Abstract
Currently, Degussa P25, with the typical mixed phases of anatase and rutile TiO2, is widely applied as the commercial photocatalysts. However, there are still some of obstacles for the P25 nanoparticles with totally high photocatalytic activities, especially for the catalytic stability due to their inevitable aggregation of the nanoparticles when used as the photocatalysts. In the present work, we reported the exploration of a novel TiO2 photocatalyst, which could offer an ideal platform for synergetic combination of the mixed-phase composition, hollow architecture and mesoporous walls for the desired excellent photocatalytic efficiency and robust stability. The mesoporous TiO2 hollow nanofibers were fabricated via a facile single capillary electrospinning technique, in which the foaming agents were used for creating mesopores throughout the walls of the hollow fibers. The obtained hollow fibers exhibit a high purity and possess the mixed phases of 94.6% anatase and 5.4% rutile TiO2. As compared to P25, the as-fabricated mesoporous TiO2 hollow fibers exhibited much higher efficient photocatalytic activities and stabilities toward the hydrogen evolution with a rate of ~499.1 μmol g−1·h−1 and ~99.5% degradation Rhodamine B (RhB) in 60 min, suggesting their promising application in efficient photocatalysts.
Semiconductor photocatalyst materials are extensively explored for the solar energy utilization, which offer a possible strategy to address the environmental problem and energy crisis1,2. Up to date, numerous interests have been focused on the titanium dioxide (TiO2) material, due to its low cost, ready commercial availability, and long-term stability against the photochemical corrosion in aggressive aqueous environments3,4.
Generally, TiO2 has four typical allotropic forms including anatase, brookite, rutile, and TiO2(B), among which the anatase has been considered as the one with the best photoactivity5. Compared with the pure TiO2 material, the mixed-phase TiO2 photocatalysts such as commercial Degussa P25 (composed by ~80% anatase and ~20% rutile) and anatase/TiO2(B) fibers, can exhibit much higher photocatalytic activity6,7,8,9,10,11, which could be mainly attributed to the enhanced charge transfer caused by the energy difference between the conduction band (CB) edges of two phases. In addition to the TiO2 crystallinity, porous structure of the photocatalyst is beneficial for the adsorption of the reactants, and thus can significantly improve the photocatalytic efficiencies. For example, one-dimensional (1D) TiO2 hollow structures are of great interest in the viewpoint of their potential various applications and fundamental phenomena specific to a confined nanostructure12,13,14,15,16,17. Particularly, the hollow TiO2 fibers with designed mesoporous walls have received remarkable attention owing to their unique hierarchical pore structure, which is helpful for improved capabilities of mass transport through the material body and maintenance of a specific surface area on the level of a satisfactory porosity18,19,20,21,22. That is to say, the efficient photocatalytic activities of the TiO2 semiconductors could be expected, once they are grown into the fiber structures together with hollow bodies, mixed phases and mesoporous walls. However, the reported works often suffered from tedious procedures or special conditions. Thereby, it is still a challenge for developing a simple and facile route to generate mesoporous TiO2 hollow nanofibers.
In the present work, we report the exploration of a novel TiO2 photocatalyst, which could offer an ideal platform for synergetic combination of the mixed-phase composition, hollow architecture and mesoporous walls for the desired excellent photocatalytic efficiency and long-term stability. The mixed-phase anatase/rutile TiO2 hollow fibers are fabricated via a simple electronspinning method. As illustrated in Fig. 1(a), the hollow structures are obtained by removing the inside paraffin oil during the air calcination, and the foaming agents (DIPA) are concomitantly decomposed into abundant vapor phases to create the thoroughly mesoporous walls. As expected, the obtained hollow fibers, with the mixed phases of anatase and rutile TiO2 as well as mesoporous walls exhibit excellent photocatalytic performances in hydrogen production and dye degradation under UV light irradiation.
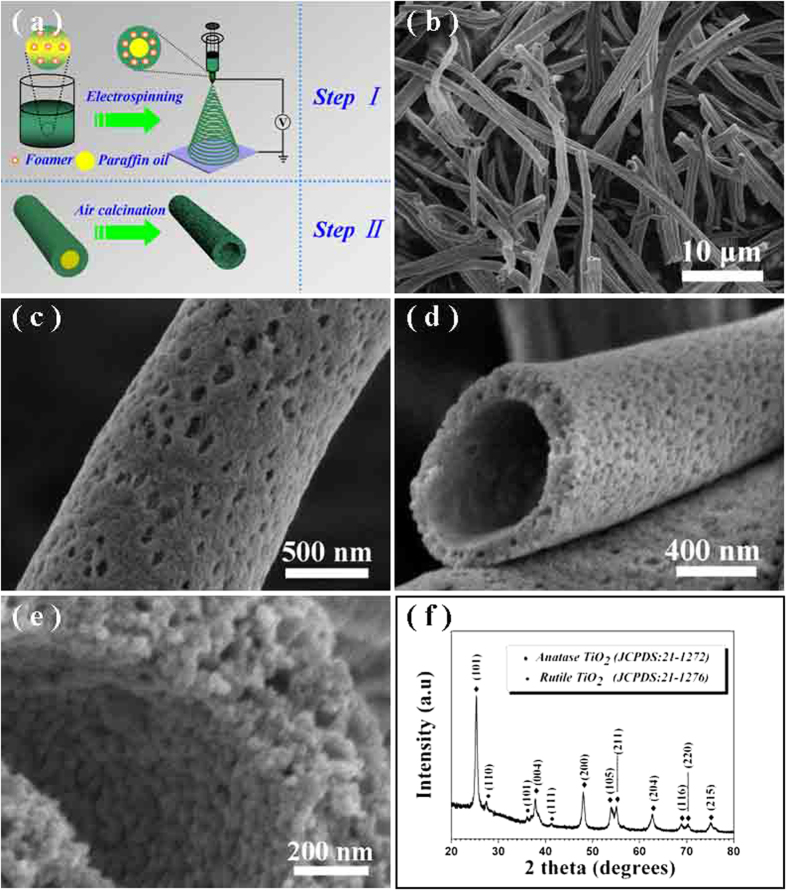
Figure 1.
