Abstract
Oral cancer which is a subtype of head and neck, cancer is any neoplastic tissue growth in the oral cavity. It comprises an abnormal mass of cells that foists genetic mutation and impedes the normal cell cycle, resulting in its unrestrained growth. Various studies on the plausible link between oral microbial flora and cancer notwithstanding, our understanding of their link remains obscure and inadequate. The multitude of mechanisms by which the microflora initiate or spur Carcinogenesis are still under study and scrutiny. As is widely known, the oral cavity is an abode to a wide assortment of microbes, each present in contrasting amounts. It is observed that increased growth of the microflora is concomitant with known clinical risk factors for oral cancer. Manifold bacterial species have been found to interfere directly with eukaryotic cellular signaling, adopting a style typical of tumor promoters. Bacteria are also known to impede apoptosis thereby potentially promoting carcinogenesis. The viral role in carcinogenesis (by annulling of p53 tumor suppressor gene and other cellular proteins with subsequent alteration in host genome function) is well documented. Furthermore, the changes occurring in the commensal microflora in accompaniment with cancer development could possibly be used as a diagnostic indicator for early cancer detection. The intention of this review is to obtain a better understanding of the “role” that micro-organisms play in oral cancer etiology.
KEY WORDS: Candida albicans, human papillomavirus, oral cancer, bacteria
Since time immemorial, micro-organisms have been presumed to have an etiological role in the evolution of cancer which by default, includes Oral cancer. The chief micro-organisms which purportedly have a role in causing cancer are bacteria, viruses, and fungi. Cancer (neoplasm) is the uncontrolled growth of abnormal cells in the body.
British Oncologist R.A. Willis (1952) defined it thus - “A neoplasm is an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of the normal tissues, and persists in the same excessive manner after cessation of the stimulus which evoked the change.”
Numerous speculations indicating a link between cancer and bacteria, fungi and viruses have been put forth in the past few decades. Different bacteria have been proposed to induce carcinogenesis either through induction of chronic inflammation or by interference with signaling pathways and cell cycles or by metabolism of potentially carcinogenic substances like acetaldehyde (ACH) causing mutagenesis. Significant salivary specificity is noted in specific bacterial species, notably Streptococcus mitis, Capnocytophaga gingivalis, Prevotella melaninogenica, in oral cancer patients. Thus, these species can be used as salivary markers for the early detection of oral cancers, improving the survival rate considerably. Such a high degree of bacterial specificity in oral cancers has also provoked the designing of new treatment options for cancer prevention by way of vaccine delivery.
In the oral cavity, candidiasis is the most frequent opportunistic fungal infection. Reports of a possible association between Candida and oral cancer have been in vogue for quite some time now although their exact role remains unclear. In this study, we examine the hypothetical causal role of candidiasis in oral precancer and cancer and suggest that Candida along with other co-factors may influence the initiation and promotion of carcinogenesis.
One of the most common virus groups in the world today affecting the skin and mucosal areas of the body is the human papillomavirus (HPV). There are strong indications that DNA viruses could be involved in oral cancers. Papillomaviruses are found in many oral cancers and are also capable of transforming cells to a malignant phenotype. We will also attempt to examine the link between viruses (chiefly Epstein–Barr virus [EBV] and HPV) and oral cancers and the plausibility of various hypotheses based on the available data.
The Bacteria-oral Cancer Nexus
It is vital to fully define the normal oral microbial flora before we begin to understand the role of bacteria in oral cancer as there is a distinctive predominant healthy Bacterial flora of the oral cavity that is site-specific and highly diverse before delving into their apparent role in tumorigenesis.
More than 700 bacterial species or phylotypes, of which over 50% have not been cultivated, have been detected in the oral cavity. Aas et al.,[1] carried out an extensive study in this regard. It is important to note that micro-organisms in the oral cavity are responsible for various oral diseases, and an existence of an inter-relationship between the two is strongly hinted at.
Takahashi[2] attempted to study the microbial ecosystem of the oral cavity and its relationship with various oral diseases.
Gendron et al.,(2000)[3] opined that oral cavity is a reservoir of bacterial pathogens that can provoke focal infections.
Hooper et al. (2006)[4] studied viable bacteria present within oral squamous cell carcinoma tissue.
Chambers et al., (2005)[5] conducted a pilot study to examine elevated levels of Mutans streptococci in xerostomic cancer patients after pilocarpine therapy.
Hooper et al. (2007)[4] studied viable bacteria present within oral squamous cell carcinoma tissue.
