Abstract
The recent advancements in the field of stem cell (SC) biology have increased the hope of achieving the definitive treatments for the diseases which are now considered incurable such as diabetes, Parkinson's disease and other chronic long standing conditions. To achieve this possibility, it is necessary to understand the basic concepts of SC biology to utilize in various advanced techniques of regenerative medicine including tissue engineering and gene therapy. This article highlights the types of SCs available and their therapeutic capacity in regenerative medical and dental fields.
KEY WORDS: Gene therapy, regenerative medicine, stem cells, tissue engineering
A stem cell (SC) is a cell which has an immense capacity for self-renewal and potency. SCs are found in all multicellular organisms, and they are characterized by the ability to renew themselves through mitotic cell division and differentiate into a diverse range of specialized cell types.[1]
Stem cells can generate daughter cells identical to their mother (self-renewal) as well as produce progeny with more restricted potential (differentiated cells).[2] By definition, “a SC is capable of self-renewal, differentiation into at least one cell type and functional reconstitution of the tissue of origin.”[3]
This self-renewal capacity underlines the ability of adult SCs such as spermatogonial SCs, and hematopoietic SCs (HSCs) to constantly renew tissues that turn over rapidly and also where cells do not turn over so rapidly in the adult brain. There are long-lived quiescent SCs that may be reactivated to repair damage.[4]
Much current research is focused on the SCs identification, their characterization and isolation from the adult, harboring the hope that such cells may be useful for therapeutic repair of adult tissues either by exogenous cell therapy or by reactivation of endogenous SCs. Biologists have explored the development of embryos of all aspects, from worms to humans, in search of the answer to the question of how a single cell, the fertilized egg forms a complex organism. Cells are initially proliferative and pluripotent during embryogenesis; they only gradually become restricted to different cell fates.[5]
During embryogenesis, SCs can differentiate into all of the specialized embryonic tissues whereas in adult organisms, progenitor cells as well as SCs act as a repair system for the body; replenishing specialized cells while also maintaining the normal turnover of regenerative organs such as blood, skin, or intestinal tissues.[6,7,8]
Commonly, SCs come from two main sources:
Embryos formed during the blastocyst phase of embryological development (embryonic stem cells [ESCs]) and
Adult tissues (adult SCs).
Stem cells can now be grown and transformed into specialized cells with characteristics consistent with cells of various tissues such as muscles or nerves through cell culture. Highly plastic adult SCs derived from a variety of sources, such as bone marrow and umbilical cord blood; are routinely used in medical treatments. Embryonic cell lines and autologous ESCs generated through therapeutic cloning have also been proposed as promising candidates for future therapies.[9]
Earlier, it was thought that only ESCs can give rise to the complete range of cells in the organism but recent studies have revealed that adult SCs are unexpectedly common and indicate that they might be more plastic in their ability to differentiate into cell types of all the three germ layers than previously appreciated.[9,10] The presence of multipotent SCs in the adult might open up new therapeutic opportunities on the basis of tissue and organ replacement.[10]
With the latest advancement in research the SCs of a particular tissue is defined as: (a) Undifferentiated cells (b) capable of proliferation, (c) able to self-maintain the population, (d) capable of producing a large number of differentiated, functional progeny, (e) capable of regenerating the injured tissues, and (f) flexible use of these options.[10,11,12,13,14,15]
Properties of Stem Cells
Stem cells differ from other kinds of cells in the body. Regardless of their source, all SCs have three general properties:[3,16,17,18]
They are unspecialized
They are capable of dividing and self-renewing themselves for long periods; and
They can give rise to specialized cell types.
Stem cells are unspecialized (relativity)
One of the fundamental properties of a SC is that it does not have any tissue-specific structures that allows it to perform specialized functions like pumping blood through the body, carrying molecules of oxygen through the bloodstream but they can give rise to specialized cells, including heart muscle cells or blood cells.[3,16,17,18]
Stem cells are capable of dividing and self-renewing themselves for long periods (self-maintenance and renewal)
Unlike the fully differentiated cells (nerve cells, blood cells, or muscle cells) which do not normally replicate themselves, SCs are capable of replicating many times. Replication of cells themselves multiple times without differentiation is called proliferation.[18]
Stem cells can give rise to specialized cells (potency)
When unspecialized SCs give rise to specialized cells, the process is called differentiation. Differentiation may be controlled by either internal signals (cell's genes) or external signals (chemicals secreted by other cells, certain cytokines molecules in the microenvironment and physical contact with the adjacent cells).[17,18]
Previously, it was considered that adult SCs typically generate the cell types of the tissue in which they reside. However, recent experiments over the last several years have proved that SCs from one tissue may be able to give rise to cell types of a completely different tissue, and this is known as plasticity. Blood cells differentiating into neurons and HSCs that can develop into heart muscles are a few examples of plasticity. Therefore, exploring the possibility of using adult SCs for cell-based therapies has become very active now-a-day.[17,18]
Classification of Stem Cells
Stem cells can be classified in the following ways: (1) According to their potency, (2) according to their origin, and/or (3) according to their origin, differentiation potency, and progeny.