(a) Schematic illustration for the formation of mesoporous TiO2 hollow fibers via the foaming assisted electrospinning. (b) A typical SEM image of the calcined products under a low magnification. (c–e) Typical SEM images of the calcined products under higher magnifications and different views. (f) A representative XRD pattern of the calcined products.
Methods
Raw materials
Polyvinylpyrrolidone (PVP, MW ≈ 1300000), butyl titanate (TBOT), diisopropyl azodiformate (DIPA), paraffin oil, absolute ethyl alcohol, acetic acid, deionized water and rhodamine B (RhB) were purchased from Aladdin (Shanghai, China). All materials were directly used as received without further treatment.
Preparation of msoporous TiO2 hollow fibers
In a typical experimental procedure, 0.6 g of PVP, 3.0 g of TBOT and 0.5g of DIPA were firstly dissolved in 7 mL absolute ethyl alcohol with stirring vigorously for 2 h. Then, 0.5 g of CTAB and 2 ml paraffin oil were added into the above solution followed by being subjected to the magnetic stirring further for 5 h. Subsequently, the above precursor microemulsions were transformed into a plastic syringe with a stainless steel nozzle (anode, diameter: 0.2 mm). The tip of the stainless steel nozzle was placed in the front of a metal cathode (collector) with a fixed distance of 20 cm between the nozzle and the collector. An electrical potential of 18 kV and a flow rate of 1 mL h−1 were applied for electrospinning precursor fibers. The as-spun polymer fibers were dried in an oven at 60 °C, followed by being located in a quartz crucible and placed at the center of a conventional tube furnace. Finally, the precursor fibers were heated up to the desired temperature of 500 °C with a heating rate of 1 °C min−1, and maintained there for 3 h in air, followed by furnace-cool to ambient temperature.
Structure characterization
The obtained products were characterized with X-ray powder diffraction (XRD, D8 Advance, Bruker, Germany) with Cu Kα radiation (λ = 1.5406 Å), field emission scanning electron microscopy (FESEM, S-4800, Hitachi, Japan), and high-resolution transmission electron microscopy (HRTEM, JEM-2010F, JEOL, Japan) equipped with energy dispersive X-ray spectroscopy (EDX, Quantax-STEM, Bruker, Germany). The porous properties of the as-prepared mesoporous fibers were characterized using N2 adsorption at −195.8 °C on a specific surface area and porosity analyzer (ASAP 2020HD88, Micromeritics, USA).
Photocatalytic activity measurements
The photocatalytic activities of the resultant products were firstly evaluated for hydrogen evolution. The photocatalytic reaction is performed in an inner-irradiation quartz annular reactor with a 300 W Xenon lamp (CEL, HUL300), a vacuum pump, a gas collection, a recirculation pump and a water-cooled condenser. The as-synthesized samples (0.1 g) were suspended in deionized water and methanol mixed solutions (40 mL, 3:1) by an ultrasonic oscillator, respectively. Then the mixture was transferred into the reactor and deaerated by the vacuum pump. The Xenon lamp was utilized as a light source, and the cooling water was circulated through a cylindrical Pyrex jacket located around the light source to maintain the reaction temperature. The reactor was sealed with ambient air during irradiation, and the hydrogen evolution were monitored by an online gas chromatography (GC, 7900) equipped with a Porapak-Q column, high-purity nitrogen carrier and a thermal conductivity detector (TCD). In order to investigate the stability and recyclability, the products were re-used in the same reaction for 3 cycles. Furthermore, degradation of rhodamine B (RhB, Aladdin, Shanghai, China) was studied to evaluate its photocatalytic ability. The photocatalytic reaction is performed at an inner-irradiation quartz annular reactor, which has a Xenon lamp (λ > 320 nm, CEL-Hx F300, Beijing, China) and a water-cooled condenser. Typically, 1.2 mg of RhB and 40 mg of the as-prepared catalysts were dispersed in 120 ml deionized water. Prior to irradiation, the suspensions were magnetically stirred in the dark for 60 min to ensure the establishment of an adsorption-desorption equilibrium between the photocatalyst and the RhB dye. An aliquot (3 mL) of the solution was taken at a certain time interval (10 min) during the reaction and analyzed on the UV-visible spectrophotometer as mentioned above. The change in RhB absorbance in the solution was used to monitor the extent of reaction at the given irradiation time intervals. For comparison, Degussa P25 was commercially available, and used directly for the photocatalytic activity for H2 generation and degradation of RhB.