Hsu et al., (2004)[6] studied the induction of apoptosis in oral cancer cells.
Lax et al., (2002)[7] attempted to explain how bacteria could cause cancer.
Lee et al. (2010)[8] explored the relevance of Human papillomavirus (HPV) infection to carcinogenesis of oral tongue cancer.
Mager et al.,(2005)[9] tried to determine if the salivary counts of 40 common oral bacteria in subjects with an oral squamous cell carcinoma (OSCC) lesion would differ from those found in cancer-free (OSCC-free) controls.
Mager et al.,(2006)[10] attempted to study the alleged relationship between bacteria and human cancers.
Mager et al.,(2005)[11] studied the various bacteria linked to oral cancers.
Nagy et al.,[12] contended that since alteration in the oral microflora demographics consequently led to local and systemic infections in patients suffering from oral neoplasms, an investigative study on the inhibition of biofilm present on the surfaces of oral squamous cell carcinomas (OSCC's) was warranted. Thus, anticancer therapy, irradiation, chemotherapy or surgery compromises the defense mechanism of the oral mucosa and is accompanied by a proliferation of the mucosal biofilm with an overgrowth of yeast and bacteria. This study was designed to probe the inhibition of the biofilm present on the surface of OSCCs. Concerning the organisms found at the tumor site before and after rinsing (with GABA Deutschland manufacture Meridol (Basel, Switzerland), S. mitis, Staphylococcus aureus and Enterococcus faecalis were isolated from at least twice as many tumor surfaces before the rinsing. Of the aerobic Gram-negative species isolated, Haemophilus influenzae, Neisseria spp., and Serratia spp. were found more frequently before rinsing than after. Furthermore, Campylobacter, Actinobacillus actinomycetemcomitans and Capnocytophaga were found more frequently and Porphyromonas at the same frequency before Meridol rinsing. Of the Gram-positive anaerobes, Clostridium was the only species isolated exclusively before rinsing from the tumor surface. Candida albicans were isolated in every sample before rinsing, but only in two patients after rinsing. This study has shown that the cancer lesion itself may greatly increase the local and systemic infection risk to oral cancer patients, even before specific tumor treatment. Sharma[13] conducted a comparative study of saliva from patients with OSCC and healthy individuals to try and establish the conjecture that the saliva of patients with oral cancers have raised concentrations of certain bacteria and these bacteria can be used as possible diagnostic tools in oral cancer detection.
A comparative study of saliva from 45 patients with OSCC and 229 healthy controls showed that levels of six common bacteria species – P. melaninogenica, Leptotrichia buccalis, Capnocytophaga ochracea, C. gingivalis, Eubacterium saburreum, and S. mitis – were significantly higher in patients than in controls. When three of these species (C. gingivalis, P. melaninogenica, and S mitis) were used as diagnostic indicators, they correctly predicted 80% of individuals with oral cancer and 83% of controls. The authors state that alterations in tumor-cell receptors could change the adhesion of some species of bacteria. Donna Mager (The Forsyth Institute, Boston, MA, USA) is unsure whether these bacteria are simple markers for OSCC or have so co-carcinogenic effects. This encouraging study gives credence to the belief that a reliable noninvasive tool for oral cancer detection may not be a pipe dream anymore.
Sixou et al.,[14] ventured out to review literature devoted to quantitative and qualitative variations in the flora of the oral cavity during immunosuppressive treatment of cancer patients.
Cloke et al.,[15] assessed patients who contracted wound infection following tissue transfer in head and neck oncological treatments.
Sakamoto et al.,[16] attempted to investigate the association between postoperative infection, colonization of certain bacterial species and its translocation to the cervical lymph nodes in oral cancer patients.
Mayrand et al., (1998)[17] studied bacterial interactions in periodontal diseases.
McCarthy et al., (1965)[18] analyzed the indigenous oral flora of man.
Meurman et al., (2010)[19] assessed the infectious and dietary risk factors of oral cancer.
Rawlinson et al., (1993)[20] discovered new data on the microbial flora associated with adult periodontitis.
Rosenquist et al., (2005)[21] described risk factors for oral and oropharyngeal squamous cell carcinoma.
Sakamoto et al., (1999)[22] isolated and studied bacteria from cervical lymph nodes in patients with oral cancer.
Muthu et al., (2004)[23] studied oropharyngeal flora changes in patients with head and neck malignancy post radiotherapy.
Fábián et al.,[24] have opined that the genomics, transcriptomics and proteomics of saliva and the oral cavity became increasingly popular subjects of research in the current era as they represent a host of advantages by being a safe, painless and inexpensive source of complex genetic information.
