Abstract
Key points
This study assessed the respective contributions of haematological and skeletal muscle adaptations to any observed improvement in peak oxygen uptake () induced by endurance training (ET).
, peak cardiac output (), blood volumes and skeletal muscle biopsies were assessed prior (pre) to and after (post) 6 weeks of ET. Following the post‐ET assessment, red blood cell volume (RBCV) reverted to the pre‐ET level following phlebotomy and and were determined again.
We speculated that the contribution of skeletal muscle adaptations to an ET‐induced increase in could be identified when offsetting the ET‐induced increase in RBCV.
, , blood volumes, skeletal muscle mitochondrial volume density and capillarization were increased after ET. Following RBCV normalization, and reverted to pre‐ET levels.
These results demonstrate the predominant contribution of haematological adaptations to any increase in induced by ET.
Abstract
It remains unclear whether improvements in peak oxygen uptake () following endurance training (ET) are primarily determined by central and/or peripheral adaptations. Herein, we tested the hypothesis that the improvement in following 6 weeks of ET is mainly determined by haematological rather than skeletal muscle adaptations. Sixteen untrained healthy male volunteers (age = 25 ± 4 years, = 3.5 ± 0.5 l min−1) underwent supervised ET (6 weeks, 3–4 sessions per week). , peak cardiac output (), haemoglobin mass (Hbmass) and blood volumes were assessed prior to and following ET. Skeletal muscle biopsies were analysed for mitochondrial volume density (MitoVD), capillarity, fibre types and respiratory capacity (OXPHOS). After the post‐ET assessment, red blood cell volume (RBCV) was re‐established at the pre‐ET level by phlebotomy and and were measured again. We speculated that the contribution of skeletal muscle adaptations to the ET‐induced increase in would be revealed when controlling for haematological adaptations. and were increased (P < 0.05) following ET (9 ± 8 and 7 ± 6%, respectively) and decreased (P < 0.05) after phlebotomy (−7 ± 7 and −10 ± 7%). RBCV, plasma volume and Hbmass all increased (P < 0.05) after ET (8 ± 4, 4 ± 6 and 6 ± 5%). As for skeletal muscle adaptations, capillary‐to‐fibre ratio and total MitoVD increased (P < 0.05) following ET (18 ± 16 and 43 ± 30%), but OXPHOS remained unaltered. Through stepwise multiple regression analysis, , RBCV and Hbmass were found to be independent predictors of . In conclusion, the improvement in following 6 weeks of ET is primarily attributed to increases in and oxygen‐carrying capacity of blood in untrained healthy young subjects.
Abbreviations
- a–vO2diff
arteriovenous oxygen difference
- BV
blood volume
- CO
carbon monoxide
- ET
endurance training
- ETS
electron transfer system capacity
- FTa
fast twitch type IIa muscle fibre
- FTx
fast twitch type IIx muscle fibre
- [Hb]
haemoglobin concentration
- %HbCO
percentage carboxyhaemoglobin
- Hbmass
haemoglobin mass
- Hct
haematocrit
- IMF
intermyofibrillar
- MitoVD
mitochondrial volume density
- N2O
nitrous oxide
- OXPHOS
maximal oxidative phosphorylation capacity
- PV
plasma volume
peak cardiac output
- RBCV
red blood cell volume
- SS
subsarcolemmal
- ST
slow twitch type I muscle fibre
- VIF
variance inflation factor
peak oxygen uptake
peak power output
Introduction
Endurance training (ET) prompts multiple physiological adaptations that may all lead to increased peak oxygen uptake () in healthy humans (Clausen, 1977; Klausen et al. 1982; Saltin, 1985; Beere et al. 1999; Nottin et al. 2002; Daussin et al. 2007; Murias et al. 2010; Jacobs et al. 2013 b; Bonne et al. 2014). These adaptations must enhance oxygen delivery to and/or extraction and utilization by metabolic active tissue in order to increase . Indeed, ET has been associated with central adaptations such as improved heart function/structure (Baggish et al. 2008), increased plasma and red blood cell volume (RBCV) (Sawka et al. 2000; Bonne et al. 2014) as well as peripheral adaptations resulting in decreased vascular resistance (Klausen et al. 1982; Weng et al. 2013), all of which may enhance peak cardiac output () and thereby oxygen delivery. Moreover, peripheral adaptations to ET comprising increases in skeletal muscle capillarization (Murias et al. 2011), mitochondrial content/function (Hoppeler et al. 1973, 1985; Jacobs et al. 2013 b; Jacobs & Lundby, 2013) and a more efficient blood flow distribution (Kalliokoski et al. 2001) could improve oxygen extraction and utilization. Nevertheless, the relative contribution of the above adaptations to the improvement in following ET remains uncertain despite related research spanning over four decades (Ekblom et al. 1968; Saltin et al. 1968). In addition, there seems to be a distinct progression of central and peripheral adaptations throughout ET (Murias et al. 2010). In this respect, 2–3 weeks of high‐intensity interval or moderate ET induced increases in that were mostly dependent on peripheral adaptations enhancing oxygen extraction in untrained subjects (Murias et al. 2010; Jacobs et al. 2013 b). On the other hand, the increase in following 6 weeks of moderate ET reverted to the baseline level after removing the ET‐induced gain in blood volume (BV) in untrained subjects (Bonne et al. 2014), which would suggest a predominant role for haematological adaptations. However, peripheral adaptations were not determined in the latter study (Bonne et al. 2014). Furthermore, increased peak oxygen extraction was reported in studies assessing ET interventions lasting more than 2 weeks in untrained subjects (Spina et al. 1992; Beere et al. 1999; Murias et al. 2010). Therefore, it is unclear to what extent peripheral adaptations to ET influence once central adaptations have taken place.
To address this question, we assessed central (haematological) and peripheral (skeletal muscle) adaptations, all relating to either the delivery or extraction/utilization of oxygen, along with and before and after 6 weeks of moderate ET in untrained healthy young subjects. After the post‐ET assessment, RBCV was re‐established at the pre‐ET level by phlebotomy, so as to offset haematological adaptations to ET while skeletal muscle adaptations remained intact, and and were determined again. We hypothesized that the improvement in following 6 weeks of ET would be primarily explained by haematological adaptations leading to increased oxygen delivery in untrained healthy young subjects.
Methods
Ethical approval
The study was approved by the Ethical Committee of the Eidgenössische Technische Hochschule Zürich (EK 2011‐N‐51) and conducted in accordance with the standards set by the Declaration of Helsinki. Prior to the start of the experiments, informed oral and written consents were obtained from all participants.
Subjects
Sixteen previously untrained healthy male volunteers (age = 25.3 ± 4.1 years, body weight = 76.4 ± 8.7 kg, height = 179.9 ± 8.3 cm, = 3.5 ± 0.5 l min−1) were recruited as study subjects. All subjects were non‐smokers and none were taking medication during the study.
Study design
The measures described below were assessed under non‐fasting conditions at baseline (pre‐ET) and after 6 weeks (post‐ET) of ET. In addition, 2–3 days after post‐ET, measures related to the incremental exercise test and haematology were determined immediately (10 min) after phlebotomy.