Results
SEM was firstly employed to meticulously study the morphology and microstructure of the precursor fibers and their corresponding calcined products. The resultant precursor fibers (Figure S1 (a), Supplementary Information) are continuous with the diameters in the range of 1 ∼ 1.5 μm and up to several hundred of millimeters in length. Figure 1(b–e) are the typical SEM images under different magnifications and views of the corresponding calcined products. It seems that the long continuous precursor fibers have been converted into hollow structures with the diameters reduced to ~900 nm, which could be mainly ascribed to the elimination of the organisms during the air calcination process (see Fig. 1(b)). Figure 1(c–e) show the interior and external surface view under different magnifications of the TiO2 hollow fibers, respectively, disclosing that numerous pores exist on the fiber surface and the hollow fiber walls are thoroughly mesoporous. The formation of the hollow and mesoporous structures could be mainly attributed to the decomposition and removing of organics existed within the as-spun polymeric fibers. The powder X-ray diffractograms (XRD) in wide angles are used to characterize the phase compositions of the calcined products. As shown in Fig. 1(f), besides the dominant diffraction peaks of anatase TiO2 (JCPDS, No. 21-1272), some weak diffraction peaks of rutile phase (JCPDS, No. 21-1276) can be also clearly detected, suggesting that the obtained products possess the mixed phases of anatase and rutile TiO2. According to the XRD data, these two phases existed in the fibers can be calculated by using semi-quantitative calculation method, disclosing that the as-synthesized hollow TiO2 fibers are composed of 94.6% anatase and 5.4% rutile. As mentioned above, the mixed-phase structure favors the exploration of efficient photocatalysts, since the photogenerated charge transfer between the involved two phases can greatly inhibit the charge recombination of the holes/electrons, thus leading to the enhancement of the photocatalytic activities23,24,25.
The nitrogen adsorption measurements (Fig. 2(a) reveal that the as-fabricated products exhibit the type IV isotherm behavior with H3 hysteresis, implying that the obtained hollow fibers are mesoporous with a BET surface area of ~27.2 m2/g. According to the Barrett–Joyner–Halenda (BJH) pore size distribution analysis determined from the adsorption branches (Fig. 2(b)), the average BJH pore diameter is centered at ~38 nm.
Figure 2.
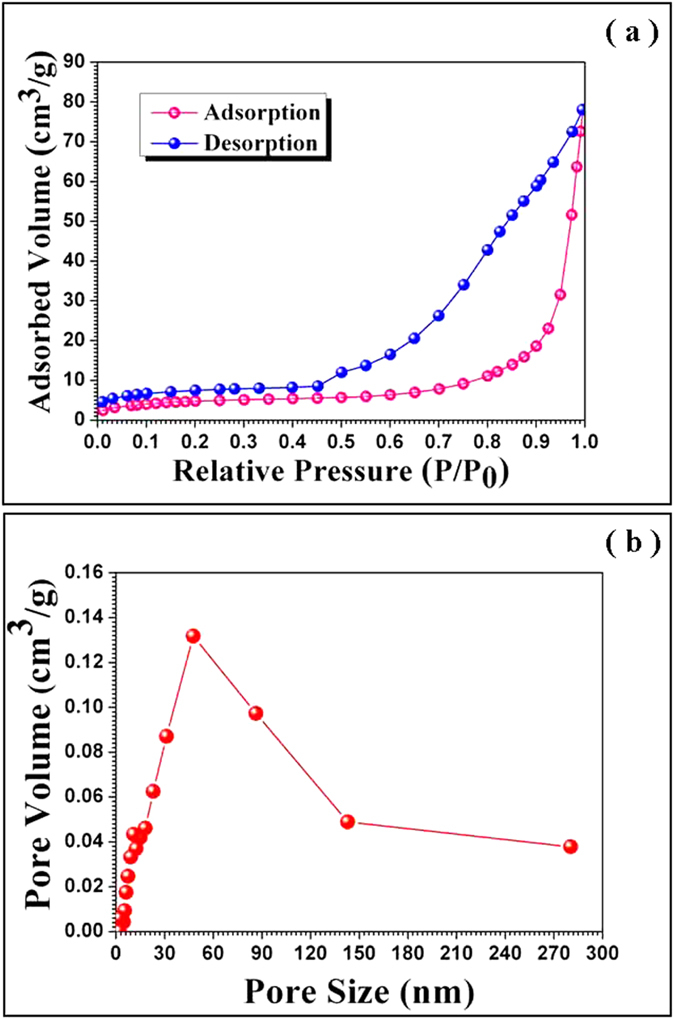
(a) Nitrogen adsorption-desorption isotherm curve of mesoporous TiO2 hollow nanofibers. (b) Pore size distribution curve of the mesoporous TiO2 hollow nanofibers.