Wijers et al., (2001)[25] tested the hypothesis that aerobic Gram-negative bacteria (AGNB) play a crucial role in the pathogenesis of radiation-induced mucositis; consequently, selective elimination of these bacteria from the oral flora should result in a reduction of the mucositis.
Meurman[26] opined that in the oral cavity, knowledge of the role of microbiota in carcinogenesis is rudimentary at best. Inflammation caused by infections may be the most important preventable cause of cancer in general. However, in the oral cavity the role of microbiota in carcinogenesis is not known.
As shown in Figure 1, several bacteria and Candida strains in the mouth convert ethanol to carcinogenic acetaldehyde thus explaining the epidemiological evidence between heavy drinking, smoking, and development of cancer. Both the commonly encountered oral streptococci and yeasts possess metabolic pathways for this conversion.
Figure 1.
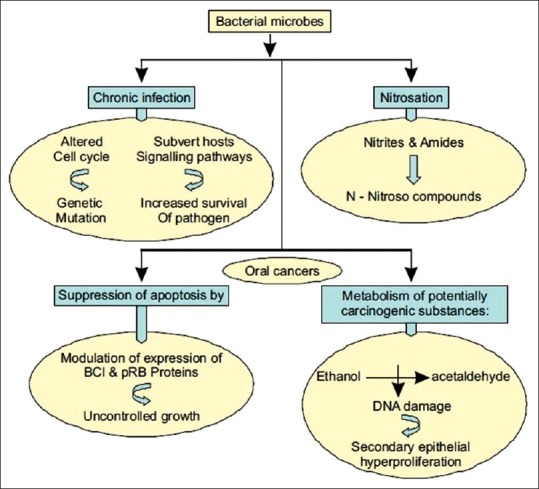
Proposed paradigm for bacterial involvement in carcinogenesis
Hooper et al.,[27] carried out an extensive study to analyze bacteria found within OSCC. In this regard, tumorous and nontumorous mucosal tissue specimens (approximately 1 cm3) were harvested from 10 OSCC patients at the time of surgery. Differences between the composition of the microbiotas within the tumorous and nontumorous mucosae were obvious which pointed to the selective growth of bacteria within carcinoma tissue. The group concluded that whether the presence of these bacteria within the mucosa has any bearing on the carcinogenic process is a concept worthy of further investigation.
Chocolatewala et al.,[28] attempted to collate all the scientific data pertinent to the apparent association between micro-organisms and oral cancer. They contended that despite the widening interest in the possible association between bacteria and different stages of the evolution of cancer, much still needs to be done in this regard.
Proposed Paradigm for the Bacterial Involvement in Carcinogenesis
In recent times, bacteria-laden with “smart polystyrene nanoparticles,” which can transport genes, drugs, nanosensors or other cargo into the interior of host cells, are being used to precisely position cargo inside the cells for the early diagnosis and treatment of cancer and other diseases.
To summarize, recent research has provided us with a host of information regarding the bacterial mechanisms purported to cause or cure cancer. However, many doubts linger. Do microbial infections initiate cancer, or is it the preexisting cancer that compromises the host's immunity facilitating secondary microbial colonization? Will the site-specific colonization of certain bacteria be of any estimable value in the diagnosis or treatment of oral cancer? Could attenuated bacteria be employed in vaccines to modulate host's immunity against cancer? This renders further exploration on this subject mandatory as it would enable us to clearly fathom the role of the micro-organisms, not only in prevention or early diagnosis of oral cancers, but also in providing an effective treatment and improving the survival of the afflicted individual.
The Viral Connection
Some Viruses reported to cause human cancers include some genotypes of: HPV, hepatitis B virus (HBV), hepatitis C virus, EBV, Kaposi's sarcoma-associated herpes virus and human T-lymphotropic virus. The recently discovered human cancer virus is actually, a polyomavirus (Merkel cell polyomavirus) purported to be responsible for a rare form of skin cancer dubbed Merkel cell carcinoma.
Saito et al.,[29] assessed the current issues and perspectives of the rapidly evolving anticancer therapy, dwelling on the potential applications of a distinctive and naturally occurring oncolytic virus.
Grinde and Olsen[30] contended that the focus has traditionally been on bacteria and fungi when dissecting the microbiological aspects of oral disease in detail.
Wu et al.,[31] have established that sensitive and reliable early diagnostic markers for OSCC remain unavailable.
