Abstract
Tooth movement by orthodontic treatment is characterized by remodeling changes in the periodontal ligament, alveolar bone, and gingiva. A reflection of these phenomenons can be found in the gingival crevicular fluid (GCF) of moving teeth, with significant elevations in the concentrations of its components like, cytokines, neurotransmitters, growth Factors, and a arachidonic acid metabolites. GCF arises at the gingival margin and can be described as a transudate or an exudate. Several studies have focused on the composition of GCF and the changes that occur during orthodontic tooth movement (OTM). GCF component analysis is a non-invasive method for studying the cellular response of the underlying periodontium. Clinically, GCF can be easily collected using platinum loops, filter paper strips, gingival washings, and micropipettes. A number of GCF biomarkers involve in bone remodeling during OTM. The data suggest that knowledge of all the biomarkers present in the GCF that can be used to mark the changes in tooth that is undergoing orthodontic treatment may be of clinical usefulness leading to proper choice of mechanical stress to improve and to shorten treatment time and avoid side effects.
KEY WORDS: Biomarkers, gingival crevicular fluid, orthodontic tooth movement, periodontium, remodeling
The key to success of orthodontic treatment needs periodontal health, oral hygiene, and optimal orthodontic forces. New methods have been developed to shorten treatment times, reduce side effects such as pain, periodontal diseases, and minimize iatrogenic damages such as root resorption and the subsequent development of nonvital teeth.
Tooth movement induced by orthodontic force application is characterized by remodeling changes in the dental and periodontal tissues.[1] Two interrelated processes involved in orthodontic tooth movement (OTM) are (1) bone bending (2) remodeling of the periodontal tissues, including the dental pulp, periodontal ligament (PDL), alveolar bone, and gingiva. The applied force causes the compression of the alveolar bone and the PDL on one side (pressure), while on the opposite side the PDL is stretched (tension).[2]
Orthodontic forces changes periodontal tissue vascularity leading to the synthesis of various signaling molecules and metabolites. The released molecules generate cellular responses around the teeth, providing a favorable microbiological environment for tissue deposition or resorption.[1] Various cell-signaling pathways are initiated, which ultimately stimulate PDL turnover, as well as localized bone resorption and bone deposition.[3]
Phases of Orthodontic Tooth Movement
In 1962, Burstone[4] said that, if the rates of tooth movement are plotted against time, there will be three phases of tooth movement:
Initial phase
Lag phase
Post lag phase.
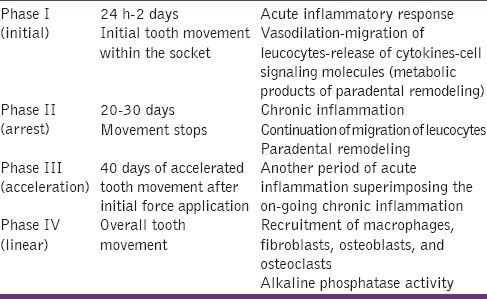
Pilon et al.[5,6] recently divided the curve of tooth movement into four phases [Table 1]. Biomarkers reflecting these phases and signaling pathways can be found in the gingival crevicular fluid (GCF) of moving teeth, where significant elevations in the concentrations of inflammatory mediators, such as cytokines and prostaglandins, occur temporally.
Table 1.
Phases of orthodontic tooth movement (Pilon et al.[5])
Gingival Crevicular Fluid
Gingival crevicular fluid is an exudate that can be harvested from the gingival sulcus, which offers a great potential as a source of factors associated with changes and destruction in the underlying periodontium due to orthodontic force application.
Orthodontic tooth movement have used GCF because of its noninvasive nature and ease of repetitive sampling from the same site with the help of platinum loops, filter paper strips, gingival washings, and micropipettes. The fluid is used to analyze various biochemical markers, which will be discussed later in this paper in detail.
