muscle resistance arteries in mice. J Appl Physiol (1985) 113, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Stralfors P (2012). Caveolins and caveolae, roles in insulin signalling and diabetes. Adv Exp Med Biol 729, 111–126. [DOI] [PubMed] [Google Scholar]
- Sumners C, de Kloet AD, Krause EG, Unger T & Steckelings UM (2015). Angiotensin type 2 receptors: blood pressure regulation and end organ damage. Curr Opin Pharmacol 21C, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio‐Fukai M & Alexander RW (2006). Caveolin‐dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension 48, 797–803. [DOI] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV & Sessa WC (2006). Direct evidence for the role of caveolin‐1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116, 1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LF ( 2001). Vascular adaptation to microgravity: what have we learned? J Appl Physiol (1985) 91, 2415–2430. [DOI] [PubMed] [Google Scholar]
- Zhang LF ( 2013). Region‐specific vascular remodeling and its prevention by artificial gravity in weightless environment. Eur J Appl Physiol 113, 2873–2895. [DOI] [PubMed] [Google Scholar]
- Zhang R, Bai YG, Lin LJ, Bao JX, Zhang YY, Tang H, Cheng JH, Jia GL, Ren XL & Ma J (2009). Blockade of AT1 receptor partially restores vasoreactivity, NOS expression, and superoxide levels in cerebral and carotid arteries of hindlimb unweighting rats. J Appl Physiol (1985) 106, 251–258. [DOI] [PubMed] [Google Scholar]
- Zhou DX, Qiu SD, Wang ZY & Zhang J (2006). [Effect of tail‐suspension on the reproduction of adult male rats]. Zhonghua Nan Ke Xue 12, 326–329. [PubMed] [Google Scholar]