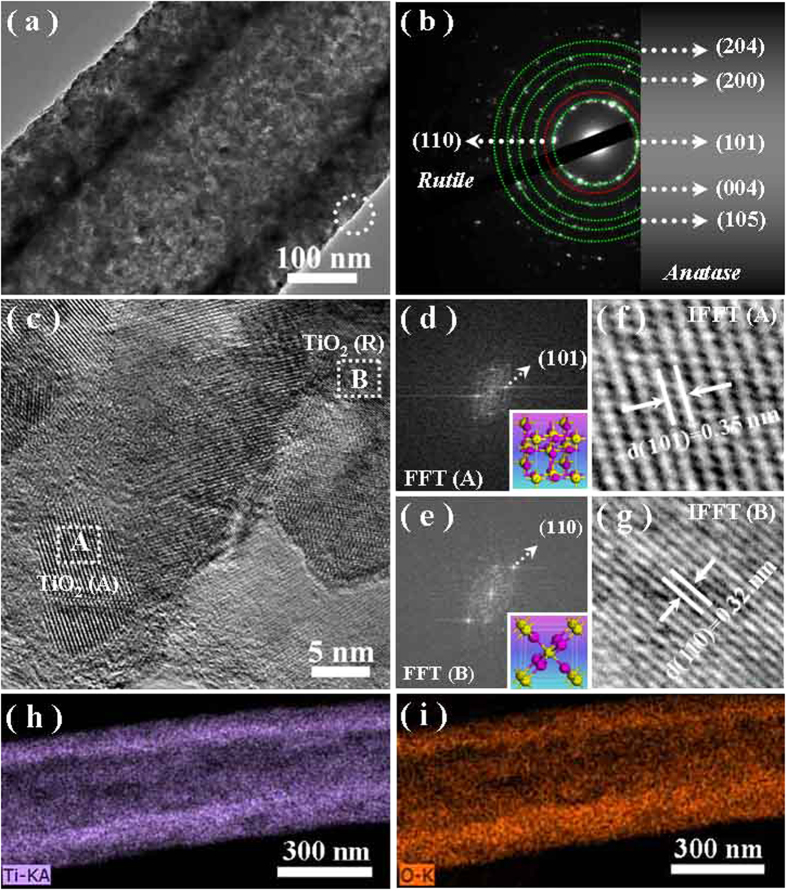
TEM was further used to investigate the structural details of the mixed-phase hollow fiber, as shown in Fig. 3(a). In agreement with the SEM observations, the inner tunnel is clearly observed by the sharp contrast between TiO2 mesoporous wall and the hollow interior. The wall thickness and inner diameter are ~100 nm and ~215 nm, respectively, suggesting the large exposed surface of the products. Figure 3(b) presents the corresponding selected area electron diffraction (SAED) pattern recorded from the single fiber in Fig. 3(a), suggesting its polycrystalline nature. The dominant diffraction spot rings could be sequentially indexed to the crystal planes of (101), (004), (105), (200) and (204) of anatase TiO2 (JCPDS, No. 21-1272), and the low-intensity diffraction spot ring matches to the (110) plane of rutile TiO2 (JCPDS, No. 21-1276). Furthermore, a representative high-resolution TEM (HRTEM) image (Fig. 2(c)) recorded from the marked area in Fig. 3(a) discloses the mixed-phase structures existed within the hollow fiber bodies. Accordingly, the two fast fourier transformation (FFT) images (Fig. 3(d,e)) provide the electron diffraction signals corresponding to the local lattice patterns recorded from the marked areas of A and B in Fig. 3(c), respectively. Their crystalline structure and crystalline face are determined from the distances between the reciprocal lattice points and center point in the FFT images23, implying that both squared regions possess the tetragonal characteristic. Hoverer, the measured distance between the highest intensity point and center one is unequal, suggesting the different phases of these two given selected areas, where the marked areas of A and B belong to anatase (the inset of Fig. 3(d)) and rutile (the inset of Fig. 3(e)), respectively. Figure 3(f,g) are the corresponding fast flourier transformation images (IFFT), further confirmed that d-spacing of 0.35 nm matches to the (101) plane of anatase (Fig. 3(f)) and that of 0.32 nm corresponds to the (110) plane of rutile (Fig. 3(g)), respectively. This is in good agreement with the XRD diffraction results, confirming that the obtained products should be composed by the mixed-phase of anatase and rutile TiO2. The energy-dispersive X-ray (EDX) spectroscopy analyses (typically shown in Figure S2, Supplementary Information), recorded from a number of fibers and different positions along a single one, suggest that the chemical compositions are identical and mainly composed by Ti and O elements. The Cu and C signal should be from the TEM grid used to support the sample. The element mapping images of Ti (Fig. 3(h)) and O (Fig. 3(i)) exhibit a harmonious ravine distribution throughout the fiber body, verifying the hollow nature of the as-fabricated TiO2 nanofibers.
Figure 3.
(a) A representative TEM image of the mesoporous TiO2 hollow fibers. (b) The corresponding SAED pattern. (c) A representative HRTEM image of the mesoporous hollow fibers recorded from the marked area in (a). (d,e) Fast fourier transformation (FFT) images of the marked areas of A and B in (c). The insets are the partial geometry models of anatase and rutile TiO2, respectively. (f,g) The corresponding inverse fast fourier transformation (IFFT) images of (d,e), respectively. (h,i) The element mappings of Ti and O within a single nanofiber.