Biomarkers of Orthodontic Tooth Movement
A biomarker is a substance that is measured and evaluated objectively as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.[7]
A good biomarker should be specific and sensitive and have the ability to inform about the biological condition in terms of periodontal tissue changes and their relationships with the particular phase of OTM. Knowledge about the type of cellular process will give a good idea of giving proper mechanical loading and thus shorten the period of treatment, which can also aid in avoiding adverse effects associated with orthodontic treatment.
In this paper, the importance of evaluating the levels of substances as valid biomarkers of periodontal effects of an orthodontic treatment is shown, through an proper description of the specific role of each of them [Table 2]. Citations to the various markers were done by going through numerous journals, PubMed, databases, and various scientific textbooks. We also did some journal search and discussed with experts in this field to provide sufficient literature.
Table 2.
List of GCF biomarkers and their role in orthodontic tooth movement

Metabolic Products of Paradental Remodeling
Glycosaminoglycans
Glycosaminoglycans (GAG) in GCF were investigated by Last et al. as a major band of hyaluronic acid and a minor band of chondroitin sulfate.[8]
Samuels et al.[9] studied the GCF in children around a canine tooth, before and during OTM to identify and quantify the glycosaminoglycan components of GCF and relate them to tooth movement, gingivitis, plaque accumulation, pocket probing depth, and GCF volume recorded at the site of sampling.
Pender et al.[10] investigated the changes in the flow of GCF and its glycosaminoglycan components at three stages of orthodontic treatment, before orthodontic treatment, during canine retraction, and in retention, to relate them to tooth movement.
Both the studies concluded that the increase in GCF volume during OTM and the decrease during retention were only partly due to changes in the severity of gingival inflammation.
Pyridinium derivatives
The pyridinium derivatives, pyridinoline (Pyr), and deoxypyridinoline (dPyr), are structural elements that bind together collagen chains. Pyr is abundant in skeletal tissues, whereas dPyr is a minor component found predominantly in bone and dentin. These two molecules are used as markers to evaluate bone resorption in such cases as Paget's disease and primary hyperparathyroidism.[11]
Pentraxin-3, also known as tumor necrosis factor-stimulated gene 14
Pentraxin-3 (PTX-3), also known as tumor necrosis factor (TNF)-stimulated gene 14 (TSG-14), is a 45-kDa glycoprotein with a 202 amino acids Surlin et al.[12] measured the levels of PTX-3 in GCF in orthodontic young and adult patients in the first 2 weeks after the orthodontic appliance showing an increased GCF levels of PTX-3 suggesting PTX-3 involvement in periodontal orthodontic remodeling and the aseptic inflammation induced by the orthodontic forces.
N-telopeptide type 1 and osteocalcin
N-telopeptide (NTx) is a specific marker of bone resorption because of its cross-linked a-2 (I) NTx. When multiple biochemical markers of bone turnover were compared, NTx was found to be a more sensitive measure of bone resorption. Hence, NTx might be an important marker of active periodontal bone loss and could be useful for analyzing site-specific responses to periodontal therapy.
Osteocalcin is a noncollagenous matrix protein of calcifying and calcified tissue. It is produced by osteoblasts and has been described as the most specific marker of osteoblast function.
Osteocalcin has been found in GCF from patients with periodontal disease: And increases in GCF osteocalcin concentration were associated with high bone turnover, assessed by digitized radiography and bone-seeking radiopharmaceutical uptake. Therefore, it remains to be established whether osteocalcin GCF levels can be used as markers of bone turnover.[13]
Matrix metalloproteins 1 and 8
Matrix metalloproteins (MMPs) are chemokines may contribute to differential bone remodeling in response to orthodontic forces through the establishment of distinct microenvironments in the sites of both compression and tension.[14] MMP protein was induced by compression and increased significantly with time, reaching a peak after 8 h of application of force. On the tension side, MMP was significantly increased after 1-h but gradually returned to basal levels within 8 h.[15] This result indicates that MMP-2 could be used during very early stages of orthodontic treatment as a marker for active tooth movement.