Herrero et al.,[32] attempted an extensive exploration on the alleged link between HPV and oral cancer. HPV, the causal agent of cervical cancer, apparently has a role to play in the etiology of cancer of the oral cavity and oropharynx. The group conducted a multicenter case–control study of cancer of the oral cavity and oropharynx in nine countries. HPV is consistently and more frequently detected in cancers of the oropharynx and tonsil than at other head and neck sites, and HPV-16 tends to be the predominant type detected. Results of a few case–control studies and a cohort study point to a likely role of HPV in some cancers at certain anatomical sites. However, many aspects of the association remain to be investigated to better define the precise contribution of HPV to the etiology of these tumors. In addition to mounting epidemiologic evidence, extensive laboratory evidence[27] supports the association between HPV and a subset of cancers of the oropharynx. The mechanism of transmission of HPV to the oral cavity warrants further investigation. The prospect of HPV vaccine development[29] offers hope for prevention of cervical and anogenital cancers and possibly also for a substantial number of cancers of the oropharynx and oral cavity.
Scully[33] explored the potential viral hypothesis as a chief etiological factor in oral cancer. Recent studies from the MD Anderson Center support an etiologic role for HPVs in at least a sub-set of head and neck squamous cell carcinoma, especially in tonsillar carcinoma. There have been numerous studies on OSCC over the past 15 years. DNA technology has shown that a substantial portion of OSCC and premalignant lesions contain HPV sequences, often of the “high risk” genotypes (mainly HPV-16 and HPV-18). HPV detection is higher when analyzed by in situ hybridization and polymerase chain reaction (PCR), and studies with these techniques have disclosed HPV 11, 16 or 18 DNA sequences in up to 60% of OSCC. Analysis of HPV E7 mRNA in OSCC and cell lines by reverse transcriptase-PCR showed that HPV E7 mRNA was present in 90% of patients with OSCC. They concluded that though HPV may be implicated in some tumors but clearly not in all OSCC where tobacco and alcohol are more important etiological factors. Thus, HPV is clearly neither necessary nor sufficient for all tumor production, and it must be remembered that much OSCC is induced by the known risk habits involving tobacco and alcohol.
Paz et al.,[34] delved into the HPV-oral cancer connection. They maintained that certain strains of HPV have been shown to be etiologically related to the development of uterine cervical and other genital cancers, but their plausible involvement in the development of malignancies at other sites is still a question mark. The team concluded that in the head and neck region, HPV-associated SCC was site specific with the viral DNA commonly found in tumors of the Waldeyer's tonsillar ring. Patients with HPV-positive tumors were reported to have been afflicted with a higher stage of disease than patients with HPV-negative tumors, but there was no discernable difference in the 3-year survival rates between these two groups of patients.
Laborde et al.,[35] argued that there is a possibility that viral agents other than HPV could contribute to the development of OSCC as squamous cell carcinoma of the oral cavity has not been associated with HPV DNA, which suggested alternate etiologic factors. Hermann et al.,[36] reported and reviewed a case highlighting the Presence of HPV-18 and EBV in a squamous cell carcinoma of the tongue in a 20-year-old patient. They acknowledged that this was the first case of co-infection in carcinoma of the tongue to be reported, and they attempted to review the present data and theories concerning viral oncogenesis of oral carcinomas. To find evidence for the presence of HPV or EBV, the team performed HPV-PCR-ELISA and EBV-PCR-ELISA. The PCR-ELISA technique gave evidence for infection with HPV-18 and EBV.
Role of Epstein–Barr Virus
Data concerning the role of EBV in oral squamous cell carcinogenesis are conflicting. Viral DNA is found in 0–70% of these carcinomas. An explanation for the failure to detect any EBV DNA in some studies may be the “hit and run” theory. It suggests that only the viral DNA is needed to induce malignant transformation. After triggering the carcinoma, the viral DNA is lost during the uncontrolled cell cycles of the host cell.
A co-infection of high-risk HPV and EBV is mainly found in nasopharyngeal carcinomas. Assuming that EBV plays a role in oral carcinogenesis, an interesting hypothesis of viral interaction in triggering malignant transformation has been propounded. Interleukin 10 is known to suppress cellular immune responses. A protein homolog, encoded by EBV, might result in a local immunosuppression and lead to or facilitate an infection of epithelial cells by high-risk HPVs.