Phlebotomy
The ET‐induced increase in RBCV was reset to pre‐training values by means of phlebotomy. The amount of whole blood to be withdrawn was estimated (on the basis of the Hct) to result in a RBCV loss that counterbalanced (±50 ml) the gain in RBCV with ET for each individual subject. For this purpose, a 20 G venflon (BD, USA) was placed in an antecubital vein and the blood was withdrawn and discarded. The subjects then rested for 10 min in the supine position and subsequently initiated the incremental exercise test.
Exercise training
All subjects underwent 6 weeks of supervised ET consisting of 60 min of cycle ergometer exercise. Subjects performed three or four training sessions/week over this period. Three different intensity profiles were alternated to facilitate participant motivation and compliance. Profile 1 consisted of a steady‐state exercise, i.e. 60 min at 65% of peak power output () attained with the incremental exercise test. Profile 2 started with 3 min at 50% of followed by 3X60, 3X65, 3X70, 3X75, 3X70, 3X65, 3X60, 3X50, 3X65, 3X50, 3X60, 3X65, 3X70, 3X75, 3X70, 3X65, 3X60, 3X50 and 3X65. Profile 3 started with 6 min at 65% of followed by 4X75, 6X65, 4X75, 6X65, 4X75, 6X65, 4X75, 6X65, 4X75, 6X65 and 4X75. Workloads were calculated from individual determined during the incremental exercise test at baseline. Exercise intensity was on average 65% of .
Measurements
Incremental exercise test
and were determined on an electronically braked bicycle ergometer (Monark, Sweden) with continuous measurements of using an online gas collection system (Innocor M400, Innovision, Denmark). The test started with a warm‐up period of 5 min at 50–150 W workloads. Thereafter, the workload was increased by 30 W every 60–90 s until exhaustion. The gas analysers and the flowmeter of the applied spirometer were calibrated prior to each test. Breath‐by‐breath values were averaged over 30 s. The highest average value was taken as the provided that standard criteria were fulfilled (American Thoracic Society, 2003). was calculated as + 30 × (t/90); is the last fully completed workload and t is the number of seconds in the final workload. To evaluate cycling economy, was determined during submaximal cycling. In this regard, breath‐by‐breath values during warm‐up (100 W) were averaged.
Cardiac output was measured by an inert gas re‐breathing technique (Innocor M400). The method has been described and validated (Siebenmann et al. 2014). Briefly, the measurement is based on the assumption that pulmonary uptake of a blood soluble testing gas is proportional to pulmonary blood flow (Krogh & Lindhard, 1912). The Innocor uses a test gas mixture containing 5% nitrous oxide (N2O, soluble in blood and physiologically inert), 1% of the insoluble sulphur hexafluoride and 94% oxygen. This is filled, together with ambient air, into the re‐breathing bag before the onset of a measurement. The volume of the re‐breathing bag is predetermined according to the tidal volume to allow for unrestricted ventilation. When a measurement is initiated, the subject is switched from breathing room air to the closed circuit and re‐breaths the testing gas. In the present study, re‐breathings were separated by several minutes with the intent to limit confounding effects related to re‐circulating inert gas (Siebenmann et al. 2014). Pulmonary N2O uptake was determined by a regression line over the N2O concentration curve in three consecutive expirations as soon as complete mixture was obtained between residual pulmonary air and the gas in the re‐breathing bag. Subjects were familiarized with the re‐breathing technique through two familiarization tests and further conducted two or three practice procedures before each test. was assessed during incremental exercise with the re‐breathing procedure initiated five heart rate beats below the previously assessed maximal heart rate. The peak arteriovenous oxygen difference (a–vO2diff) was calculated from the Fick equation (a–vO2diff = /).
Blood
Haemoglobin mass (Hbmass) was measured as previously described (Siebenmann et al. 2012), using a modified version of the carbon monoxide (CO) re‐breathing technique (Burge & Skinner, 1995). All subjects rested for 20 min in a semi‐recumbent position before each measurement. Thereafter, 2 ml of blood was sampled from an antecubital vein via a 20 G venflon (BD, USA) and analysed immediately in quadruplicate for (i) percentage carboxyhaemoglobin (%HbCO) and Hb concentration ([Hb]) using a haemoximeter (ABL800, Radiometer, Denmark), and (ii) haematocrit (Htc) with the micromethod (4 min at 13,500 rpm). Subsequently, the subject breathed 100% oxygen for 4 min to flush the nitrogen from the airways. After closing the oxygen input, a bolus 1.5 ml kg−1 of 99.997% chemically pure CO (CO N47, Air Liquide, France) was administrated into the breathing circuit. The subjects re‐breathed this gas mixture for 10 min. Then, an additional 2 ml blood sample was obtained and analysed in quadruplicate. The change in%HbCO was used to calculate Hbmass, taking into account the amount of CO that remained in the re‐breathing circuit at the end of the procedure (2.2%) (Burge & Skinner, 1995). Total RBCV, BV and plasma volume (PV) were derived from measures of Hbmass and haematocrit (Burge & Skinner, 1995).
Skeletal muscle biopsy
Using the Bergström technique (Bergstrom, 1962) with a needle modified for suction, skeletal muscle biopsies from m. vastus lateralis were obtained under local anaesthetics while the subject was at rest with a minimum of 24 h following the last exercise training bout. The biopsy specimen was dissected free of fat and connective tissue, divided into sections and immediately prepared for analysis as stated below.
Fibre typing
Muscle samples were embedded in Tissue Tek (Sakura Finetek, USA) and serial transverse sections of10–12 μm (Leica CM 1850, Leica Biosystems, Germany) were used for histochemical analysis. A myofibrillar ATPase reaction was carried out at pH 9.4. By applying pre‐incubations at pH 4.3, 4.6, and 10.3, the fibres were classified as slow twitch type I (ST), fast twitch type IIa (FTa) and type IIx (FTx) (Brooke & Kaiser, 1970; Gollnick et al. 1972). A slide scanner (Zeiss Mirax Midi, Germany) connected to a 3‐CCD colour camera (Hitachi HV‐F22(1360 × 1024 pixels), Japan) was used for digitizing the cryo‐sections at a ×20 magnification and further analysed by using the free version of Panoramic viewer (3DHISTECH Ltd, Hungary). The relative occurrence of fibre types for each subject was determined from a mean of 207 ± 117 (range 79–729) fibres. Fibre typing was not conducted in four subjects due to poor tissue preparation.
Muscle capillarization
Serial transverse sections (8 μm) prepared as described above were used for immunohistochemical analysis for capillary density. Primary antibodies against caveolin‐1 (Cat 610060) and collagen IV (Dako M0785) in conjunction with biotinylated secondary antibodies (Dako E032 and E033) and the VECTASTAIN ABC‐AP KIT (Vector laboratories, USA) were used for capillary identification. Sections were visualized as described above. Capillary density was determined by counting the number of capillaries surrounding a minimum of 50 coherent fibres and expressed as the capillary‐to‐fibre ratio. Counting was performed manually using Photoshop CS6 and Panoramic Viewer (3DHISTECH Ltd). The number of capillaries in the outer circumference of each section was divided by 2 and then added to the number of capillaries in the inner section. The total number of capillaries was divided by the number of counted fibres. Capillary‐to‐fibre ratio was missing for six subjects due to poor tissue preparations.