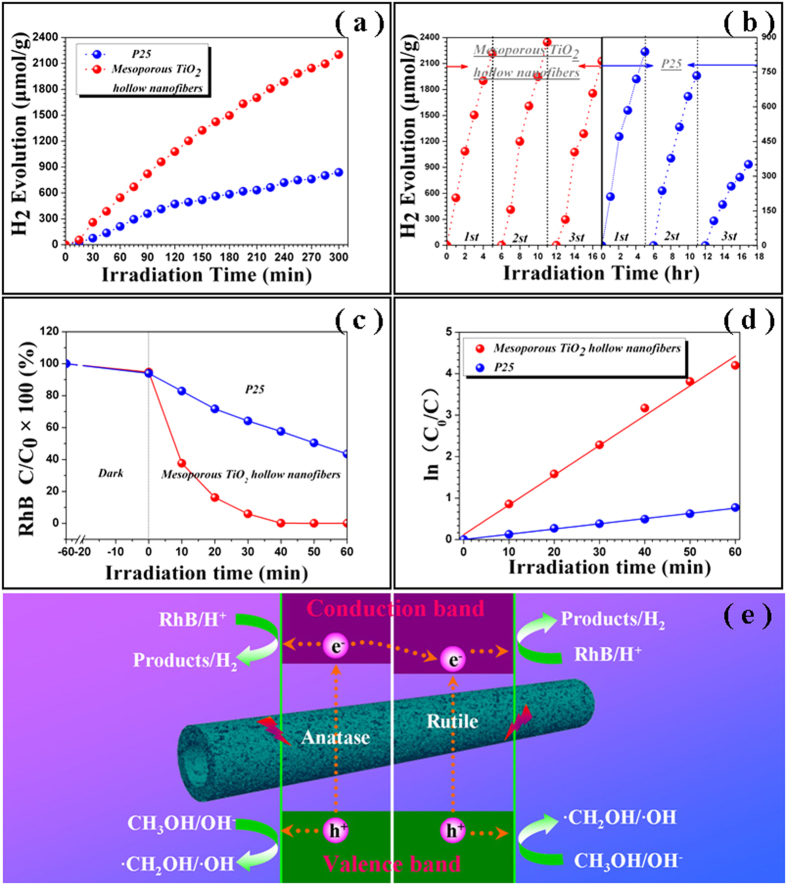
Photocatalytic evolution of H2 on the as-prepared products as well as P25 (the typical SEM image and EDX spectrum, see Figure S3, Supplementary Information) is carried out by using methanol as sacrificial agents and irradiation under a 300 W Xenon arc lamp. Figure 4(a) plots the typical H2 evolution kinetic lines over these two photocatalysts. For the mesoporous TiO2 hollow fibers, their photocatalytic reactions exhibit a stable H2 release rate of ~499.1 μmol g−1·h−1, which is much higher than that of P25 (i.e., 197.8 μmol g−1·h−1) and those reported for solid and porous TiO2 nanofibers always lower than 400 μmol g−1·h−1 (see Table 1). In order to investigate their reusability, these two photocatalysts are recovered and re-used for photocatalytic H2 production under the identical experimental conditions. As shown in Fig. 4(b), the activity of the mesoporous TiO2 hollow fibers is still maintained with no noticeable decrease observed after 3 cycles. However, the photocatalytic ability of P25 has evidently declined after 3 cycles. Obviously, the 1D mesoporous hollow structure exhibits a much better long-term photocatalytic stability than the commercial P25. Additionally, the photocatalytic properties of the as-prepared TiO2 hollow nanofibers are evaluated by the decomposition of RhB dye. Figure 4(c) shows their time-dependent degradation of RhB, which are monitored by the maximum band absorbance (also see Figure S4, Supplementary Information), and corrected one by one based on the standard plots of the dye with various RhB concentrations (see Figure S5, Supplementary Information). It seems that, without the light irradiation, the concentration of RhB suspension over the mesoporous TiO2 hollow fibers as well as P25 undergoes a tiny change during 60 min magnetic stirring for the sample preparation, suggesting that the dark physics adsorption have little influence on the dye concentration change. The colors of the suspensions catalyzed by the mesoporous TiO2 hollow products (the inset in Figure S4(a), Supplementary Information) change gradually from pink to almost colorless, implying the nearly complete photodegradation of RhB. Nevertheless, the suspensions catalyzed by P25 (the inset of Figure S4(b), Supplementary Information) still maintain an obvious light pink color after 60 min illumination, declaring that the some part of the RhB still remained in the suspension without decomposition. The overall degradation efficiency is 99.5% for mesoporous TiO2 hollow fibers, which is ~2.5 times to that of P25. To further study the photocatalytic reaction, the pseudo-first-order kinetic model is adopted according to the equation ln(C0/C) = k, where C0 is the adsorption equilibrium concentration of pollutant before irradiation, C is the instantaneous concentration, and k is the apparent rate constant with determined reaction time, respectively5,26. As shown in Fig. 4(d), the corresponding reaction rate constants (k) are calculated to be 0.071 and 0.010 min−1 for the mesoporous TiO2 hollow fibers and P25, respectively. Notably, the present mesoporous hollow products own superior photodegradation efficiency to that of P25. These experimental results verify that, in comparison to the commercial products of P25, the present mesoporous TiO2 hollow fibers could be a much higher efficient photocatalyst candidate for both photocatalytic evolution H2 production and degradation of hazardous materials.