Classification according to their potency
Stem cells are categorized by their potential to differentiate into other types of cells. The full classification includes.
Totipotent
Totipotency is the ability of a single cell to divide and produce all the differentiated cells including extraembryonic tissues of an organism. Spores and zygotes are examples for totipotent cells. Human development begins with a sperm fertilizes an egg to produce a single totipotent cell (zygote) which in turn divides into identical totipotent cells in the first hours after fertilization.[19,20,21]
Pluripotent
Pluripotent SC that has the potential to differentiate into any of the three germ layers: Ectoderm, endoderm or mesoderm. E.g., ESCs isolated from inner cell mass (ICM) of blastocysts. Any fetal or adult cell types can be derived from pluripotent SCs, but they lack the ability to contribute to extraembryonic tissue like the placenta.[19,20,21]
Multipotent
Multipotent progenitor cells have the potential to give rise to cells from multiple, but a limited number of lineages. E.g., HSCs - A blood SC that can develop into several types of blood cells, but lack the potential to develop into brain cells and other types of cells. Scientists have long held the opinion that differentiated cells cannot be altered or induced to behave in any way other than the way in which they have been naturally committed. In recent SC experiments, scientists have been able to persuade blood SCs to behave like neurons or brain cells - A process known as transdifferentiation.[19,20,21]
Oligopotent
An oligopotent cell has the potency to differentiate into a few cell types. Examples of progenitor cells are vascular SCs which have the capacity to become either endothelial or smooth muscle cells.[19,20,21]
Unipotent
A unipotent cell or precursor cell is one that has the capacity to differentiate into only one type of cell/tissue type. The most common example of these cells in humans is skin cells. This cell has a unique property: Self-renewal.[19,20,21]
Classification according to the origin
Human embryonic stem cells
The ICM of the 5–6-day old human blastocyst is the source of pluripotent ESCs. The ICM is composed of 30–34 cells. During embryonic development, the ICM develops into two distinct cell layers, the epiblast and hypoblast. The hypoblast forms the yolk sac which later becomes redundant in the human and the epiblast differentiates into the three primordial germ layers (ectoderm, mesoderm, and endoderm). Human embryonic germ cells which are also SCs, develop from the primordial germ cells of the gonadal ridge of 5–9-week old fetuses. These SCs are pluripotent and are able to produce cells of all three germ layers.[22,23]
Fetal stem cells
Fetal SCs are primitive cell types found in the organs of fetuses. Neural crest SCs, fetal HSCs, and pancreatic islet progenitors have been isolated in abortuses.[24]
Infant stem cells
Infant SCs are isolated from umbilical cord blood and Wharton's jelly. Umbilical cord blood contains circulating SCs, and the cellular contents of umbilical cord blood appear to be quite distinct from those of the bone marrow and adult peripheral blood. Cord blood SCs have been shown to be multipotent by being able to differentiate into neurons and liver cells. Matrix cells from the umbilical cord contain potentially useful SCs. This matrix termed as Wharton's jelly, has been a source for isolation of mesenchymal SCs (MSCs).[25]
Adult stem cells
-
Hematopoietic SCs (bone marrow and peripheral blood)
Bone marrow possesses SCs that are both hematopoietic and mesenchymal in origin. The HSC is derived early in embryogenesis from the mesoderm and becomes deposited in very specific hematopoietic sites within the embryo. These sites include the bone marrow, liver, and yolk sac.[26,29,31]
-
MSCs (bone marrow stroma)
Mesenchymal SCs are found postnatally in the nonhematopoietic bone marrow stroma. MSCs are multipotent cells that are capable of differentiating into cartilage, bone, muscle, tendon, ligament and fat tissues.[27,32,34]
Bone and cartilage SCs
-
Epidermal SCs (skin and hair)
The epidermis harbors SCs at the base of the hair follicle and in the basal layer of the epidermis.[31,32]
-
Neuronal SCs
The subventricular zone of the forebrain and the central gyrus of the hippocampus which are considered reservoirs of new neural cells.[27,28]
-
Pancreatic SCs
Human pancreatic islets contained an unrecognized population of cells that expressed the neural SC (NSC)-specific marker nestin.[19,24]
-
Eye SCs (pigmented ciliary margin cells).[27,33]
Classification according to their origin, differentiation potency and progeny[35] [Table 1].