Inflammatory mediators
Prostaglandin E
Prostaglandin E (PGE2), specially, is able to mediate inflammatory responses and induce bone resorption by activating osteoclastic cells.[16] They directly stimulate osteoclast production and form ruffled border to affect bone resorption. In addition, the GCF level of PGE2 reflect the biologic activity in periodontium during OTM, and it is significantly high in both tension and compression sides.[17] Clinical and animal studies[18,19] by various authors have identified PGE1 and PGE2 role in stimulating bone resorption.
Neuropeptides (calcitonin related gene peptide and substance p)
The peripheral sensory nervous system contributes to the development of acute and chronic inflammatory processes through the local release of neuropeptides. Various neuropeptides, including substance P (SP) and calcitonin gene-related peptide, are known to be present in the nerve fibers in the tooth pulp and the periodontium in rats, cats, monkeys, and humans.[20,21,22,23,24,25] With the application of physiologic orthodontic force, SP increases production of proinflammatory cytokines and formation of osteoclasts in dental pulp fibroblasts in patients with severe orthodontic root resorption.[26] Sohn[27] reported that SP activated the osteoclastogenesis in osteoclast precursors (bone marrow macrophages).
Transforming growth factor-α1
Transforming growth factor is a family of polypeptides produced by cells within the periodontium involved in many biologic activities, including cell growth, differentiation, and apoptosis, as well as in developmental processes and bone remodeling.[28] Uematsu et al.[29] reported the presence of TGF- α1 in GCF during OTM. His study showed a rapid and transient increase associated with elevation of cytokine levels and may reflect early stages of tooth mobilization.
Epidermal growth factor
Epidermal growth factor (EGF) is another cytokine possibly associated with bone remodeling. Fibroblasts and stromal cells produce it.[30] Uematsu et al.[31] in a study reported a transient elevation of EGF levels in GCF after application of mechanical stress of an experimental tooth.
α2 Microglobulin and insulin-like growth factor-1
α2 microglobulin (α2MG) is considered a mediator with a considerable role in the inflammatory response, because of its association with major histocompatibility complex, class I, as well as because of its similarity in amino acid sequence to the constant region of the immunoglobulin chain. Although it is produced in other tissues besides bone, α1MG enhances the biologic action of insulin-like growth factor-I (lGF).[30] They are a family of peptides that promote cell proliferation and differentiation and have insulin-like metabolic effects. They have been associated with stimulation of the osteoblasts and its functions.[32]
Therefore, an increase in α2-MG concentration leads to enhancement of bone deposition activity. Uematsu et al.[30]-found an increase in αMG levels in the GCF after mechanical orthodontic stimulation.
Interleukin-1 (receptor antagonist) 1β, 2, 6, 8
Interleukin-1 (IL-1) are cytokines that affect bone metabolism and OTM, has 2 forms – α and β – that code different genes have similar actions. These actions include attracting leukocytes and stimulating endothelial cells, fibroblasts, osteoclasts, and osteoblasts to enhance bone resorption and inhibit bone formation.[33] Osteoblasts are target cells for IL-1, which in turn conveys messages to osteoclasts to resorb bone.[34] Tuncer et al.[35] reported increased levels of IL-8 at PDL tension sites and proposed it to be a triggering factor for bone remodeling. Another cytokine of the IL family with a stimulatory effect on bone remodeling and osteoclast formation is Il-6.[30]
Tumor necrosis factor
Tumor necrosis factor-α, another pro-inflammatory cytokine, was shown to elicit acute or chronic inflammation and stimulate bone resorption. Studies[36,37,38,39] have shown that TNFα directly stimulates the differentiation of osteoclast progenitors to osteoclasts in the presence of macrophage colony-stimulating factor (M-CSF). Davidovitch et al.[36] and Saito et al.[37] demonstrated marked increases in TNFα in cells of the PDL and alveolar bone during OTM in cats.