Figure 4.
(a) The hydrogen production photocatalyzed by the as-fabricated mesoporous TiO2 hollow nanofibers as well as p25 under different irradiation times. (b) Reusability experiment for photocatalytic H2 generation of mesoporous TiO2 hollow nanofibers and p25. (c) Photocatalytic degradation of RhB (C0 = 10 mg/L) of mesoporous TiO2 hollow nanofibers and p25 under UV-visible light irradiation. (d) The plot of ln(C0/C) with irradiation time for mesoporous TiO2 hollow nanofibers and p25. (e) The proposed mechanism for the enhanced photocatalytic activities of the mesoporous TiO2 hollow fibers with mixed phases of anatase and rutile.
Table 1. Comparison of the related typical works for H2 production using TiO2 nanofibers as the Photocatalyst.
| Material | Preparation | Morphology | Irradiation conditions | Reaction solution | Activity (μmol g−1h−1) | Reference |
|---|---|---|---|---|---|---|
| TiO2 (B) | hydrothermal | Nanofibers | 15 W UV lamp | Neat ethanol | 238 | 34 |
| TiO2 | Electrospinning | Nanofibers | 450 W Hg | Water+MeOH | 54 | 20 |
| TiO2 (B)/Pt | hyndrothermal | Nanofibers | 15 W UV lamp | Neat ethanol | 257 | 35 |
| TiO2/Pt | Electrospinning | Nanofibers | 300 W Xe | Water+MeOH | 910 | 36 |
| TiO2/Pt | hydrothermal | Nanofibers | 300 W Hg | Water+MeOH | 310 | 37 |
| TiO2 | hydrothermal | Nanofibers | 6 UVB lamps | Water +ethanol | 30 | 28 |
| TiO2 | Electrospinning | Porous Nanofibers | 400 W Hg | Water+MeOH | 80 | 17 |
| TiO2 | Electrospinning | Nanofibers | 400 W Hg | Water+AO7 | 21 | 38 |
| TiO2 | Electrospinning | Porous fibers | 300 W Xe | Water+MeOH | 198 | 27 |
| TiO2 | foaming-assisted electrospinning | Mesoporous nanofibers | 300 W Xe | Water+MeOH | 399 | 29 |
| TiO2 | foaming-assisted electrospinning | Mesoporous nanofibers | 300 W Xe | Water+MeOH | 499 | Current work |
Discussion
The growth of mesoporous TiO2 hollow fibers could be assigned to follow issues: i) The formation of the hollow interior. As stated in the experimental procedure, the used raw materials of PVP, TBOT, DIPA, absolute ethyl alcohol, CTAB and paraffin oil would cause the formation of microemulsions. As schematically illustrated in Fig. 1(a), the core-shell structure of the as-spun polymeric fibers would be then formed, due to the distinctively different phase interfaces between the mixtures dissolved in the solvent, which cause the formation of a core-shell jet driven by the electrical forces during electrospinning27. The core is mainly made up by the trapped paraffin oil, and the shell should be the remained materials of PVP/TBOT/CTAB/DIPA. Once subjected to be calcined at high temperature, the inside paraffin oil core would be completely decomposed into gas phases such as CO2 and H2O, leading to the formation of the hollow interior of the fiber. ii) The formation of the thoroughly mesoporous walls of the hollow fibers. It should be mainly ascribed to the thermal decomposition of foaming agents of DIPA assembled within the fibers, which would be converted into abundant vapor phases such as CO2, NO2 and H2O28, making the creation of mesoporous throughout the walls of the hollow fibers.
To account for the enhanced photocatalytic ability mesoporous TiO2 hollow fibers, a tentative mechanism is proposed, as schematically illustrated in Fig. 4(e). It is well known that the crystallite phase and material architecture play a significant role on the photocatalytic performance of TiO2 photocatalysts2,3,29. Thus, the excellent photocatalytic performance of mesoporous TiO2 hollow fibers might be explained by the following points. i) The mixed phases of anatase and rutile TiO2 within the fibers. The present products possess the similar functional mix-phase junction of P25, which leads to the improvement of interfacial charge transfer and effectively limits the charge recombination20,21,22. ii) The well-defined hollow mesoporous nanofibers. The presence of favorable hollow formation with big inner diameters could make the reactant more and easier to contact the photocatalysts, owing to the more active sites offered by the larger exposed surface areas30,31. iii) The 1D mesoporous architecture walls. This can not only remarkably inhibit the agglomeration of nanoparticles, but also improve the surface adsorption capacity of the reactants. Furthermore, the mesoporous channels existed throughout the fiber bodies also could facilitate the effective transportation of products32,33. In brief words, the as-fabricated mesoporous TiO2 hollow fibers could be considered as the assembly of P25 nanoparticles with the mixed phases of anatase and rutile phases into hollow one-dimensional counterparts with thoroughly mesoporous walls, which offer the platform for synergetic combination of the mix-phase composition, hollow architecture and mesoporous walls to bring a significant enhancement on the photocatalytic activities.