Mitochondrial volume density
Four 1 mm3 pieces of each muscle biopsy were fixed in 2.5% glutaraldehyde at RT and processed according to standard electron‐microscopy protocols. TEM images were obtained in a FEI Tecnai G2 Spirit electron microscope (FEI, USA) with an Orius SC1000 CCD camera (Gatan, USA) and interfaced with the TEM User software from FEI. Two hundred and sixteen images per biopsy were acquired in a random systematic order from 24 meshes distributed on 8 grids from 4 blocks. The Cavalieri feature in the Stereo‐Investigator software (MBF Bioscience, USA) was used to estimate mitochondrial volume density (MitoVD) by point counting (West, 2012) The grid spacing was 1 μm along both x‐ and y‐axis. Mitochondria boundaries were recognized at the ×8200 magnification. Each point was assigned as either intermyofibrillar (IMF) mitochondria, subsarcolemmal (SS) mitochondria, muscle or ‘nothing’. SS mitochondria were defined as the mitochondria that were not separated by myofibrils from the sarcolemma. MitoVD determinations were not performed in three subjects due to lack of tissue.
Mitochondrial respiration
For analysis of oxidative phosphorylation a piece of the biopsy (∼20 mg) was placed in an ice‐cold biopsy preservation solution (BIOPS) and processed as previously described (Jacobs et al. 2013 b). Measurements of oxygen consumption for the evaluation of oxidative phosphorylation capacity (OXPHOS) and electron transfer capacity (ETS) were performed in duplicate at 37°C and in a hyperoxygenated environment using the high‐resolution Oxygraph‐2k (Oroboros, Austria) previously described in detail (Jacobs et al. 2013 b). A more complete listing of and thorough explanations for the standard nomenclature regarding various respiratory states, titration protocols, coupling control and flux control ratios can be found elsewhere (Pesta & Gnaiger, 2012; Jacobs et al. 2013 b). Mitochondrial respiration measures were missing for two subjects.
Hexokinase and lactate dehydrogenase enzyme content
Snap frozen muscle sections were freeze dried for 16 h at −55 °C (ScanVac CoolSafe55‐4, Denmark), thereafter homogenized (Precellys 24 Tissue Homogenizer, Bertin Technologies, France) and prepared as previously described (Robach et al. 2012). Total protein concentrations were determined by BCA assay (Pierce, USA). Standard western blotting procedures (Jacobs et al. 2013 a) were applied for quantification of hexokinase II (2867S, Cell Signalling Technology, USA) lactate dehydrogenase (ab135396, Abcam, UK) and actin (A2066, Sigma Aldrich, USA) detection. Protein bands were detected with LuminataTM Classico (Millipore, USA) using the Las‐4000 image analyser system (Fujifilm Life Science, USA). Quantification of band intensity was done using Image J software (NIH, USA) and determined as the total band intensity minus the background intensity. The primary antibodies were optimized by use of a pool of human muscle lysates. Pre‐ and post‐ samples were loaded on the same gel. Signal intensity from each muscle sample was normalized to the mean signal intensity of all samples on the same gel.
Statistical analysis
The paired Student's t test and Wilcoxon signed‐rank test were respectively used to assess normally and non‐normally distributed differences between pre‐ET, post‐ET and phlebotomy pairs. Bivariate associations were determined by Pearson's correlation coefficients. A stepwise multiple regression analysis was used to identify variables independently associated with . Variables significantly associated with in bivariate analysis were entered into the regression model as independent variables. Additionally, multiple regression analyses forcing the inclusion of potential causative variables (, haemotological variables, capillary‐to‐fibre ratio, total MitoVD, ST cross‐sectional area) based on established underlying physiology (di Prampero & Ferretti, 1990; Bassett & Howley, 2000; Levine, 2008) were performed. In the event of high correlation between independent variables, each of these were separately entered into the regression model in order to avoid high multicollinearity (variance inflation factor (VIF) > 10). A two‐tailed P value less than 0.05 was considered significant. Variables were expressed as means ± SD, unless otherwise stated. All statistical analyses were performed using MedCalc software (bvba, Mariakerke, Belgium).
In addition, an intuitive approach using mean effects and confidence intervals was used to assess mechanistic magnitude‐based inferences (Hopkins, 2007; Hopkins et al. 2009). Such an approach determines the effect as unclear if the confidence interval, which represents uncertainty about the true value, overlaps values that are substantial in a positive and negative sense as regards the smallest physiologically worthwhile effect; otherwise, the effect is characterized with a qualitative statement about the chance that it is positive or negative. In order to limit the potential experimenter's bias, any mean effect distinct from the zero value was considered as a physiologically worthwhile effect. The qualitative probabilistic terms are assigned following the following scale (Hopkins, 2007; Hopkins et al. 2009): most unlikely, < 0.5%; very unlikely, 0.5‐5%; unlikely, 5–25%; possibly, 25–75%; likely, 75–95%; very likely, 95–99.5%; and most likely, > 99.5%, all of them referred to a given positive or negative effect.
Results
All subjects completed 18–20 endurance exercise sessions over the 6 week intervention period. Body weight was unchanged by ET.
Incremental exercise test (Table 1)
Table 1.
attained with an incremental exercise test until exhaustion and related variables before (pre) and after (post) 6 weeks of endurance training and the subsequent phlebotomy (phle)
| Pre | Post | Phle | % Pre–post | % Post–phle | |
|---|---|---|---|---|---|
| (W) | 290.0 ± 45.8 | 332.0 ± 38.6* | 316.1 ± 33.4†, ‡ | 15.80 ± 13.44 | −4.98 ± 4.20 |
| (l min–1) | 3.52 ± 0.51 | 3.84 ± 0.60* | 3.60 ± 0.58† | 9.22 ± 8.15 | −6.88 ± 7.40 |
| (l min–1) | 18.67 ± 2.97 | 19.93 ± 2.89* | 18.19 ± 3.07† | 7.19 ± 6.24 | −10.11 ± 6.71 |