Butel, (1999)[37] attempted to establish an etiologic role for Virus in causing cancers. The RNA and DNA tumor viruses have made fundamental contributions to two major areas of cancer research. Viral systems support the concept that cancer development occurs by the accumulation of multiple cooperating events. Viruses are now scientifically accepted as a credible etiologic factor in the development of human cancer. The infectious nature of viruses distinguishes them from all other cancer-causing factors; tumor viruses establish long-term persistent infections in human beings, with cancer occurring as a potentially lethal side effect of viral replication strategies. More information needs to be learned about environmental co-factors that synergize with viral infections to stimulate tumor production. Both the identification of carcinogenic co-factors in specific virus systems and studies of mechanisms of action of such factors in target tissues warrant investigation. Guiding examples are the probable role of aflatoxin in cooperation with HBV chronic infections on induction of hepatocellular carcinoma in certain geographic areas and the involvement of dietary nitrosamines in EBV-induced neoplasia.
Cruz et al., (1997)[38] carried out a study using PCR technique to assess the prevalence of Epstein-Barr Virus in oral squamous carcinomas, premalignant lesions and normal mucosa.
Higa et al., (2003)[39] analyzed EBV-related oral squamous carcinoma patients simultaneously infected with HPV in Okinawa (Southern Japan).
Maeda et al., (1998)[40] investigated the presence of EBV DNA in forty-five cases of oral squamous cell carcinoma.
Shamaa et al., (2008)[41] gave a detailed exposition of the significance of Epstein Barr virus (EBV) and DNA topoisomerase II alpha (DNA-Topo II alpha) immunoreactivity in normal oral mucosa, oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC).
The Fungal Link
Sanjaya et al.,[42] scrutinized the data that allegedly links Candida and oral cancer. The team hypothesized a causal role for candidiasis in oral precancer and cancer albeit an indirect one while also implying that Candida along with other co-factors has a say in initiation and promotion of carcinogenesis. The authors suggest that nitrosation potential of the C. albicans results in the production of carcinogenic nitrosamine thus predisposing the oral epithelium to dysplastic changes leading o carcinoma. Further contributing factors include the integrity of the oral mucosa and tobacco smoking habits, which might enhance the virulence of the organism.
The literature review has suggested a role of Candida in causing preneoplastic changes of the oral mucosa. Cawson (1973) demonstrated the ability of C. albicans to elicit epithelial hyperplasia in chick chorioallantoic membrane. Several other studies have confirmed the hyperplastic response of the epithelium when invaded by Candida. Barrett et al. (1998) demonstrated a significant positive correlation between fungal infection and moderate and severe epithelial dysplasia. Their study also showed significant worsening of epithelial dysplasias associated with fungal infection.
Krogh (1990) commented on the role of yeasts in oral cancer by means of endogenous nitrosation. Findings by Krogh et al. (1987) and Field et al. (1989) suggested a possible role in the initiation of carcinogenesis. Field et al. (1989) postulated that nitrosamine compounds produced by Candida species may directly or in combination with other chemical carcinogenesis, activate specific proto-oncogenes and thus initiate the development of a malignant lesion. They further postulated that the progression of the activated cell into a tumorigenic cell might be linked to the amplification and over-expression of oncogenes.
The authors’ propose a pathogenesis for the role of Candida in oral precancer and cancer, wherein certain factors such as immunocompromised state may lead to the activation of various biotypes of C. albicans that have nitrosation ability to form nitrosamines from their precursors. These nitrosamines then act on the normal epithelium leading to dysplasia and further development of oral carcinoma thereby suggesting a causal role of Candida species. Tobacco products are known to cause epithelial changes through the nitrosation potential. Thus, it can be postulated that C. albicans in association with tobacco will enhance the process of carcinogenesis.
Nieminen et al.,[43] explored the ability of non-C. albicans, Candida species to produce ACH in vitro. Major environmental risk factors for upper digestive tract cancers are excessive smoking of tobacco, increased alcohol consumption and unsatisfactory maintenance of oral hygiene. Consequently, this led to raised ACH levels in saliva, which has been shown to be carcinogenic. They postulated that colonization of oral mucosa with Candida glabrata (a non-C. albicans species) which is widely deemed to have the potential to produce carcinogenic amounts of ACH from both ethanol and glucose may play a considerably vital role in the development of oral cancer.
Nagy et al., (1998)[44] and (2000)[12] investigated the inhibition of the biofilm present on the surface of OSCCs.
Bakri et al., (2010)[45] revisited the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma.
Napenas et al., (2007)[46] studied the relationship between mucositis and changes in oral microflora during cancer chemotherapy.