Conclusions
In summary, we have demonstrated the investigation of mesoporous TiO2 hollow nanofiber photocatalysts, which are fabricated via a facile single capillary electrospinning technique39,40,41. The obtained fibers are composed of anatase and rutile mixed phases with well-defined hollow bodies and thoroughly mesoporous walls. The as-fabricated mesoporous TiO2 hollow fibers exhibit efficient photocatalytic activities and robust stabilities toward the hydrogen evolution and degradation of RhB, which are ~2.5 times to those of commercial P25, suggesting their promising applications as excellent photocatalysts. It is promising that the mesoporous TiO2 hollow nanofibers could offer an ideal platform for synergetic combination of the mixed-phase composition, hollow architecture and mesoporous walls for the desired excellent photocatalytic efficiency and stability, which could provide a novel strategy for the exploration of novel TiO2 photocatalysts with high performances.
Additional Information
How to cite this article: Hou, H. et al. Efficient Photocatalytic Activities of TiO2 Hollow Fibers with Mixed Phases and Mesoporous Walls. Sci. Rep. 5, 15228; doi: 10.1038/srep15228 (2015).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC, Grant Nos. 51372122 and 51372123), Program of Shanghai Outstanding Technical Leaders (Grant No. 14XD1425400), and Special Fund for Nanotechnology of Shanghai Science and Technology Commission (Grant No. 12nm0503100).
Footnotes
Author Contributions W.L., B.T. and W.Y. conceived and directed the experiments. H.H., M.S. and L.W. performed the experiments. H.H., B.T. and W.Y. co-wrote the manuscript. All authors discussed the results and helped with the preparation of the final manuscript.
References
- Pang C. L., Lindsay R. & Thornton G. Structure of clean and adsorbate-covered single-crystal rutile TiO2 surfaces. Chem. Rev. 113, 3887–3948 (2013). [DOI] [PubMed] [Google Scholar]
- Hisatomi T., Kubota J. & Domen K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7025–7035 (2014). [DOI] [PubMed] [Google Scholar]
- Linsebigler A. L., Lu G. & Yates J. T. Jr. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995). [Google Scholar]
- Chen H., Nanayakkara C. E. & Grassian V. H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 112, 5919–5948 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Heterojunctions in g-C3N4/TiO2 (B) nanofibres with exposed (001) plane and enhanced visible-light photoactivity. J. Mater. Chem. A 2, 2071–2078 (2014). [Google Scholar]
- Hurum D. C., Agrios A. G., Gray K. A., Rajh T. & Thurnauer M. C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 107, 4545–4549 (2003). [Google Scholar]
- Yang D. et al. An efficient photocatalyst structure: TiO2 (B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 131, 17885–17893 (2009). [DOI] [PubMed] [Google Scholar]
- Yang D. et al. Enhancing photoactivity of TiO2 (B)/anatase core–shell nanofibers by selectively doping cerium ions into the TiO2 (B) core. Chem. Eur. J. 19, 5113–5119 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Heterojunctions in g-C3N4/TiO2 (B) nanofibres with exposed (001) plane and enhanced visible-light photoactivity. J. Mater. Chem. A 2, 2071–2078 (2014) [Google Scholar]
- Zhang S. et al. Enhanced photodynamic therapy of mixed phase TiO2 (B)/anatase nanofibers for killing of HeLa cells. Nano Res. 7, 1659–1669 (2014). [Google Scholar]
- Wang R., et al.Tuning and understanding the phase interface of TiO2 nanoparticles for more efficient lithium ion storage. Nanoscale, 10.1039/C5NR02582F (2015). [DOI] [PubMed] [Google Scholar]
- Hoyer P. Semiconductor nanotube formation by a two-step template process. Adv. Mater. 8, 857–859 (1996). [Google Scholar]
- Kasuga T., Hiramatsu M., Hoson A., Sekino T. & Niihara K. Titania nanotubes prepared by chemical processing. Adv. Mater. 11, 1307–1311 (1999). [Google Scholar]
- Rao C., Govindaraj A., Deepak F. L., Gunari N. & Nath M. Surfactant-assisted synthesis of semiconductor nanotubes and nanowires. Appl. Phys. Lett. 78, 1853–1855 (2001). [Google Scholar]
- Goldberger J. et al. Single-crystal gallium nitride nanotubes. Nature 422, 599–602 (2003). [DOI] [PubMed] [Google Scholar]
- Martinson A. B., Elam J. W., Hupp J. T. & Pellin M. J. ZnO nanotube based dye-sensitized solar cells. Nano Lett. 7, 2183–2187 (2007). [DOI] [PubMed] [Google Scholar]
- Lee S. S., Bai H., Liu Z. & Sun D. D. Electrospun TiO2/SnO2 nanofibers with innovative structure and chemical properties for highly efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 37, 10575–10584 (2012). [Google Scholar]