| Peak a–vO2diff (ml O2 (100 ml)–1) | 18.99 ± 1.73 | 19.30 ± 1.46 | 19.90 ± 1.55‡ | 2.17 ± 9.26 | 2.82 ± 5.92 |
| Haematological adaptations | |||||
| RBCV (ml) | 2437 ± 395 | 2624 ± 362* | 2451 ± 399† | 8.12 ± 4.38 | −7.49 ± 4.05 |
| PV (ml) | 3161 ± 485 | 3284 ± 454* | 3098 ± 478†, ‡ | 4.26 ± 6.42 | −6.29 ± 4.52 |
| BV (ml) | 5598 ± 861 | 5907 ± 784* | 5549 ± 858† | 5.93 ± 5.06 | −6.83 ± 4.03 |
| Hbmass (g) | 833 ± 125 | 878 ± 102* | – | 5.96 ± 4.86 | – |
| [Hb] (g dl–1) | 14.90 ± 0.73 | 14.97 ± 0.67 | 14.90 ± 0.65 | 0.51 ± 3.18 | 1.13 ± 1.71 |
| Htc (%) | 43.52 ± 1.68 | 44.44 ± 1.96* | 44.83 ± 2.09‡ | 2.12 ± 2.57 | 0.85 ± 2.12 |
| Skeletal muscle adaptations | |||||
| Capillary‐to‐fibre ratio | 1.59 ± 0.28 | 1.88 ± 0.45* | – | 17.55 ± 16.22 | – |
| Total MitoVD (%) | 4.68 ± 1.02 | 6.50 ± 1.14* | – | 42.69 ± 29.53 | – |
| SS MitoVD (%) | 0.68 ± 0.31 | 1.38 ± 0.54* | – | 184 ± 328 | – |
| IMF MitoVD (%) | 4.00 ± 1.08 | 5.12 ± 0.92* | – | 35.27 ± 40.27 | – |
| OXPHOS (pmol s–1 (mg ww)–1) | 105.4 ± 25.7 | 110.8 ± 15.6 | – | 10.54 ± 28.57 | – |
| OXPHOS/total MitoVD | 22.61 ± 5.69 | 17.40 ± 2.74* | – | −17.78 ± 28.49 | – |
| ETS pmol s–1 (mg ww)–1) | 130.6 ± 32.4 | 138.1 ± 29.6 | – | 9.83 ± 27.07 | – |
| ETS/MitoVD | 28.00 ± 5.60 | 21.70 ± 3.89* | – | −20.03 ± 19.92 | – |
| OXPHOS/ETS | 0.82 ± 0.16 | 0.82 ± 0.12 | – | 1.82 ± 17.50 | – |
| ST cross‐sectional area (%) | 56.93 ± 10.91 | 49.02 ± 12.30* | – | −13.43 ± 19.45 | – |
| FTa cross‐sectional area (%) | 36.29 ± 11.07 | 39.85 ± 11.67 | – | 13.69 ± 27.05 | – |
| FTx cross‐sectional area (%) | 6.77 ± 5.20 | 11.13 ± 5.24* | – | 140 ± 240 | – |
Values are means ± SD. Skeletal muscle adaptations were measured in a subgroup of the total sample (n<16). *, † and ‡ denote statistical significant differences (P < 0.05) from pre to post, post to phlebotomy and pre to phlebotomy, respectively. BV, blood volume; ETS, electron transport system capacity in skeletal muscle; FTa, fast twitch type IIa muscle fibre; FTx, fast twitch type IIx muscle fibre; [Hb], haemoglobin concentration; Hbmass, haemoglobin mass; Hct, haematocrit; IMF, intermyofibrillar; MitoVD, mitochondrial volume density; OXPHOS, maximal oxidative phosphorylation capacity in skeletal muscle; PV, plasma volume; , peak cardiac output; RBCV, red blood cell volume; SS, subsarcolemmal; ST, slow twitch type I muscle fibre; , peak oxygen uptake; , peak power output.
was increased (P < 0.05) by 42 ± 31 W (16 ± 13%) from pre‐ to post‐ET and was reduced (P < 0.05) by 16 ± 13 W (5 ± 4%) following the phlebotomy procedure when compared with post‐ET. Compared with pre‐ET, remained 26 ± 23 W (10 ± 10%) higher (P < 0.05) after the phlebotomy. was increased (P < 0.05) by 0.32 ± 0.30 l min−1 (9 ± 8%) from pre‐ to post‐ET and reduced (P < 0.05) by 0.24 ± 0.26 l min−1 (7 ± 7%) with phlebotomy compared with post‐ET. There was no difference in between pre‐ET and after the phlebotomy procedure. was enhanced (P < 0.05) by 1.26 ± 1.09 l min−1 (7 ± 6%) from pre‐ to post‐ET and reduced (P < 0.05) by 1.74 ± 1.02 l min−1 (10 ± 7%) following phlebotomy compared with post‐ET. There was no difference in between pre‐ET and after the phlebotomy procedure. The calculated peak a–vO2diff was not altered by ET. After phlebotomy, peak a–vO2diff was increased compared to pre‐ET (P < 0.05) but not with respect to post‐ET. Cycling economy, determined by the average during the 100 W workload remained unchanged throughout the study. Magnitude‐based inferences determined the above significant differences as ‘most likely’, except for that regarding peak a–vO2diff which was deemed ‘very likely’.
Haematological adaptations (Table 1)
ET resulted in 187 ± 94 (8 ± 4%), 123 ± 188 (4 ± 6%) and 310 ± 258 ml (6 ± 5%) increases (P < 0.05) in RBCV, PV and BV, respectively. The phlebotomy procedure normalized RBCV to the pre‐ET level and at the same time induced 186 ± 133 ml and 359 ± 198 ml respective decreases (P < 0.05) in PV and BV (i.e. very mild hypovolaemia) compared with post‐ET. Therefore, after phlebotomy PV and BV were 63 ± 103 (2 ± 4%) and 49 ± 111 ml (1 ± 2%) lower (P < 0.05 only for PV) than pre‐ET. Hbmass was enhanced (P < 0.05) by 45 ± 40 g (6 ± 5%) after ET. Htc was increased (P < 0.05) by 0.92 ± 1.12% (2 ± 3%) from pre‐ to post‐ET and remained higher (P < 0.05) after phlebotomy when compared with pre‐ET. [Hb] did not change as a function of ET or phlebotomy. The above significant differences ranged from ‘very likely’ to ‘most likely’ according to magnitude‐based inferences.
Skeletal muscle adaptations (Table 1)
Capillary‐to‐fibre ratio was increased (P < 0.05) by 0.28 ± 0.28 (18 ± 16%) from pre‐ to post‐ET. Total, SS and IMF MitoVD were increased (P < 0.05) by 1.82 ± 0.96 (43 ± 30%), 0.70 ± 0.63 (184 ± 328%) and 1.12 ± 0.82% (35 ± 40%) from pre‐ to post‐ET, respectively. OXPHOS and ETS did not change in absolute terms but were decreased (P < 0.05) by 18 ± 28 and 20 ± 20% when normalized to total MitoVD following ET. Regarding muscle fibre types, ST cross‐sectional % area was decreased (P < 0.05) by 7.92 ± 10.12% (13 ± 19%) while FTx cross‐sectional % area was increased (P < 0.05) by 4.36 ± 4.62% (140 ± 240%) after ET. FTa muscle fibre cross‐sectional area was not modified by ET. Muscle hexokinase and lactate dehydrogenase protein content were not modified by ET. The above significant differences ranged from ‘very likely’ to ‘most likely’ according to magnitude‐based inferences.
Bivariate associations
Table 2 displays the bivariate associations with using pre‐ET, post‐ET and phlebotomy values. and haematological variables such as PV, RBCV, BV and Hbmass were closely correlated with (r > 0.70, P < 0.0001). Htc was moderately correlated with (r = 0.40, P = 0.005). ST and FTa muscle fibre cross‐sectional % areas presented a positive and a negative moderate correlation with (r = 0.43 P = 0.04; r = −0.42, P = 0.04, respectively). No other peripheral variable correlated with . In addition, peripheral variables potentially affecting oxygen extraction such as capillary‐to‐fibre ratio, total MitoVD and OXPHOS were not correlated with peak a–vO2diff (r = 0.02, P = 0.93; r = 0.11, P = 0.60; r = −0.24, P = 0.21, respectively).
Table 2.
