Abstract
Aim:
This study evaluating N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) and high-sensitivity C-reactive protein (hs-CRP) relationship with features of the metabolic syndrome (MS) in high risk subgroups for cardiovascular disease (CVD) in Trinidad.
Materials and Methods:
The sample population consisted of 160 subjects, 78 of whom were African and 82 East Indian attending medical outpatient clinics of regional health authority hospitals of Trinidad.
Results:
Systolic blood pressure, triglycerides, glucose and insulin as well as NT-pro-BNP were elevated among the East Indian sub-population, with only systolic blood pressure being significantly elevated among the African sub-population. NT-pro-BNP and hs-CRP demonstrated significant correlations with respect to the majority of independent risk factors inclusive of Adult Treatment Panel III and American Association of Clinical Endocrinologists defined criteria for MS. NT-pro-BNP demonstrated stronger association among the East Indian sub-population as compared to that of the African sub-population.
Conclusions:
Our study showed that the East Indian subgroup was more at risk for CVD as evidenced by the fulfillment of the criteria for diagnosis of MS and therefore NT-pro-BNP and hs-CRP can be deemed a suitable marker for MS.
Keywords: Cardiovascular disease, high-sensitivity C-reactive protein, metabolic syndrome, N-terminal pro-B-type natriuretic peptide
INTRODUCTION
In recent years, Trinidad has seen the defining parameters of metabolic syndrome (MS), such as waist circumference (WC), ethnicity, triglyceride (TG), high-density lipoprotein (HDL) cholesterol, blood pressure and blood glucose level (International Diabetes Federation criteria), increase in prevalence, indicating a great risk for diabetes and cardiovascular outcomes.[1] Collectively, of the 35 million deaths that occur annually worldwide, 32% are attributable to cardiovascular coupled with diabetes.[2] Traditionally, TG/HDL-C ratio predicts coronary heart disease (CHD) and cardiovascular disease (CVD) mortality as well as or better than do MS. Blood glucose levels and more recently, the TG index[3] for insulin resistance have impact on diabetes and CVD risk. Etiologically, atherosclerotic plaque deposition has been suggested as the causal pathologic mechanism linking the MS to CVDs, including; CHD, myocardial infarction, and sudden cardiac death.[4]
Meticulous control of glycemic levels is paramount for diabetics in the avoidance of microvascular complications of diabetes such as, retinopathy, nephropathy, and neuropathy. However, it has been shown that macrovascular complications such as coronary artery disease, for which studies have shown, to be the major determinant of mortality,[5] is not as strongly related to glycemic control and therein, the problem lies in “predicting” the risk among diabetics, especially given the multisystemic nature of symptoms.[6] This need is becoming increasingly important, especially taking into consideration the growing prevalence of diabetics, with likely numbers in developing countries reaching over 82 million by 2030.[7,8]
The proposition to achieve this is partly through a biomarker called N-terminal pro-B-type natriuretic peptide (NT-pro-BNP). NT-pro-BNP is the biologically inactive 76 amino acid peptide product of the cleavage of a 108 amino acid peptide called pro-BNP.[9] The other product of said process is the biologically active BNP. Location for this process is primarily in the left ventricle[10] and an increased level of NT-pro-BNP is synonymous with conditions causing increased tension on the ventricular walls. This is indicative of various forms of CVDs such as cardiac hypertrophy, ischemia, coronary endothelial dysfunction, fibrosis, and arrhythmia. This, coupled with the 60–120 min half-life of NT-pro-BNP versus the 15–20 min half-life of BNP, supports claims proposed that NT-pro-BNP has diagnostic value as an early biomarker for CVD and related pathologies.[11,12]
C-reactive protein (CRP) is a global marker of inflammation and MS. Levels are elevated in obese patients[13] and may well be altered in patients with the MS due to its inflammatory nature from dyslipidemia and hyperuricemia. Serum biomarkers CRP and total plasma homocysteine have also demonstrated significant relation to the development of atherosclerotic plaques.[14,15] Adiposite generated cytokines like tumor necrosis factor-α, interleukin-1 (IL-1) and IL-6, are responsible for eliciting a systemic acute phase response, and promoting the release of CRP; an acute phase reactant and inflammatory mediator.[16] CRP functions to propagate atherosclerogenesis via induction of endothelial cell adhesion molecule expression associated with macrophage and T-lymphocyte margination and infiltration at atherosclerotic loci.[17]
MATERIALS AND METHODS
Data collection was based on a stratified cross-sectional study design. The sample population consisted of subjects attending medical outpatient clinics at two major hospitals; Eric Williams Medical Sciences Complex and the San Fernando General Hospital in the Republic of Trinidad, West Indies during the period of August 2012 to May 2013.