- Lin H. P., Mou C. Y. & Liu S. B. Formation of mesoporous silica nanotubes. Adv. Mater. 12, 103–106 (2000). [Google Scholar]
- Yu D. et al. Mesoporous nanotubes of iron phosphate: synthesis, characterization, and catalytic property. Langmuir 23, 382–386 (2007). [DOI] [PubMed] [Google Scholar]
- Chuangchote S., Jitputti J., Sagawa T. & Yoshikawa S. Photocatalytic activity for hydrogen evolution of electrospun TiO2 nanofibers. ACS Appl. Mater. Inter. 1, 1140–1143 (2009). [DOI] [PubMed] [Google Scholar]
- Xiang H. et al. A novel and facile method to prepare porous hollow CuO and Cu nanofibers based on electrospinning. Cryst Eng Comm 13, 4856–4860 (2011). [Google Scholar]
- Hua G., Zhang L., Fei G. & Fang M. Enhanced catalytic activity induced by defects in mesoporous ceria nanotubes. J. Mater. Chem. 22, 6851–6855 (2012). [Google Scholar]
- Ohno T., Tokieda K., Higashida S. & Matsumura M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal., A 244, 383–391 (2003). [Google Scholar]
- Li G. et al. Synergistic effect between anatase and rutile TiO2 nanoparticles in dye-sensitized solar cells. Dalton Trans. 45, 10078–10085 (2009). [DOI] [PubMed] [Google Scholar]
- Kho Y. K. et al. Photocatalytic H2 evolution over TiO2 nanoparticles. The synergistic effect of anatase and rutile. J. Phys. Chem. C 114, 2821–2829 (2010). [Google Scholar]
- Chen W. et al. Enhanced visible-light activity of titania via confinement inside carbon nanotubes. J. Am. Chem. Soc. 133, 14896–14899 (2011). [DOI] [PubMed] [Google Scholar]
- Hou H. et al. Fabrication of porous titanium dioxide fibers and their photocatalytic activity for hydrogen evolution. Int. J. Hydrogen Energy 39, 6837–6844 (2014). [Google Scholar]
- Wu M. C. et al. Enhanced photocatalytic activity of TiO2 nanofibers and their flexible composite films: Decomposition of organic dyes and efficient H2 generation from ethanol-water mixtures. Nano Res. 4, 360–369 (2011). [Google Scholar]
- Hou H. et al. General strategy for fabricating thoroughly mesoporous nanofibers. J. Am. Chem. Soc. 136, 16716–16719 (2014). [DOI] [PubMed] [Google Scholar]
- Zhan S., Chen D., Jiao X. & Tao C. Long TiO2 hollow fibers with mesoporous walls: sol-gel combined electrospun fabrication and photocatalytic properties. J. Phys. Chem. B 110, 11199–11204 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang X., Thavasi V., Mhaisalkar S. & Ramakrishna S. Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials. Nanoscale 4, 1707–1716 (2012). [DOI] [PubMed] [Google Scholar]
- Yu J. C., Wang X. & Fu X. Pore-wall chemistry and photocatalytic activity of mesoporous titania molecular sieve films. Chem. Mater. 16, 1523–1530 (2004). [Google Scholar]
- Zhou W. et al. Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst. J. Am. Chem. Soc. 136, 9280–9283 (2014). [DOI] [PubMed] [Google Scholar]
- Lin C. H., Chao J. H., Liu C. H., Chang J. C. & Wang F. C. Effect of calcination temperature on the structure of a Pt/TiO2 (B) nanofiber and its photocatalytic activity in generating H2. Langmuir 24, 9907–9915 (2008). [DOI] [PubMed] [Google Scholar]
- Wang F. C., Liu C. H., Liu C. W., Chao J. H. & Lin C. H. Effect of Pt loading order on photocatalytic activity of Pt/TiO2 nanofiber in generation of H2 from neat ethanol. J. Phys. Chem. C 113, 13832–13840 (2009). [Google Scholar]
- Choi S. K., Kim S., Lim S. K. & Park H. Photocatalytic comparison of TiO2 nanoparticles and electrospun TiO2 nanofibers: effects of mesoporosity and interparticle charge transfer. J. Phys. Chem. C 114, 16475–16480 (2010). [Google Scholar]
- Li W. et al. Single-crystalline and reactive facets exposed anatase TiO2 nanofibers with enhanced photocatalytic properties. J. Mater. Chem. 21, 6718–6724 (2011). [Google Scholar]
- Lee S. S., Bai H., Liu Z. & Sun D. D. Novel-structured electrospun TiO2/CuO composite nanofibers for high efficient photocatalytic cogeneration of clean water and energy from dye wastewater. Water Res. 47, 4059–4073 (2013). [DOI] [PubMed] [Google Scholar]
- Seo M., Kim S. K., Han J. H. & Hwang C. S. Permittivity enhanced atomic layer deposited HfO2 thin films manipulated by a rutile TiO2 interlayer. Chem. Mater. 22, 4419–4425 (2010). [Google Scholar]
- Chen H. et al. Fabrication of hierarchically porous inorganic nanofibers by a general microemulsion electrospinning approach. Small 7, 1779–1783 (2011). [DOI] [PubMed] [Google Scholar]
- Chen X. & Mao S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.