Correlations between and related variables in bivariate analyses using pre training, post training and phlebotomy values
| r | P | ||
|---|---|---|---|
|
|
0.84 | <0.0001 | |
| Peak a–vO2diff | 0.24 | 0.10 | |
| RBCV | 0.86 | <0.0001 | |
| PV | 0.71 | <0.0001 | |
| BV | 0.80 | <0.0001 | |
| Hbmass | 0.78 | <0.0001 | |
| [Hb] | −0.19 | 0.25 | |
| Htc | 0.40 | 0.005 | |
| Capillary‐to‐fibre ratio | 0.19 | 0.43 | |
| Total MitoVD | 0.22 | 0.27 | |
| SS MitoVD | 0.12 | 0.54 | |
| IMF MitoVD | 0.22 | 0.28 | |
| OXPHOS | −0.15 | 0.46 | |
| OXPHOS/total MitoVD | −0.35 | 0.07 | |
| ETS | 0.05 | 0.80 | |
| ETS/MitoVD | −0.18 | 0.36 | |
| OXPHOS/ETS | −0.32 | 0.10 | |
| ST cross‐sectional area | 0.43 | 0.04 | |
| FTa cross‐sectional area | −0.42 | 0.04 | |
| FTx cross‐sectional area | −0.08 | 0.73 |
BV, blood volume; ETS, electron transport system capacity in skeletal muscle; FTa, fast twitch type IIa muscle fibre; FTx, fast twitch type IIx muscle fibre; [Hb], haemoglobin concentration; Hbmass, haemoglobin mass; Hct, haematocrit; IMF, intermyofibrillar; MitoVD, mitochondrial volume density; OXPHOS, maximal oxidative phosphorylation capacity in skeletal muscle; PV, plasma volume; , peak cardiac output; RBCV, red blood cell volume; SS, subsarcolemmal; ST, slow twitch type I muscle fibre; , peak oxygen uptake.
Multiple regression analysis
All variables significantly associated with in bivariate analysis were entered into a stepwise multivariable regression model to determine the independent predictors of (Table 3). RBCV, PV BV and Hbmass were closely correlated with each other in bivariate analysis (r > 0.85, P < 0.0001) and their concurrent inclusion in multiple regression analysis resulted in a VIF > 10, indicating high multicollinearity. Accordingly, each of the aforementioned haematological variables were separately entered into the regression model. , Hct, ST cross‐sectional % area, FTa cross‐sectional % area and PV, RBCV, BV or Hbmass were entered into the regression model as potential independent predictors of . remained the only independent predictor of when PV or BV were included in the regression model (adjusted R 2 = 0.65, P < 0.0001). Moreover, RBCV was the only independent predictor of when RBCV was included in the regression model (adjusted R 2 = 0.74, P < 0.0001). Finally, along with Hbmass were the independent predictors of when Hbmass was included in the regression model (adjusted R 2 = 0.73, P < 0.0001).
Table 3.
Multiple linear regression with as the dependent variable
| Model | b | r partial | P | Adjusted R 2 | P | |
|---|---|---|---|---|---|---|
| All variables entered | ||||||
|
|
0.0585 | 0.33 | 0.15 | 0.74 | <0.0001 | |
| RBCV | 0.0008 | 0.56 | 0.01 | — | — | |
| Htc | 0.0336 | 0.23 | 0.33 | — | — | |
| ST cross‐sectional area | −0.0013 | −0.03 | 0.91 | — | — | |
| FTa cross‐sectional area | 0.0006 | 0.01 | 0.96 | — | — | |
| Stepwise | ||||||
| RBCV | 0.0012 | 0.87 | <0.0001 | 0.74 | <0.0001 | |
| Stepwise (including PV instead of RBCV) | ||||||
|
|
0.1574 | 0.82 | <0.0001 | 0.65 | <0.0001 | |
| Stepwise (including BV instead of RBCV) | ||||||
|
|
0.1574 | 0.82 | <0.0001 | 0.65 | <0.0001 | |
| Stepwise (including Hbmass instead of RBCV) | ||||||
|
|
0.0811 | 0.45 | 0.03 | 0.73 | <0.0001 | |
| Hbmass | 0.0023 | 0.51 | 0.01 | — | — |
RBCV, PV, BV and Hbmass were closely correlated with each other in bivariate analysis (r > 0.85, P < 0.0001); only the model including RBCV is presented for the initial (all variables entered) multiple regression analysis. BV, blood volume; FTa, fast twitch type IIa muscle fibre; Hbmass, haemoglobin mass; Hct, haematocrit; PV, plasma volume; , peak cardiac output; RBCV, red blood cell volume: ST, slow twitch type I muscle fibre.
In addition, potentially causative variables according to accepted underlying physiology were forced into multiple regression models with as the dependent variable. Following this approach, the highly inter‐correlated haematological variables (RBCV, PV, BV and Hbmass) were the independent predictors of .
Discussion
This study assessed the respective role of central and peripheral adaptations on following 6 weeks of ET and subsequent phlebotomy in previously untrained healthy young subjects. As expected, ET led to increases in RBCV, PV, Hbmass, Htc, skeletal muscle capillarization and MitoVD, as well as enhanced and . The key observations were: (i) and , but not , were normalized to pre‐ET levels when the ET‐induced increase in RBCV was abolished by means of phlebotomy and (ii) oxygen delivery‐related variables such as , RBCV, BV and Hbmass were independent predictors of .
Accumulating evidence corroborates as the main factor contributing to the increase in after 5–13 weeks of ET in untrained/moderately trained healthy young humans (Klausen et al. 1982; Spina et al. 1992; Helgerud et al. 2007; Murias et al. 2010; Weng et al. 2013; Bonne et al. 2014; Wang et al. 2014). In the current study, the concomitant increase of , RBCV and with 6 weeks of ET, and subsequent reversal with phlebotomy supports a major role of oxygen delivery on . Moreover, the ET‐induced increase in (∼320 ml) was accompanied by a lower than predicted Hbmass gain (∼45 g), considering the proposed 4 ml min−1 increase in per 1 g rise in Hbmass (Schmidt & Prommer, 2010). This highlights the impact of the increase of on oxygen delivery after ET. Given that both central and peripheral adaptations to ET may influence (Klausen et al. 1982; Calbet et al. 2006; Baggish et al. 2008; Weng et al. 2013; Bonne et al. 2014), it could be questioned whether the increase in oxygen delivery was led by central adaptations alone or along with peripheral adaptations. The fact that the increase in was abolished after RBCV (and BV) normalization strongly suggests that haematological adaptations to 6 weeks of ET primarily underlay the increase in oxygen delivery and , which was confirmed in multiple regression analysis. Nonetheless, whilst speculative, it is possible that after phlebotomy an increase in sympathetic activation due to moderate hypovolaemia (Fortrat et al. 1998; Zollei et al. 2004) may have masked a possible decrease in vascular resistance at peak exercise which would contribute as a peripheral adaptation to the increase in and following ET (Klausen et al. 1982; Weng et al. 2013).