Macrophages colony stimulating factors
Colony-stimulating factors are specific glycoproteins, which interact to regulate production, maturation, and function of monocyte-macrophages-CSF (M-CSF) as well as granulocytes-CSF (G-CSF). They might have implications in bone remodeling and thereby during tooth movement.[16] An important implication in tooth movement is played by the M-CSF through an increased early osteoclastic recruitment and differentiation.[40] In the future, optimal dosages of M-CSF already correlated with measurable changes in tooth movement and gene expression will provide a great potential in accelerating clinically the rate of tooth movement.
Receptor activator of nuclear factor-kappa/receptor activator of nuclear factor-kappa ligand/osteoprotegerin system
The TNF-related ligand receptor activator of nuclear factor-kappa ligand (RANKL) and its two receptors, receptor activator of nuclear factor-kappa (RANK), and osteoprotegerin (OPG), are known for involvement in bone remodeling process.
Receptor activator of nuclear factor-kappa ligand is a downstream regulator of osteoclast formation and activation, through which hormones and cytokines produce their osteoresorptive effect. In the bone system, RANKL is expressed on osteoblast cell lineage and exerts its effect by binding the RANK receptor on osteoclast lineage cells. This binding leads to rapid differentiation of hematopoietic osteoclast precursors to mature osteoclasts.
Osteoprotegerin is a decoy receptor produced by osteoblastic cells, which compete with RANK for RANKL binding. The biologic effects of OPG on bone cells include inhibition of terminal stages of osteoclast differentiation, suppression of the activation of matrix osteoclasts, and induction of apoptosis.[41,42] Kanzaki et al.[43] reported recently that OPG gene transfer to periodontal tissues inhibited RANKL-mediated osteoclastogenesis and inhibited experimental tooth movement in rats. Thus, the inhibition of the activity of RANKL in its promoting osteoclast differentiation could be very helpful in preventing movement of anchor teeth during orthodontic treatment and a relapse during the post treatment period.
Myeloperoxidase
Myeloperoxidase (MPO) is an enzyme found in polymorphonuclear neutrophil (PMN) granules and can be used to estimate the number of PMN granules in the tissues. Mean MPO activity increased in both the GCF and saliva of orthodontic patients 2 h after appliance activation and they might be a good biomarker to assess inflammation in orthodontic movement.[44]
Markers of root resorption
Early detection of root resorption during orthodontic treatment is essential for identifying teeth at risk of severe resorption.[45] At present, detection of root resorption is obtained using radiographic techniques which are technique sensitive require radiation exposure.
Dentin consists of noncollagenous proteins such as dentine matrix protein 1, dentin phosphoprotein (DPP), and dentin sialoprotein (DSP). DPP and DSP are products of mRNA transcription and are portions of one expressed protein known as dentin sialophosphoprotein. Examination of patients undergoing active orthodontic treatment showed elevated levels of DPP relative to the control group.[46] Kereshanan et al.[47] reported the potential for measuring DSP in GCF as a biomarker to monitor root resorption. Results showed that DSP was raised at sites that were undergoing physiological resorption compared with the nonresorbing controls.
Enzymes and enzyme inhibitors
Cathepsin B
Cathepsin B, an intracellular lysosomal enzyme is known to play an important role in the initiation and perpetuation of inflammatory processes. The accumulation of cathepsin B in GCF has been shown to increase with OTM. They were increased around osteoclasts and played a role in the decomposition of exposed collagen fibers and collagen degradation byproducts.[48]
Acid phosphatase and alkaline phosphatase
Bone metabolism is associated with alkaline phosphatase (ALP) and acid phosphatase (ACP), expressed, respectively, by osteoblasts and osteoclasts.
Alkaline phosphatase is a ubiquitous tetrameric enzyme, localized outside the cell membrane.[49] ALP activity is found at much higher levels in PDL than in other connective tissues.[50] In conjunction with bone formation increased levels of ALP were detected in human GCF collected from orthodontically treated patients. Therefore, they might have biological activities in the early stages of tooth movement.[51,52] Insoft et al.[53] performed a longitudinal study on three subjects, with data showing early elevations in ALP activity during the time when little tooth movement occurs and an increase in ACP activity that coincided with maximum tooth movement.