All subjects included were aged 45–75 years, African/East Indian ethnicity and no past history of heart, liver or kidney disease. These subjects were either patients attending medical OPC (n > 89.4%) for management of comorbidities including diabetes mellitus, hypertension and hyperlipidemia or elective subjects (n >10.6%) with nil other significant comorbid factors. The protocol for the study was approved by the ethics committee of the faculty of medical sciences, University of the West Indies, St. Augustine Campus, Trinidad. Written consent was obtained from all participating subjects.
Independent risk factors for CVD selected were based on the Adult Treatment Panel III (ATP III) and American Association of Clinical Endocrinologists (AACE) defined criteria for MS, which includes; hypertension (systolic blood pressure >130 mmHg and diastolic blood pressure > 85 mmHg), impaired glucose tolerance (fasting blood glucose [FBS] >110 mg/dL), hyperlipidemia; HDL; (<40 males and <50 females) and TGs (>150 mg/dL), elevated body mass index (BMI) (>25 kg/m2) and elevated WC (>102 cm in males and >88 cm in females). NT-pro-BNP levels (≥125 pg/ml) and high-sensitivity CRP (hs-CRP) levels (≥1.0 mg/dL) were considered elevated.
Venous blood samples of 10.0 ml were collected from all subjects after overnight fasting, centrifuged and the serum was stored at −20°C pending subsequent analysis. All biochemical tests; serum glucose and lipid profile were conducted with the Vitros 950 Clinical Chemistry Analyzer (Johnson and Johnson Vitros 250, Ortho-Clinical Diagnostic Inc., Rochester, NY 14626, USA). NT-pro-BNP levels were measured via Elecys 2012 fully automated immunoassay system by using the NT-pro-BNP reagent kit, supplied by (Johnson and Johnson Vitros 250, Ortho-Clinical Diagnostic Inc., Rochester, NY 14626, USA). The hs-CRP levels were measured with Hitachi 9000 (Rochester, NY 14626, USA) system.
Statistical analysis
Statistical analyses were conducted via SPSS version 23. Data sets consisted of interval ratio data; BMI, WC, systolic blood pressure, diastolic blood pressure, TGs, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, fasting serum; glucose and insulin as well as NT-pro-BNP and hs-CRP. Data was subsequently divided ordinally into dichotomous groups based on whether values were considered normal or elevated. Arithmetic means and standard deviations were assessed to determine the distribution of considered variables. Pearson's rank correlation coefficients were used to assess the univariate significance between NT-pro-BNP and hs-CRP among the African and East Indian sub-populations. Binary logistic regression adjusted for cofounders of age, gender and cigarette smoking was employed to determine if there were any significant odds between the African and East Indian sub-populations and considered features of the MS.