The erythropoietic response to ET merits particular attention. Although debated two decades ago (Shoemaker et al. 1996), it is currently accepted that ET facilitates RCBV expansion (Schmidt & Prommer, 2010). According to the present and preceding reports, RBCV may be enhanced by 8–13% after 6–12 weeks (Warburton et al. 2004; Helgerud et al. 2007; Bonne et al. 2014) and longer (Sawka et al. 2000; Schmidt & Prommer, 2008) normoxic ET interventions in previously un‐ to moderately trained subjects. Yet, the mechanisms stimulating erythropoiesis with exercise training remain unclear. In this regard, the expression of hypoxia‐inducible factor‐2 was transiently increased in untrained skeletal muscle in response to acute exercise and returned to pre‐exercise values after 24 h of recovery (Lundby et al. 2006), while plasma erythropoietin concentration is increased (Schwandt et al. 1991) or unchanged (Schmidt et al. 1991; Weight et al. 1992; Bodary et al. 1999) up to 24–48 h following acute exercise in un‐ and trained subjects. In the longer term, the increase in PV relative to RBCV within the first few days of ET might propel the erythropoietic system to approach a new equilibrium in that haematocrit is re‐established at the pre training level (Sawka et al. 2000; Schmidt & Prommer, 2008; Jelkmann & Lundby, 2011). Herein, the observation of increased haematocrit after 6 weeks of ET suggests that the early RBCV expansion might be, at least in part, non‐hypoxically regulated. Alternatively or additionally to hypoxia‐related signals, post‐exercise reduced central venous pressure (Halliwill et al. 2000) might contribute to upregulate erythropoietin production (Gunga et al. 1996). Furthermore, exercise may alter the circulating levels of catecholamines, peptides (growth hormone, insulin‐like growth factor) and steroid hormones (testosterone, cortisol) known to modulate red blood cell production and/or release from the bone marrow (Hu & Lin, 2012).
Skeletal muscle adaptations to the 6 week ET were observed (Table 1). Capillary‐to‐fibre ratio and MitoVD were increased by 18 and 43%, respectively, but were not associated with . Earlier reports have shown comparable increases in muscle capillarization and MitoVD after 6 weeks of ET (Hoppeler et al. 1985; Turner et al. 1997), which, however, were associated with in a group of 10 untrained young men and women (Hoppeler et al. 1985). Differences in methodology, sample size and gender distribution might account for the discrepancy in the associations. Besides, it should be noted that, in the present study, skeletal muscle OXPHOS was similar prior to and after ET, which is consistent with findings from 6 week ET interventions in young men (Ponsot et al. 2006; Robach et al. 2014) but contrasts with studies demonstrating increased skeletal muscle OXPHOS in shorter‐ (2 weeks) and longer‐term (≥10 weeks) endurance‐trained male subjects (Pesta et al. 2011; Jacobs et al. 2013 b; Jacobs & Lundby, 2013). Likewise, oxygen extraction, as determined by a–vO2diff, was increased following ET interventions lasting 2–3 and 12–13 but not 5–8 weeks in young (primarily male) subjects (Klausen et al. 1982; Spina et al. 1992; Beere et al. 1999; Helgerud et al. 2007; Murias et al. 2010; Jacobs et al. 2013 b; Weng et al. 2013; Bonne et al. 2014; Wang et al. 2014), including the current findings. Thus, the unchanged oxygen extraction could be paralleled, not necessarily caused (Boushel et al. 2011), by the transitory normalization of OXPHOS in skeletal muscle in the presence of increased oxygen delivery within the 5 to ∼8 week period of ET. On the other hand, the enhanced muscle capillarization might prevent a decrease in oxygen extraction after ET if oxygen diffusion is hampered by an increase in capillary blood flow velocity associated with that of (Spurway et al. 2012). In addition, we found a positive association between ST muscle fibre cross‐sectional % area and , even though, paradoxically, the ST cross‐sectional % area was decreased whereas FTx cross‐sectional % area was increased after ET. This could suggest that the FTx cross‐sectional absolute area was increased with ET provided that leg muscle mass is, if anything, augmented following ET in healthy young subjects (Spence et al. 2013), corresponding with the notion that the increase in fast‐twitch fibre size occurs during the first 8 weeks of ET while slow‐twitch fibres are increased after 8–24 weeks of ET (Abernethy et al. 1990). Taken together, the impact of skeletal muscle adaptations (measured in this study) on may be dynamically related to the duration of ET.
It should be noted that peak a–vO2diff was higher following phlebotomy than prior to ET. As previously mentioned, it could be speculated that the phlebotomy‐induced decrease in and plausible lower leg blood flow, along with the increase in muscle capillarization, may have augmented red blood cell transit time and thus peak a–vO2diff. However, the lack of association between peak a–vO2diff and capillary‐to‐fibre ratio (r = 0.02, P = 0.93), even when adjusted for (r = 0.09, P = 0.73), weakens this hypothesis. Likewise, this speculation conflicts with the existence of a considerable reserve in muscle O2 diffusing capacity in normoxia in healthy individuals (Calbet et al. 2003, 2009). Alternatively, the higher , but similar , following phlebotomy compared with pre‐ET – if these reflect an increase in exercise intensity relative to leg O2 delivery – could lead to enhanced peak a–vO2diff (Richardson et al. 1993). In this respect, peak a–vO2diff correlated with the ‐to‐ ratio (r = 0.41, P = 0.004) in the present study. Additionally, oxygen extraction might be augmented via improved blood flow distribution (Heinonen et al. 2013) related to the increase in sympathetic drive prompted by phlebotomy (Fortrat et al. 1998; Zollei et al. 2004).
The effects of phlebotomy on and peak power output during incremental exercise () were dissociated in that was normalized but remained increased compared to pre‐ET levels (Fig. 1). Hence, the increase in after ET was not exclusively driven by the improved aerobic capacity, as previously suggested (Jacobs et al. 2011). Additional adaptations potentially underlying the increase in after training include, but are not limited to, changes in biochemical and neuromuscular factors (Noakes, 1988; Paavolainen et al. 1999). With regard to the former, the increase in after ET could be secondary to, among other things, an enhanced muscle buffering capacity (Weston et al. 1997) associated with the increase in FTx cross‐sectional area (Nakagawa & Hattori, 2002). In turn, we did not detect an increase in muscle hexokinase and lactate dehydrogenase protein content after ET. Ultimately, submaximal cycling economy was not modified by ET, although evidence for this is lacking at peak exercise.
Figure 1. Effects of endurance training and subsequent phlebotomy on RBCV, and .
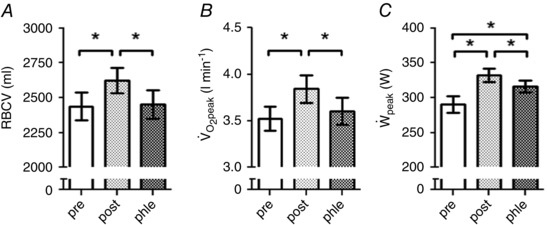
RBCV, and before (pre) and after (post) 6 weeks of endurance training and the subsequent phlebotomy (phle). Bars represent mean ± SEM. *P < 0.05. RBCV, red blood cell volume; , peak oxygen uptake; , peak power output.
Limitations
Findings were obtained from a relatively small number of untrained healthy young males, thus our conclusions should be taken with caution and limited to this population. Moreover, adaptations to ET related to vascular resistance were not assessed, and furthermore, the measured peripheral adaptations were not available in all study subjects, which might have reduced the statistical power to detect their influence on . Nevertheless, equivalent results were found in a subgroup (n = 10) of the total sample with complete phenotyping. Also, the observed adaptations could be partly specific to the intensity, rather than the duration, of training that was applied (Daussin et al. 2007, 2008), though the contribution of the latter seems predominant in our study population (Murias et al. 2010). Finally, despite RBCV being restored to the resting pre‐ET level after phlebotomy, it is uncertain whether the normalization was preserved during exercise.