Table 1.
Classification of stem cells
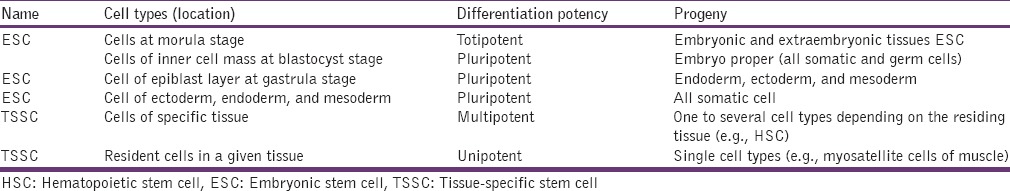
Sources of Tissue-Specific Stem Cells
(1) Endodermal origin (pulmonary epithelial SCs, gastrointestinal tract SCs, pancreatic SCs, hepatic oval cells, mammary and prostate gland SCs, ovarian and testicular SCs), (2) Mesodermal origin (hematopoietic SCs, mesenchymal stromal SC, cardiac SC, and satellite cells of muscles), (3) Ectodermal origin (NSCs, skin SCs, and ocular SCs).[31]
Basic Biology
Early mammalian embryogenesis is characterized by a gradual restriction in the developmental potential of the cells that constitute the embryo. The zygote and single blastomeres from a two to four cell embryo are totipotent. As the embryo continues to cleave, the blastomeres lose the potential to differentiate into all lineages. The blastocyst is the first landmark of the embryo in which lineage restriction is apparent. At this stage, the outer cells of the embryo compact into the trophectoderm, from which the placenta will derive. The inner cells, termed ICM, will give rise to all cell lineages of the embryo proper, but cannot contribute to the trophectoderm, and thus are considered pluripotent. As the embryo develops, the potency of the cells are more restricted, and they become multipotent, oligopotent, and unipotent.[36,37,38]
The four areas mainly concentrated on understanding the molecular mechanisms of ESC potency are: (1) Influence of interleukin-6 family cytokines, (2) extrinsic determinants of pluripotency, (3) intrinsic determinants of pluripotency and (4) epigenetic configurations. Cytokines and extrinsic determinants like leukemia inhibitory factor and other cytokines, ERK-MAPK pathway, fibroblast growth factor-4 (FGF-4), Wnt signaling pathway, transforming growth factor-β (TGF-β) and BMP, activin, and PI3/AKT pathway control the ESCs by up or down-regulating the potency and self-renewing properties.[39,40,41,42,43] Intrinsic determinants like transcription factors OCT-4, SOX-2, FOX-D3 and NANOG, and epigenetic mechanisms like polycomb-related complex-2, RNA interference system, nucleosome remodeling and histone deacetylation complex, and epigenetic modifiers like Ronin play a vital role in the self-renewal of ESCs. Likewise, by understanding the mechanism revolving around all types of SCs (fetal, infant and adult), it is possible to generate SCs with desired therapeutic properties.[36,44,45]
Applications of Stem Cells
The tremendous advancement in genetic, molecular and SC biology has led to the use of SCs in almost all the aspects of medical and dental fields to treat cancer, neurodegenerative diseases, heart diseases, muscular dystrophies, diabetes, burns, and skin ulcers, and applied in orthopedic and bone marrow transplantation treatments. The rapidly evolving techniques like tissue engineering and gene therapy use the SC concept as their basis to produce tissue organs for organ replacement and gene modification treatments.