RESULTS
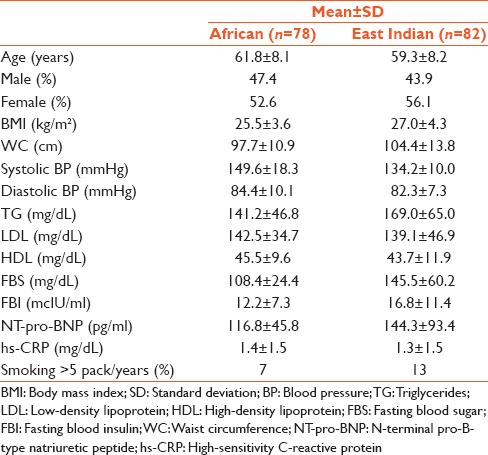
Table 1 illustrates the comparison of various clinical parameters associated with the MS among 160 subjects, 78 of whom were African and 82 East Indian. Both subgroups were well matched for age, gender, BMI, WC, diastolic blood pressure, LDL cholesterol, HDL cholesterol, and hs-CRP. Systolic blood pressure, serum fasting; TGs, glucose and insulin as well as NT-pro-BNP were all elevated among the East Indian sub-population, with only systolic blood pressure being significantly elevated among the African sub-population.
Table 1.
Arithmetic means, SD and percentages for selected variables
Pearson's rank correlation was performed to measure the strength of the association between these independent risk factors for CVD in relation to serum NT-pro-BNP and hs-CRP in both sub-populations. The results are illustrated in Table 2. Both serum NT-pro-BNP and hs-CRP demonstrated significant correlations with respect to the majority of these independent risk factors inclusive of ATP III and AACE defined criteria for MS. NT-pro-BNP as compared to hs-CRP demonstrated stronger association among the East Indian sub-population as compared to that of the African sub-population.
Table 2.
Pearson's correlation coefficients and P values

Binary logistic regression adjusted for confounders of age, gender and cigarette smoking are illustrated in Table 3a and b. hs-CRP [Table 3a] demonstrated significant odds with respect to Systolic and diastolic blood pressure, insulin, cholesterol, TG, HDL, LDL and both ATP III and AACE defined criteria for MS among Africans with only BMI and WC yielding significant odds among the East Indian sub-population. In contrast NT-pro-BNP [Table 3b] demonstrated significant odds with respect to the majority of risk factors BMI, WC, systolic blood pressure, diastolic blood pressure, serum TG, FBS and fasting blood insulin among East Indian sub-population with only systolic blood pressure, diastolic blood pressure and serum TG demonstrating significant odds among the African sub-population. Both sub-populations also demonstrated statistically, significant and equivalent odds with respect to NT-pro-BNP and ATP III and AACE defined criteria for the MS.
Table 3a.
Binary logistic regression hs-CRP adjusted for age, smoking and gender
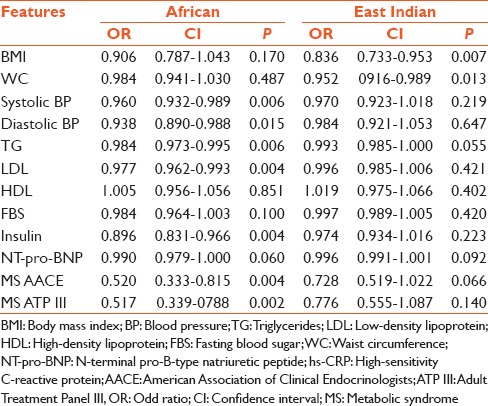
Table 3b.
Binary logistic regression NT-pro-BNP adjusted for age, smoking and gender

DISCUSSION
The impact of ethnicity toward the manifestation of disease, in general, populous holds ground with the advent of various studies proving or disproving, as the case may be, the detectable factors.
Continuing on the early precedence set that serum lipoprotein concentrations correlate with ethnic composition, NT-pro-BNP and hs-CRP from initial evidence, has begun to emerge as a deciding element of diagnosis and thus detection for cardiovascular outcomes.[18,19,20,21] This study was based on the ATP III and AACE defined criteria for MS.[22]
In our study, the MS did not raise the risk for CHD to more than 20% in the absence of diabetes. The presence of MS was highly predictive of new onset diabetes.[22] The Framingham study[23] depicted that the MS is capable of prediction of approximately 25% of all new onset CVD. This being said, by improving risk evaluation, and diagnosis for CVD, sensitivity and specificity of diagnostic regimes are improved, thereby leading to effective management.