Conclusion
The effect of 6 weeks of ET on is primarily explained by an increase in and oxygen‐carrying capacity of blood in previously untrained healthy young subjects. Skeletal muscle adaptations related to muscle capillarization and mitochondrial volume density do not substantially contribute to the improvement following 6 weeks of ET. Further research is needed to elucidate whether the predominant effect of haematological adaptations on persists with longer term ET in healthy subjects.
Additional information
Competing interests
The authors declare no conflict of interest with the present study.
Author contributions
Conception and design of experiments: C.L.; collection, analysis and interpretation: D.M., A.C., R.A.J., D.F., J.L., S.K., T.B., N.K., A.L., C.L.; drafting the article or revising it critically for important intellectual content: D.M., A.C., R.A.J., D.F., J.L., S.K., T.B., N.K., A.L., C.L.
Funding
This study was conducted with a grant obtained from the Swiss National Science Foundation (SNF grant 320030_143745/1).
Acknowledgements
The authors acknowledge the assistance and support of the Centre for Microscopy and Image Analysis, University of Zurich for performing scanning electron microscopy experiments.
References
- Abernethy PJ, Thayer R & Taylor AW (1990). Acute and chronic responses of skeletal muscle to endurance and sprint exercise. A review. Sports Med 10, 365–389. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society (2003). ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167, 211–277. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM Jr & Wood MJ (2008). Training‐specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985) 104, 1121–1128. [DOI] [PubMed] [Google Scholar]
- Bassett DR Jr & Howley ET (2000). Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32, 70–84. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW & Higginbotham MB (1999). Aerobic exercise training can reverse age‐related peripheral circulatory changes in healthy older men. Circulation 100, 1085–1094. [DOI] [PubMed] [Google Scholar]
- Bergstrom J (1962). Muscle electrolytes in man. Scand J Clin Lab Invest, 1–110. [Google Scholar]
- Bodary PF, Pate RR, Wu QF & McMillan GS (1999). Effects of acute exercise on plasma erythropoietin levels in trained runners. Med Sci Sports Exerc 31, 543–546. [DOI] [PubMed] [Google Scholar]
- Bonne TC, Doucende G, Fluck D, Jacobs RA, Nordsborg NB, Robach P, Walther G & Lundby C (2014). Phlebotomy eliminates the maximal cardiac output response to six weeks of exercise training. Am J Physiol Regul Integr Comp Physiol 306, R752–R760. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Calbet JA, Gonzalez‐Alonso J, Wright‐Paradis C, Sondergaard H, Ara I, Helge JW & Saltin B (2011). Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion 11, 303–307. [DOI] [PubMed] [Google Scholar]
- Brooke MH & Kaiser KK (1970). Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem 18, 670–672. [DOI] [PubMed] [Google Scholar]
- Burge CM & Skinner SL (1995). Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol (1985) 79, 623–631. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD & Saltin B (2003). Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol 284, R304–R316. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B & Boushel R (2006). Effects of ATP‐induced leg vasodilation on VO2peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291, R447–R453. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R & Saltin B (2009). On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol 587, 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen JP (1977). Effect of physical training on cardiovascular adjustments to exercise in man. Physiol Rev 57, 779–815. [DOI] [PubMed] [Google Scholar]
- Daussin FN, Ponsot E, Dufour SP, Lonsdorfer‐Wolf E, Doutreleau S, Geny B, Piquard F & Richard R (2007). Improvement of VO2max by cardiac output and oxygen extraction adaptation during intermittent versus continuous endurance training. Eur J Appl Physiol 101, 377–383. [DOI] [PubMed] [Google Scholar]
- Daussin FN, Zoll J, Dufour SP, Ponsot E, Lonsdorfer‐Wolf E, Doutreleau S, Mettauer B, Piquard F, Geny B & Richard R (2008). Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol 295, R264–R272. [DOI] [PubMed] [Google Scholar]
- di Prampero PE & Ferretti G (1990). Factors limiting maximal oxygen consumption in humans. Respir Physiol 80, 113–127. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Astrand PO, Saltin B, Stenberg J & Wallstrom B (1968). Effect of training on circulatory response to exercise. J Appl Physiol 24, 518–528. [DOI] [PubMed] [Google Scholar]
- Fortrat JO, Nasr O, Duvareille M & Gharib C (1998). Human cardiovascular variability, baroreflex and hormonal adaptations to a blood donation. Clin Sci (Lond) 95, 269–275. [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saubert CWT, Piehl K & Saltin B (1972). Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol 33, 312–319. [DOI] [PubMed] [Google Scholar]
- Gunga HC, Kirsch K, Baartz F, Maillet A, Gharib C, Nalishiti W, Rich I & Rocker L (1996). Erythropoietin under real and simulated microgravity conditions in humans. J Appl Physiol (1985) 81, 761–773. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Minson CT & Joyner MJ (2000). Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol (1985) 89, 1830–1836. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Wendelin‐Saarenhovi M, Kaskinoro K, Knuuti J, Scheinin M & Kalliokoski KK (2013). Inhibition of alpha‐adrenergic tone disturbs the distribution of blood flow in the exercising human limb. Am J Physiol Heart Circ Physiol 305, H163–H172. [DOI] [PubMed] [Google Scholar]
- Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R & Hoff J (2007). Aerobic high‐intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc 39, 665–671. [DOI] [PubMed] [Google Scholar]
- Hopkins WG (2007). A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a p value. Sportscience, 16–20. [Google Scholar]
- Hopkins WG, Marshall SW, Batterham AM & Hanin J (2009). Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41, 3–13. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P & Weibel ER (1985). Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol (1985) 59, 320–327. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Luthi P, Claassen H, Weibel ER & Howald H (1973). The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well‐trained orienteers. Pflugers Arch 344, 217–232. [DOI] [PubMed] [Google Scholar]
- Hu M & Lin W (2012). Effects of exercise training on red blood cell production: implications for anemia. Acta Haematol 127, 156–164. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Diaz V, Soldini L, Haider T, Thomassen M, Nordsborg NB, Gassmann M & Lundby C (2013. a). Fast‐twitch glycolytic skeletal muscle is predisposed to age‐induced impairments in mitochondrial function. J Gerontol A Biol Sci Med Sci 68, 1010–1022. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Fluck D, Bonne TC, Burgi S, Christensen PM, Toigo M & Lundby C (2013. b). Improvements in exercise performance with high‐intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol (1985) 115, 785–793. [DOI] [PubMed] [Google Scholar]
- Jacobs RA & Lundby C (2013). Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol (1985) 114, 344–350. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Rasmussen P, Siebenmann C, Diaz V, Gassmann M, Pesta D, Gnaiger E, Nordsborg NB, Robach P & Lundby C (2011). Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J Appl Physiol (1985) 111, 1422–1430. [DOI] [PubMed] [Google Scholar]