Enzymes of high cellular activity
In general, a high enzyme activity is suggestive of a greater cellular activity.[54] An apoptotic process occurs to eliminate the hyalinized periodontal tissue formed during the early stages of orthodontic movement. Among mediators of the apoptotic response activated by alterations in the intracellular ionic milieu, the most relevant are as follows:
β-Glucuronidase
A biomarker of primary granule release from polymorphonuclear leukocytes is the lysosomal enzyme β-glucuronidase (βG). Increased levels of this enzyme have been found in the GCF of adolescents treated with the rapid maxillary expander. Moreover, βG, as other biochemical mediators like IL-1β, responds to direct and indirect application of mechanical force to teeth, with an increased level that is higher than following stronger forces.[55]
Aspartate aminotransferase and lactate dehydrogenase
Aspartate aminotransferase (AST) and lactate dehydrogenase activities in GCF have been measured to confirm the biological activity, which occurs in the periodontium during orthodontic treatment. They are soluble enzymes normally confined to the cytoplasm of cells then released to the extracellular environment after cell necrosis. The GCF AST activity is significantly elevated in both tension and compression sites at days 7 and 14. This rise is explained as a consequence of a controlled trauma, which produces cell death as a consequence of mechanical force exerted on alveolar bone and PDL.[56] It positively relates with compression sites caused by an OTM.[57]
Conclusion
On the basis of sequential reactions and released substances, numerous substances have been proposed as biomarkers. This will give a better understanding of the ongoing cellular process during orthodontic treatment. We also have to remember that the development of biomarkers will go on and will keep providing vital information of the microenvironment. Thus, the data suggest that knowledge of all the biomarkers present in the GCF that can be used to mark the changes in tooth that is undergoing orthodontic treatment may be of clinical usefulness leading to proper choice of mechanical loading, to improve and to shorten the period of treatment, avoiding adverse consequences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469.e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Dolce C, Malone JS, Wheeler TT. Current concepts in the biology of orthodontic tooth movement. Semin Orthod. 2002;8:6–12. [Google Scholar]
- 3.Bartzela T, Türp JC, Motschall E, Maltha JC. Medication effects on the rate of orthodontic tooth movement: A systematic literature review. Am J Orthod Dentofacial Orthop. 2009;135:16–26. doi: 10.1016/j.ajodo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Burstone CJ. The biomechanics of tooth movement. In: Kraus BS, Riedel RA, editors. Vistas in Orthodontics. Philadelphia: Lea and Febiger; 1962. [Google Scholar]
- 5.Pilon JJ, Kuijpers-Jagtman AM, Maltha JC. Magnitude of orthodontic forces and rate of bodily tooth movement. An experimental study. Am J Orthod Dentofacial Orthop. 1996;110:16–23. doi: 10.1016/s0889-5406(96)70082-3. [DOI] [PubMed] [Google Scholar]
- 6.van Leeuwen EJ, Maltha JC, Kuijpers-Jagtman AM. Tooth movement with light continuous and discontinuous forces in beagle dogs. Eur J Oral Sci. 1999;107:468–74. doi: 10.1046/j.0909-8836.1999.eos107608.x. [DOI] [PubMed] [Google Scholar]
- 7.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–71. doi: 10.1016/j.cden.2005.03.009. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Last KS, Donkin C, Embery G. Glycosaminoglycans in human gingival crevicular fluid during orthodontic movement. Arch Oral Biol. 1988;33:907–12. doi: 10.1016/0003-9969(88)90021-0. [DOI] [PubMed] [Google Scholar]