Results of this study were indicative of both hs-CRP and NT-pro-BNP having a correlation to MS prevalence, but NT-pro-BNP having greater correlation to the East Indian population than that of African. Based on the Multi-ethnic Study of Atherosclerosis, incident heart failure (HF) and baseline left ventricular (LV) function were related to ethnicity.[24] This allows for the possibility of ethnic differences in the physiologic biomarker response to increased myocardial stress, representative of the underlying cause of NT-pro-BNP production and release, and could be related to genetic factors or exposure to unmeasured environmental confounders.[25] The variation between subgroups could also be secondary to NT-pro-BNP gene polymorphism, genetic predisposition, or the possibility that HF in individuals with low baseline levels of NT-pro-BNP has a different pathogenesis. Therefore, there can be a correlation of elevated BNP with traditional risk factors known to be associated with increased CVD risk, indicating its usefulness as an additional screening test.[26]
The variation of NT-pro-BNP and hs-CRP among the different ethnic groups found in our study may be due to the influence of genetic loci, lifestyle and environmental factors upon the individuals.[27] Studies regarding CRP levels depict that migrant East Indians in the UK presented with higher CRP levels than native East Indians.[28] Furthermore, recent studies have indicated that diabetic Indians have higher mean CRP levels than native Europeans. This is attributed to the increased risk of obesity in Indians and hence a higher chance of adipose tissue cytokinemia.[25] In our study for the African population, only their systolic blood pressure was elevated and thus did not fulfill the criteria outlined by ATP III and AACE. Hence, it can, therefore, be inferred that they did not present with MS and hence the decreased hs-CRP and NT-pro-BNP levels. Superimposition of this information unto a Trinidad populous is reflective by the results obtained in this study.
The fact, therefore, remains that assessment of NT-pro-BNP level 6 months after STEMI remains a good indicator of infarct size and left ventricle function at long-term follow-up.[29] Therefore indicating that East Indians and among the majority of the subgroups, who have had a myocardial infarction. Furthermore, hs-CRP is a useful factor for predicting LV remodeling,[30] indicative of the African subgroup having decreased incidence of LV remodeling.
CONCLUSION
Our study indicated that hs-CRP and NT-pro-BNP are representative of CVD within a Trinidad population. It showed that the East Indian subgroup was more at risk for CVD as evidenced by the fulfillment of the criteria for the diagnosis of MS. Therefore, the hs-CRP and NT-pro-BNP levels can be deemed a suitable marker for MS.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ezenwaka CE, Nwagbara E, Seales D, Okali F, Hussaini S, Raja B, et al. A! comparative study of the prevalence of the metabolic syndrome and its components in type 2 diabetic patients in two Caribbean Islands using the new International Diabetes Federation definition. Arch Physiol Biochem. 2007;113:202–10. doi: 10.1080/13813450701475201. [DOI] [PubMed] [Google Scholar]
- 2.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–38. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 3.Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wilson PW, D’Agostino RB, Levy D, Belanger AM. Sudden coronary death in women. Am Heart J. 1998;136:205–12. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman JC, Donahue RP, Harris MI, Finucane FF, Madans JH, Brock DB. Mortality among diabetics in a national sample. Am J Epidemiol. 1988;128:389–401. doi: 10.1093/oxfordjournals.aje.a114979. [DOI] [PubMed] [Google Scholar]
- 6.Butler R, MacDonald TM, Struthers AD, Morris AD. The clinical implications of diabetic heart disease. Eur Heart J. 1998;19:1617–27. doi: 10.1053/euhj.1998.1284. [DOI] [PubMed] [Google Scholar]