Soysa et al.,(2004)[47] discussed the clinical and laboratory findings on the relationship between cytotoxics, radiotherapy and oral candidiasis, possible mechanisms of pathogenicity following such therapy, as well as precautions that could be taken to minimize such recalcitrant yeast infections.
Siikala et al., (2011)[48] explored the ability of the oral microbiome to produce acetaldehyde in ethanol incubation.
Martin et al., (1981)[49] studied the yeast flora of the mouth and skin during and after irradiation for oral and laryngeal cancer.
Yamamoto et al.,[50] reported some interesting findings on a patient suffering from oral carcinoma. A patient with oral carcinoma underwent chemo radioimmunotherapy for the same and, approximately 4 weeks right from the start of the therapy, the patient had to grapple with severe oral mucositis evidently caused by the chemoradiotherapy, consequently leading to candidal pneumonia. Randomly amplified polymorphic DNA analysis and DNA sequence examination of strains isolated from the oral cavity 1-week before the onset of pneumonia and autopsied lung revealed the identity of both strains as C. albicans, and the DNA analysis confirmed aspiration of oral Candida.
Pärnänen et al.,[51] set out to study oral mucosal E-cadherin (E-Cad) degradation by clinical and reference strains of C. albicans and C. glabrata. E-Cad is a 120-kDa cell adhesive protein. This study was carried out to test the ability of two Candida strains to degrade human E-Cad from the perspective of the Candida virulence factor. Thus, degradation of E-Cad may result in a significant lack of cellular adhesion, which is often associated with cancer cells.
Conclusions
We have seen in detail, the various data and the existing literature that strongly suggest a definitive link between micro-organisms and oral cancer. All the three major types of bacteria, fungi and viruses provide certain species, which seem to agree with the aforementioned hypothesis. Notwithstanding the burgeoning interest in the possible association between bacteria and different stages of cancer development, our definitive knowledge in its relation to oral cancers remains inadequate although various theories have been put forth by various scientists. Similarly, the role of viruses and fungi in causing oral cancer has also been examined in painstaking detail and unanimously, it can be concluded that further study is warranted. New and improved scientific developments coupled with numerous precise methods of intra-cellular study hints at a future that is optimistic and full of possibilities. Hopefully, the next few years will provide definitive answers to the most pertinent questions examined here.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi N. Microbial ecosystem in the oral cavity: Metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congr Ser. 2005;1284:103–12. [Google Scholar]
- 3.Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2:897–906. doi: 10.1016/s1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 4.Hooper SJ, Crean SJ, Lewis MA, Spratt DA, Wade WG, Wilson MJ. Viable bacteria present within oral squamous cell carcinoma tissue. J Clin Microbiol. 2006;44:1719–25. doi: 10.1128/JCM.44.5.1719-1725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers MS, Keene HJ, Toth BB, Lemon JC, Gallagher SC, Martin CG, et al. Mutans streptococci in xerostomic cancer patients after pilocarpine therapy: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:180–4. doi: 10.1016/j.tripleo.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Hsu S, Singh B, Schuster G. Induction of apoptosis in oral cancer cells: Agents and mechanisms for potential therapy and prevention. Oral Oncol. 2004;40:461–73. doi: 10.1016/j.oraloncology.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Lax AJ, Thomas W. How bacteria could cause cancer: One step at a time. Trends Microbiol. 2002;10:293–9. doi: 10.1016/s0966-842x(02)02360-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Cho NH, Choi EC, Baek SJ, Kim WS, Shin DH, et al. Relevance of human papilloma virus (HPV) infection to carcinogenesis of oral tongue cancer. Int J Oral Maxillofac Surg. 2010;39:678–83. doi: 10.1016/j.ijom.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mager DL. Bacteria and cancer: Cause, coincidence or cure? A review. J Transl Med. 2006;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mager DL. Bacteria linked to oral cancer. J Transl Med. 2005 doi: 10.1186/1479-5876-4-14. [Google Scholar]