- 9.Samuels RH, Pender N, Last KS. The effects of orthodontic tooth movement on the glycosaminoglycan components of gingival crevicular fluid. J Clin Periodontol. 1993;20:371–7. doi: 10.1111/j.1600-051x.1993.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 10.Pender N, Samuels RH, Last KS. The monitoring of orthodontic tooth movement over a 2-year period by analysis of gingival crevicular fluid. Eur J Orthod. 1994;16:511–20. doi: 10.1093/ejo/16.6.511. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths GS, Moulson AM, Petrie A, James IT. Evaluation of osteocalcin and pyridinium crosslinks of bone collagen as markers of bone turnover in gingival crevicular fluid during different stages of orthodontic treatment. J Clin Periodontol. 1998;25:492–8. doi: 10.1111/j.1600-051x.1998.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 12.Surlin P, Rauten AM, Silosi I, Foia L. Pentraxin-3 levels in gingival crevicular fluid during orthodontic tooth movement in young and adult patients. Angle Orthod. 2012;82:833–8. doi: 10.2319/072911-478.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfaqeeh SA, Anil S. Osteocalcin and N-telopeptides of type I collagen marker levels in gingival crevicular fluid during different stages of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2011;139:e553–9. doi: 10.1016/j.ajodo.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Garlet TP, Coelho U, Repeke CE, Silva JS, Cunha Fde Q, Garlet GP. Differential expression of osteoblast and osteoclast chemmoatractants in compression and tension sides during orthodontic movement. Cytokine. 2008;42:330–5. doi: 10.1016/j.cyto.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Cantarella G, Cantarella R, Caltabiano M, Risuglia N, Bernardini R, Leonardi R. Levels of matrix metalloproteinases 1 and 2 in human gingival crevicular fluid during initial tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:568.e11–6. doi: 10.1016/j.ajodo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Grieve WG, 3rd, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369–74. doi: 10.1016/s0889-5406(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 17.Dudic A, Kiliaridis S, Mombelli A, Giannopoulou C. Composition changes in gingival crevicular fluid during orthodontic tooth movement: Comparisons between tension and compression sides. Eur J Oral Sci. 2006;114:416–22. doi: 10.1111/j.1600-0722.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee WC. Experimental study of the effect of prostaglandin administration on tooth movement – WSith particular emphasis on the relationship to the method of PGE1 administration. Am J Orthod Dentofacial Orthop. 1990;98:231–41. doi: 10.1016/s0889-5406(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 19.Klein DC, Raisz LG. Prostaglandins: Stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–40. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- 20.Olgart L, Gazelius B. Effects of adrenaline and felypressin (octapressin) on blood flow and sensory nerve activity in the tooth. Acta Odontol Scand. 1977;35:69–75. doi: 10.3109/00016357709055992. [DOI] [PubMed] [Google Scholar]
- 21.Wakisaka S, Nishikawa S, Ichikawa H, Matsuo S, Takano Y, Akai M. The distribution and origin of substance P-like immunoreactivity in the rat molar pulp and periodontal tissues. Arch Oral Biol. 1985;30:813–8. doi: 10.1016/0003-9969(85)90136-0. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JD, Kruger L. An interpretation of dental innervation based upon the pattern of calcitonin gene-related peptide (CGRP)-immunoreactive thin sensory axons. Somatosens Res. 1987;5:157–75. doi: 10.3109/07367228709144624. [DOI] [PubMed] [Google Scholar]
- 23.Byers MR, Mecifi KB, Kimberly CL. Numerous nerves with calcitonin gene-related peptide-like immunoreactivity innervate junctional epithelium of rats. Brain Res. 1987;419:311–4. doi: 10.1016/0006-8993(87)90598-1. [DOI] [PubMed] [Google Scholar]