- 7.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 8.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs FD, Davis RC, Roalfe AK, Hare R, Davies MK, Kenkre JE. Reliability of N-terminal pro-brain natriuretic peptide assay in diagnosis of heart failure: Cohort study in representative and high risk community populations. BMJ. 2002;324:1498. doi: 10.1136/bmj.324.7352.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omland T, Aakvaag A, Vik-Mo H. Plasma cardiac natriuretic peptide determination as a screening test for the detection of patients with mild left ventricular impairment. Heart. 1996;76:232–7. doi: 10.1136/hrt.76.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis M, Espiner EA, Yandle T, Richards G, Town I, Neill A, et al. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet. 1994;343:440–4. doi: 10.1016/s0140-6736(94)92690-5. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of NT-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. 2004;27:1929–35. doi: 10.2337/diacare.27.8.1929. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 14.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple risk factor intervention trial. Am J Epidemiol. 1996;144:537–47. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 15.Eikelboom JW, Lonn E, Genest J, Jr, Hankey G, Yusuf S. Homocyst (e) ine and cardiovascular disease: A critical review of the epidemiologic evidence. Ann Intern Med. 1999;131:363–75. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 16.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 17.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–8. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 18.Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62:345–9. doi: 10.2310/JIM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 19.Mahadavan G, Nguyen TH, Horowitz JD. Brain natriuretic peptide: A biomarker for all cardiac disease? Curr Opin Cardiol. 2014;29:160–6. doi: 10.1097/HCO.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 20.Maries L, Manitiu I. Diagnostic and prognostic values of B-type natriuretic peptides (BNP) and N-terminal fragment brain natriuretic peptides (NT-pro-BNP) Cardiovasc J Afr. 2013;24:286–9. doi: 10.5830/CVJA-2013-055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JJ, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, et al. Prognostic value of C-reactive protein as an inflammatory and N-terminal Probrain natriuretic peptide as a neurohumoral marker in acute heart failure (from the Korean Heart Failure registry) Am J Cardiol. 2014;113:511–7. doi: 10.1016/j.amjcard.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 23.Robins SJ, Lyass A, Zachariah JP, Massaro JM, Vasan RS. Insulin resistance and the relationship of a dyslipidemia to coronary heart disease: The Framingham heart study. Arterioscler Thromb Vasc Biol. 2011;31:1208–14. doi: 10.1161/ATVBAHA.110.219055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-ethnic study of atherosclerosis. Circ Heart Fail. 2012;5:727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandyopadhyay R, Paul R, Basu AK, Chakraborty PP, Mitra S. Study of C reactive protein in type 2 diabetes and its relation with various complications from Eastern Indian. J Appl Pharm Sci. 2013;3:156–9. [Google Scholar]
- 26.Shivananda Nayak B, Teelucksingh S, Jagessar A, Maharaj S, Maharaj N. A cross sectional study comparing traditional risk factors with N-terminal pro-BNP in high risk groups for cardiovascular disease in Trinidad, West Indies. Diabetes Metab Syndr. 2013;7:8–11. doi: 10.1016/j.dsx.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408. doi: 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS, et al. Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis. 2006;185:297–306. doi: 10.1016/j.atherosclerosis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Kleczynski P, Legutko J, Rakowski T, Dziewierz A, Siudak Z, Zdzienicka J, et al. Predictive utility of NT-pro BNP for infarct size and left ventricle function after acute myocardial infarction in long-term follow-up. Dis Markers. 2013;34:199–204. doi: 10.3233/DMA-120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehara K, Nomura M, Ozaki Y, Fujinaga H, Ikefuji H, Kimura M, et al. High-sensitivity C-reactive protein and left ventricular remodeling in patients with acute myocardial infarction. Heart Vessels. 2003;18:67–74. doi: 10.1007/s10380-003-0692-2. [DOI] [PubMed] [Google Scholar]