- 12.Nagy K, Szöke I, Sonkodi I, Nagy E, Mari A, Szolnoky G, et al. Inhibition of microflora associated with oral malignancy. Oral Oncol. 2000;36:32–6. doi: 10.1016/s1368-8375(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 13.Sharma DC. Salivary bacteria linked to oral cancers. Lancet Oncol. 2005;6:547. doi: 10.1016/s1470-2045(05)70266-7. [DOI] [PubMed] [Google Scholar]
- 14.Sixou JL, de Medeiros-Batista O, Bonnaure-Mallet M. Modifications of the microflora of the oral cavity arising during immunosuppressive chemotherapy. Eur J Cancer B Oral Oncol. 1996;32B:306–10. doi: 10.1016/0964-1955(96)00006-1. [DOI] [PubMed] [Google Scholar]
- 15.Cloke DJ, Green JE, Khan AL, Hodgkinson PD, McLean NR. Factors influencing the development of wound infection following free-flap reconstruction for intra-oral cancer. Br J Plast Surg. 2004;57:556–60. doi: 10.1016/j.bjps.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto H, Sasaki J, Nord CE. Association between bacterial colonization on the tumor, bacterial translocation to the cervical lymph nodes and subsequent postoperative infection in patients with oral cancer. Clin Microbiol Infect. 1999;5:612–6. doi: 10.1111/j.1469-0691.1999.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 17.Mayrand D, Grenier D. Bacterial interactions in periodontal diseases. Pasteur Inst Bull. 1998;96:125–33. [Google Scholar]
- 18.McCarthy C, Snyder ML, Parker RB. The indigenous oral flora of man. I. The newborn to the 1-year-old infant. Arch Oral Biol. 1965;10:61–70. doi: 10.1016/0003-9969(65)90058-0. [DOI] [PubMed] [Google Scholar]
- 19.Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010;46:411–3. doi: 10.1016/j.oraloncology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Rawlinson A, Duerden BI, Goodwin L. New findings on the microbial flora associated with adult periodontitis. J Dent. 1993;21:179–84. doi: 10.1016/0300-5712(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 21.Rosenquist K, Wennerberg J, Schildt EB, Bladström A, Göran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–36. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto H, Naito H, Ohta Y, Tanakna R, Maeda N, Sasaki J, et al. Isolation of bacteria from cervical lymph nodes in patients with oral cancer. Arch Oral Biol. 1999;44:789–93. doi: 10.1016/s0003-9969(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 23.Muthu K, Raman R, Gopalakrishnan G. Oropharyngeal flora changes in patients with head and neck malignancy post radiotherapy. Med J Malaysia. 2004;59:585–90. [PubMed] [Google Scholar]
- 24.Fábián TK, Fejérdy P, Csermely P. Salivary Genomics, Transcriptomics and Proteomics: The Emerging Concept of the Oral Ecosystem and their Use in the Early Diagnosis of Cancer and other Diseases. Curr Genomics. 2008;9:11–21. doi: 10.2174/138920208783884900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijers OB, Levendag PC, Harms ER, Gan-Teng AM, Schmitz PI, Hendriks WD, et al. Mucositis reduction by selective elimination of oral flora in irradiated cancers of the head and neck: A placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys. 2001;50:343–52. doi: 10.1016/s0360-3016(01)01444-4. [DOI] [PubMed] [Google Scholar]
- 26.Meurman JH. Oral microbiota and cancer. J Oral Microbiol. 2010:2. doi: 10.3402/jom.v2i0.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper SJ, Crean SJ, Fardy MJ, Lewis MA, Spratt DA, Wade WG, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56:1651–9. doi: 10.1099/jmm.0.46918-0. [DOI] [PubMed] [Google Scholar]
- 28.Chocolatewala N, Chaturvedi P, Desale R. The role of bacteria in oral cancer. Indian J Med Paediatr Oncol. 2010;31:126–31. doi: 10.4103/0971-5851.76195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito K, Shirasawa H, Isegawa N, Shiiba M, Uzawa K, Tanzawa H. Oncolytic virotherapy for oral squamous cell carcinoma using replication-competent viruses. Oral Oncol. 2009;45:1021–7. doi: 10.1016/j.oraloncology.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Grinde B, Olsen I. The role of viruses in oral disease. J Oral Microbiol. 2010:2. doi: 10.3402/jom.v2i0.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JY, Yi C, Chung HR, Wang DJ, Chang WC, Lee SY, et al. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol. 2010;46:226–31. doi: 10.1016/j.oraloncology.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 33.Scully C. Oral squamous cell carcinoma; from an hypothesis about a virus, to concern about possible sexual transmission. Oral Oncol. 