- 24.Luthman J, Dahllöf G, Modèer T, Johansson O. Immunohistochemical study of neuronal markers in human gingiva with phenytoin-induced overgrowth. Scand J Dent Res. 1988;96:339–46. doi: 10.1111/j.1600-0722.1988.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 25.Casasco A, Calligaro A, Casasco M, Springall DR, Polak JM, Poggi P, et al. Peptidergic nerves in human dental pulp. An immunocytochemical study. Histochemistry. 1990;95:115–21. doi: 10.1007/BF00266583. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi M, Ozawa Y, Mishima H, Aihara N, Kojima T, Kasai K. Substance P increases production of proinflammatory cytokines and formation of osteoclasts in dental pulp fibroblasts in patients with severe orthodontic root resorption. Am J Orthod Dentofacial Orthop. 2008;133:690–8. doi: 10.1016/j.ajodo.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Sohn SJ. Substance P upregulates osteoclastogenesis by activating nuclear factor kappa B in osteoclast precursors. Acta Otolaryngol. 2005;125:130–3. doi: 10.1080/00016480410017710. [DOI] [PubMed] [Google Scholar]
- 28.Derringer KA, Linden RW. Vascular endothelial growth factor, fibroblast growth factor 2, platelet derived growth factor and transforming growth factor beta released in human dental pulp following orthodontic force. Arch Oral Biol. 2004;49:631–41. doi: 10.1016/j.archoralbio.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Uematsu S, Mogi M, Deguchi T. Increase of transforming growth factor-beta 1 in gingival crevicular fluid during human orthodontic tooth movement. Arch Oral Biol. 1996;41:1091–5. doi: 10.1016/s0003-9969(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 30.Uematsu S, Mogi M, Deguchi T. Cytokine levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. In: Davidovitch Z, Norton LA, editors. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Boston: Harvard Society for the Advancement of Orthodontics; 1996. pp. 223–32. [Google Scholar]
- 31.Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562–7. doi: 10.1177/00220345960750010801. [DOI] [PubMed] [Google Scholar]
- 32.Davidovitch Z. Cell histology associated with orthodontic tooth movement. In: Berkovitz BB, Moxham BJ, Newman HN, editors. The Periodontal Ligament in Health and Disease. St. Louis: Moshy Wolfe; 1995. [Google Scholar]
- 33.Sabatini M, Boyce B, Aufdemorte T, Bonewald L, Mundy GR. Infusions of recombinant human interleukins 1 alpha and 1 beta cause hypercalcemia in normal mice. Proc Natl Acad Sci U S A. 1988;85:5235–9. doi: 10.1073/pnas.85.14.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidovitch Z. Cell biology associated with orthodontic tooth movement. In: Berkovitz BB, Moxham BJ, Newman HN, editors. The Periodontal Ligament in Health and Disease. St. Louis: Mosby; 1995. [Google Scholar]
- 35.Tuncer BB, Ozmeriç N, Tuncer C, Teoman I, Cakilci B, Yücel A, et al. Levels of interleukin-8 during tooth movement. Angle Orthod. 2005;75:631–6. doi: 10.1043/0003-3219(2005)75[631:LOIDTM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Davidovitch Z, Nicolay OF, Ngan PW, Shanfeld JL. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988;32:411–35. [PubMed] [Google Scholar]
- 37.Saito S, Ngan P, Saito M, Kim K, Lanese R, Shanfeld J, et al. Effects of cytokines on prostaglandin E and cAMP levels in human periodontal ligament fibroblasts in vitro. Arch Oral Biol. 1990;35:387–95. doi: 10.1016/0003-9969(90)90186-e. [DOI] [PubMed] [Google Scholar]
- 38.Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. 2001;119:307–12. doi: 10.1067/mod.2001.110809. [DOI] [PubMed] [Google Scholar]
- 39.Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic movement induces high numbers of cells expressing IFN-gamma at mRNA and protein levels. J Interferon Cytokine Res. 2000;20:7–12. doi: 10.1089/107999000312685. [DOI] [PubMed] [Google Scholar]
- 40.Brooks PJ, Heckler AF, Wei K, Gong SG. M-CSF accelerates orthodontic tooth movement by targeting preosteoclasts in mice. Angle Orthod. 2011;81:277–83. doi: 10.2319/051210-258.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Low E, Kharbanda O, Zoellner H, Darendeliler A. Genetic Expression of RANK/RANKL and OPG During Root Resorption Following Orthodontic Treatment. [Last accessed on 2005 Oct 01]. Available from: http://www.chsusydeduau/conf2002/minipost/av-low.pdf .