- Jelkmann W & Lundby C (2011). Blood doping and its detection. Blood 118, 2395–2404. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J & Nuutila P (2001). Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance‐trained men. Am J Physiol Endocrinol Metab 280, E1015–E1021. [DOI] [PubMed] [Google Scholar]
- Klausen K, Secher NH, Clausen JP, Hartling O & Trap‐Jensen J (1982). Central and regional circulatory adaptations to one‐leg training. J Appl Physiol Respir Environ Exerc Physiol 52, 976–983. [DOI] [PubMed] [Google Scholar]
- Krogh A & Lindhard J (1912). Measurements of the blood flow through the lungs of man. Skand Arch Physiol, 100–125. [Google Scholar]
- Levine BD (2008). : what do we know, and what do we still need to know? J Physiol 586, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Gassmann M & Pilegaard H (2006). Regular endurance training reduces the exercise induced HIF‐1alpha and HIF‐2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol 96, 363–369. [DOI] [PubMed] [Google Scholar]
- Murias JM, Kowalchuk JM & Paterson DH (2010). Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol (1985) 108, 621–627. [DOI] [PubMed] [Google Scholar]
- Murias JM, Kowalchuk JM, Ritchie D, Hepple RT, Doherty TJ & Paterson DH (2011). Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J Gerontol A Biol Sci Med Sci 66, 957–964. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y & Hattori M (2002). Relationship between muscle buffering capacity and fiber type during anaerobic exercise in human. J Physiol Anthropol Appl Human Sci 21, 129–131. [DOI] [PubMed] [Google Scholar]
- Noakes TD (1988). Implications of exercise testing for prediction of athletic performance: a contemporary perspective. Med Sci Sports Exerc 20, 319–330. [DOI] [PubMed] [Google Scholar]
- Nottin S, Vinet A, Stecken F, N'Guyen LD, Ounissi F, Lecoq AM & Obert P (2002). Central and peripheral cardiovascular adaptations to exercise in endurance‐trained children. Acta Physiol Scand 175, 85–92. [DOI] [PubMed] [Google Scholar]
- Paavolainen L, Hakkinen K, Hamalainen I, Nummela A & Rusko H (1999). Explosive‐strength training improves 5‐km running time by improving running economy and muscle power. J Appl Physiol (1985) 86, 1527–1533. [DOI] [PubMed] [Google Scholar]
- Pesta D & Gnaiger E (2012). High‐resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810, 25–58. [DOI] [PubMed] [Google Scholar]
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M & Gnaiger E (2011). Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301, R1078–R1087. [DOI] [PubMed] [Google Scholar]
- Ponsot E, Dufour SP, Zoll J, Doutrelau S, N'Guessan B, Geny B, Hoppeler H, Lampert E, Mettauer B, Ventura‐Clapier R & Richard R (2006). Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol (1985) 100, 1249–1257. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Robach P, Bonne T, Fluck D, Burgi S, Toigo M, Jacobs RA & Lundby C (2014). Hypoxic training: effect on mitochondrial function and aerobic performance in hypoxia. Med Sci Sports Exerc 46, 1936–1945. [DOI] [PubMed] [Google Scholar]
- Robach P, Siebenmann C, Jacobs RA, Rasmussen P, Nordsborg N, Pesta D, Gnaiger E, Diaz V, Christ A, Fiedler J et al (2012). The role of haemoglobin mass on VO2max following normobaric ‘live high–train low’ in endurance‐trained athletes. Br J Sports Med 46, 822–827. [DOI] [PubMed] [Google Scholar]
- Saltin B (1985). Hemodynamic adaptations to exercise. Am J Cardiol 55, 42D–47D. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K & Chapman CB (1968). Response to exercise after bed rest and after training. Circulation 38, VII1–VII78. [PubMed] [Google Scholar]
- Sawka MN, Convertino VA, Eichner ER, Schnieder SM & Young AJ (2000). Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 32, 332–348. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Eckardt KU, Hilgendorf A, Strauch S & Bauer C (1991). Effects of maximal and submaximal exercise under normoxic and hypoxic conditions on serum erythropoietin level. Int J Sports Med 12, 457–461. [DOI] [PubMed] [Google Scholar]
- Schmidt W & Prommer N (2008). Effects of various training modalities on blood volume. Scand J Med Sci Sports 18 (Suppl. 1), 57–69. [DOI] [PubMed] [Google Scholar]
- Schmidt W & Prommer N (2010). Impact of alterations in total hemoglobin mass on VO2max. Exerc Sport Sci Rev 38, 68–75. [DOI] [PubMed] [Google Scholar]
- Schwandt HJ, Heyduck B, Gunga HC & Rocker L (1991). Influence of prolonged physical exercise on the erythropoietin concentration in blood. Eur J Appl Physiol Occup Physiol 63, 463–466. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Green HJ, Coates J, Ali M & Grant S (1996). Failure of prolonged exercise training to increase red cell mass in humans. Am J Physiol Heart Circ Physiol 270, H121–H126. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Rasmussen P, Sorensen H, Zaar M, Hvidtfeldt M, Pichon A, Secher NH & Lundby C (2014). Cardiac output during exercise: A comparison of four methods. Scand J Med Sci Sports 25, e20–27. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Robach P, Jacobs RA, Rasmussen P, Nordsborg N, Diaz V, Christ A, Olsen NV, Maggiorini M & Lundby C (2012). "Live high–train low" using normobaric hypoxia: a double‐blinded, placebo‐controlled study. J Appl Physiol (1985) 112, 106–117. [DOI] [PubMed] [Google Scholar]
- Spence AL, Carter HH, Naylor LH & Green DJ (2013). A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise. Conduit artery adaptation in humans. J Physiol 591, 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina RJ, Ogawa T, Martin WH, Coggan AR, Holloszy JO & Ehsani AA (1992). Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J Appl Physiol 72, 2458–2462. [DOI] [PubMed] [Google Scholar]
- Spurway NC, Ekblom B, Noakes TD & Wagner PD (2012). What limits ? A symposium held at the BASES Conference, 6 September 2010. J Sports Sci 30, 517–531. [DOI] [PubMed] [Google Scholar]
- Turner DL, Hoppeler H, Claassen H, Vock P, Kayser B, Schena F & Ferretti G (1997). Effects of endurance training on oxidative capacity and structural composition of human arm and leg muscles. Acta Physiol Scand 161, 459–464. [DOI] [PubMed] [Google Scholar]
- Wang E, Naess MS, Hoff J, Albert TL, Pham Q, Richardson RS & Helgerud J (2014). Exercise‐training‐induced changes in metabolic capacity with age: the role of central cardiovascular plasticity. Age (Dordr) 36, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Taylor DA, McGavock J & Humen DP (2004). Blood volume expansion and cardiorespiratory function: effects of training modality. Med Sci Sports Exerc 36, 991–1000. [DOI] [PubMed] [Google Scholar]
- Weight LM, Alexander D, Elliot T & Jacobs P (1992). Erythropoietic adaptations to endurance training. Eur J Appl Physiol Occup Physiol 64, 444–448. [DOI] [PubMed] [Google Scholar]
- Weng TP, Huang SC, Chuang YF & Wang JS (2013). Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One 8, e80248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ (2012). Estimating volume in biological structures. Cold Spring Harb Protoc 2012, 1129–1139. [DOI] [PubMed] [Google Scholar]
- Weston AR, Myburgh KH, Lindsay FH, Dennis SC, Noakes TD & Hawley JA (1997). Skeletal muscle buffering capacity and endurance performance after high‐intensity interval training by well‐trained cyclists. Eur J Appl Physiol Occup Physiol 75, 7–13. [DOI] [PubMed] [Google Scholar]
- Zollei E, Paprika D, Makra P, Gingl Z, Vezendi K & Rudas L (2004). Human autonomic responses to blood donation. Auton Neurosci 110, 114–120. [DOI] [PubMed] [Google Scholar]