2002;38:227–34. doi: 10.1016/s1368-8375(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 34.Paz BI, Cook NB, Maryon TO, Xie Y, Wilcyzinski SP. Human papillomavirus (HPV) in head and neck cancer. Am Cancer Soc. 1997;79:595–604. doi: 10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 35.Laborde RR, Novakova V, Olsen KD, Kasperbauer JL, Moore EJ, Smith DI. Expression profiles of viral responsive genes in oral and oropharyngeal cancers. Eur J Cancer. 2010;46:1153–8. doi: 10.1016/j.ejca.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Hermann RM, Füzesi L, Pradier O, Christiansen H, Schmidberger H. Presence of human papillomavirus-18 and Epstein-Barr virus in a squamous cell carcinoma of the tongue in a 20-year-old patient. Case report and review of the current literature. Cancer Radiother. 2004;8:262–5. doi: 10.1016/j.canrad.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Butel JS. Viral carcinogenesis: Revelation of molecular mechanisms and etiology of human disease. Oxf J Life Sci Med. 1999;21:405–26. doi: 10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- 38.Cruz I, Van den Brule AJ, Steenbergen RD, Snijders PJ, Meijer CJ, Walboomers JM, et al. Prevalence of Epstein-Barr virus in oral squamous cell carcinomas, premalignant lesions and normal mucosa - A study using the polymerase chain reaction. Oral Oncol. 1997;33:182–8. doi: 10.1016/s0964-1955(96)00054-1. [DOI] [PubMed] [Google Scholar]
- 39.Higa M, Kinjo T, Kamiyama K, Chinen K, Iwamasa T, Arasaki A, et al. Epstein-Barr virus (EBV)-related oral squamous cell carcinoma in Okinawa, a subtropical island, in southern Japan – Simultaneously infected with human papillomavirus (HPV) Oral Oncol. 2003;39:405–14. doi: 10.1016/s1368-8375(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 40.Maeda T, Hiranuma H, Matsumura S, Furukawa S, Fuchihata H. Epstein-Barr virus infection and response to radiotherapy in squamous cell carcinoma of the oral cavity. Cancer Lett. 1998;125:25–30. doi: 10.1016/s0304-3835(97)00485-0. [DOI] [PubMed] [Google Scholar]
- 41.Shamaa AA, Zyada MM, Wagner M, Awad SS, Osman MM, Abdel Azeem AA. The significance of Epstein Barr virus (EBV) and DNA topoisomerase II alpha (DNA-Topo II alpha) immunoreactivity in normal oral mucosa, oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC) Diagn Pathol. 2008;3:45. doi: 10.1186/1746-1596-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanjaya PR, Gokul S, Gururaj Patil B, Raju R. Candida in oral pre-cancer and oral cancer. Med Hypotheses. 2011;77:1125–8. doi: 10.1016/j.mehy.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Nieminen MT, Uittamo J, Salaspuro M, Rautemaa R. Acetaldehyde production from ethanol and glucose by non-Candida albicans yeasts in vitro. Oral Oncol. 2009;45:e245–8. doi: 10.1016/j.oraloncology.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–8. [PubMed] [Google Scholar]
- 45.Bakri M, Hussaini HM, Holmes AR, Cannon RD, Rich AM. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. Journal of Oral Microbiology. 2010 doi: 10.3402/jom.v2i0.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Napeñas JJ, Brennan MT, Bahrani-Mougeot FK, Fox PC, Lockhart PB. Relationship between mucositis and changes in oral microflora during cancer chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:48–59. doi: 10.1016/j.tripleo.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Soysa NS, Samaranayake LP, Ellepola AN. Cytotoxic drugs, radiotherapy and oral candidiasis. Oral Oncol. 2004;40:971–8. doi: 10.1016/j.oraloncology.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Siikala E, Uittamo J, Rusanen P, Rautemaa R. Microbial colonization and acetaldehyde production in oral cancer and oral lichen planus lesions. J Oral Maxillofac Surg. 2011;69:70–1. [Google Scholar]
- 49.Martin MV, Al-Tikriti U, Bramley PA. Yeast flora of the mouth and skin during and after irradiation for oral and laryngeal cancer. J Med Microbiol. 1981;14:457–67. doi: 10.1099/00222615-14-4-457. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Ueta E, Kamatani T, Osaki T. DNA identification of the pathogen of candidal aspiration pneumonia induced in the course of oral cancer therapy. J Med Microbiol. 2005;54:493–6. doi: 10.1099/jmm.0.45769-0. [DOI] [PubMed] [Google Scholar]
- 51.Pärnänen P, Meurman JH, Samaranayake L, Virtanen I. Human oral keratinocyte E-cadherin degradation by Candida albicans and Candida glabrata. J Oral Pathol Med. 2010;39:275–8. doi: 10.1111/j.1600-0714.2009.00866.x. [DOI] [PubMed] [Google Scholar]