- 42.Drugarin D, Drugarin M, Negru S, Cioace R. RANK-RANKL/OPG molecular complex-control factors in bone remodelling. TMJ. 2003;53:296–302. [Google Scholar]
- 43.Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83:920–5. doi: 10.1177/154405910408301206. [DOI] [PubMed] [Google Scholar]
- 44.Marcaccini AM, Amato PA, Leão FV, Gerlach RF, Ferreira JT. Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am J Orthod Dentofacial Orthop. 2010;138:613–6. doi: 10.1016/j.ajodo.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Levander E, Malmgren O. Evaluation of the risk of root resorption during orthodontic treatment: A study of upper incisors. Eur J Orthod. 1988;10:30–8. doi: 10.1093/ejo/10.1.30. [DOI] [PubMed] [Google Scholar]
- 46.Mah J, Prasad N. Dentine phosphoproteins in gingival crevicular fluid during root resorption. Eur J Orthod. 2004;26:25–30. doi: 10.1093/ejo/26.1.25. [DOI] [PubMed] [Google Scholar]
- 47.Kereshanan S, Stephenson P, Waddington R. Identification of dentine sialoprotein in gingival crevicular fluid during physiological root resorption and orthodontic tooth movement. Eur J Orthod. 2008;30:307–14. doi: 10.1093/ejo/cjn024. [DOI] [PubMed] [Google Scholar]
- 48.Rhee SH, Kang J, Nahm DS. Cystatins and cathepsin B during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2009;135:99–105. doi: 10.1016/j.ajodo.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Groeneveld MC, Everts V, Beertsen W. Alkaline phosphatase activity in the periodontal ligament and gingiva of the rat molar: Its relation to cementum formation. J Dent Res. 1995;74:1374–81. doi: 10.1177/00220345950740070901. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi M, Shimizu N, Shibata Y, Abiko Y. Effects of different magnitudes of tension-force on alkaline phosphatase activity in periodontal ligament cells. J Dent Res. 1996;75:889–94. doi: 10.1177/00220345960750030501. [DOI] [PubMed] [Google Scholar]
- 51.Perinetti G, Paolantonio M, D’Attilio M, D’Archivio D, Tripodi D, Femminella B, et al. Alkaline phosphatase activity in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2002;122:548–56. doi: 10.1067/mod.2002.126154. [DOI] [PubMed] [Google Scholar]
- 52.Asma AA, Megat R, Wahab A, Zainal Ariffin SH. Crevicular alkaline phosphatase activity during orthodontic tooth movement: Canine retraction stage. J Med Sci. 2008;8:228–33. [Google Scholar]
- 53.Insoft M, King GJ, Keeling SD. The measurement of acid and alkaline phosphatase in gingival crevicular fluid during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1996;109:287–96. doi: 10.1016/s0889-5406(96)70152-x. [DOI] [PubMed] [Google Scholar]
- 54.Watts NB. Clinical utility of biochemical markers of bone remodeling. Clin Chem. 1999;45:1359–68. [PubMed] [Google Scholar]
- 55.Tzannetou S, Efstratiadis S, Nicolay O, Grbic J, Lamster I. Comparison of levels of inflammatory mediators IL-1beta and betaG in gingival crevicular fluid from molars, premolars, and incisors during rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2008;133:699–707. doi: 10.1016/j.ajodo.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 56.Perinetti G, Paolantonio M, D’Attilio M, D’Archivio D, Dolci M, Femminella B, et al. Aspartate aminotransferase activity in gingival crevicular fluid during orthodontic treatment. A controlled short-term longitudinal study. J Periodontol. 2003;74:145–52. doi: 10.1902/jop.2003.74.2.145. [DOI] [PubMed] [Google Scholar]
- 57.Perinetti G, Serra E, Paolantonio M, Bruè C, Meo SD, Filippi MR, et al. Lactate dehydrogenase activity in human gingival crevicular fluid during orthodontic treatment: A controlled, short-term longitudinal study. J Periodontol. 2005;76:411–7. doi: 10.1902/jop.2005.76.3.411. [DOI] [PubMed] [Google Scholar]