Cancer Stem Cells: A Therapeutic Target in Cancer Therapy
The cancer SC (CSC) hypothesis has postulated that each tumor contains a subset of cells – the CSCs – that are uniquely responsible for tumor growth, heterogeneity, and metastasis. These specialized cells possess two key features that together distinguish them from the remainder of the tumor cells: Self-renewal and differentiation potential. The finding that leukemic SCs in acute myeloid leukemia share the same immunophenotype with normal HSCs, CD341, CD382, might suggest that cancers result from the oncogenic transformation of normal SCs. Similarly, observations about the time from carcinogenic exposure to the development of cancer also seem to support the notion that cancers are derived from normal tissue SCs.[46,47,48,49]
Traditionally, the only cells in a tissue thought to persist long enough to accumulate several oncogenic mutations and exhibit such a delayed effect, were the tissue SCs. Due to their rapid turnover, non-SCs that acquire mutations would be expected to die off before enough mutations could accumulate to transform them into cancer cells.[46,47,48,49]
The cancer stem cell model
At least two different models attempt to explain both clonality and tumor heterogeneity: The stochastic model; and the CSC model. The stochastic model is based on the notion that the transforming events that endow the cell of origin with a malignant phenotype cause all progeny of that cell also to be tumorigenic. In this model, tumor heterogeneity is caused by subclones of tumor cells that result from a combination of different microenvironments and random genetic changes (including point mutations, chromosomal rearrangements, aneuploidy, and gene amplifications). Ultimately, this model implies that effective treatment for cancer must involve eradication of all cancer cells.[46,47,48,49]
The CSC model postulates that only some tumor cells are tumorigenic - The CSC. When they divide, these malignant cells self-renew and also give rise to the non-self-renewing cells that go on to elaborate the heterogeneous cells within a cancer. In this way, CSCs drive tumor growth.[50]
Cancer stem cells and their clinical implications
The CSC model has many important clinical implications which might not only contribute a novel pathophysiological mechanism to explain tumors’ resistance to treatment, but it might also improve clinicians’ ability to more effectively diagnose, prognose, and treat cancer.[46,47,48,49]
Since the CSC model suggests that CSCs are responsible for the sustained and uncontrolled growth of malignancies, treatment failures in the clinic may be due partly to the resistance of CSCs to therapy. If CSCs are intrinsically more resistant to treatment (such as chemotherapy or radiotherapy) than other cells in the tumor, then treatment should increase the percentage of CSCs relative to pretreatment levels. Conversely, if all cells are similarly sensitive to treatment, then the relative percentages of tumorigenic and nontumorigenic cells should not change as a tumor shrinks which helps us to assess the prognosis.[46,47,48,49]
Central Nervous System Repair and Neural Stem Cells
The poor regenerative ability, particularly in the adult central nervous system, may be because of the limited number and restricted location of native NSCs, and/or limitations imposed by the surrounding microenvironment, which may not be supportive or instructive for neuronal differentiation for the most devastating of injuries is inadequate or ineffective. NSCs expanded ex vivo in culture, and then implanted into regions needing repair, may overcome those limitations related simply to inadequate numbers of NSCs near the defective region.[51] However, several transplantation experiments have suggested that neurogenic cues are transiently elaborated during degenerative processes (perhaps recapitulating developmental cues) and that exogenous NSCs are able to sense, home in on and respond appropriately to those cues.[52,53]
These disorders include rodent models of genetic and acquired (e.g., traumatic and ischemic) neurodegeneration, inheritable metabolic disorders, age-related degeneration, and neoplasms. NSCs differentiate robustly into neural cells, integrate flawlessly into neural parenchyma as multiple neural cell types (both neuronal and glial), respond to normal developmental and regeneration cues and migrate (even long distances) to multiple, disseminated areas of neuropathology. NSCs appear to be ideally suited for the molecular and cellular therapies required by extensive, diffuse degenerative processes. Examples of such widespread neurodegenerative conditions include myelin disorders, motor neuron degeneration, storage diseases, dementia conditions such as Alzheimer's disease and ischemic and traumatic pathologies like stroke.[54]
Use of ESCs for neural transplantation is in its formative stage, and only a limited amount of work has been completed with the spinal cord. Overall, the studies demonstrate that ESCs have a remarkable ability to integrate into the injured region of the cord and differentiate appropriately.[54]
Heart Disease and Stem Cells
It is now well-established that cardiomyocytes can be stably transplanted into normal or injured adult hearts. Cardiomyocytes derived from embryonic and fetal SCs or bone marrow and MSCs are used as donor cells to restore lost myocardial function to enhance angiogenesis and to provide support to the tissues.[55,56]
Moreover, transplanted donor cells can form a functional syncytium with the host myocardium and also it has been proved that the cardiomyocyte cells transplanted heart showed improvement in cardiac functions.[55,57,58] It has been contented that it is possible to provide large amount of human cardiomyocytes to fulfill the requirement of cells for transplantation and also favor cardiac regeneration with expected cardiac function.[59,60,61,62]
Muscular Dystrophy and Stem Cells
Muscular dystrophies are caused by progressive degeneration of skeletal muscle fibers. Sixty-five lack of one of the several proteins at the plasma membrane or within internal membranes, raises the probability of damage during contraction, and degeneration of fibers occur eventually. Fiber degeneration is counterbalanced by the regeneration of new fibers at the expense of resident myogenic cells, located underneath the basal lamina and known as satellite cells.[63,64] These cells should also produce new satellite cells to ensure a reserve population for further cycles of regeneration, and failure of this process results in the most severe forms of dystrophy.[65]
These other progenitors are probably derived from distinct anatomic sites, such as the microvascular niche of the skeletal muscle or bone marrow itself. In the most severe forms, like Duchenne's muscular dystrophy, regeneration is totally exhausted, and skeletal muscle is progressively replaced by fat and fibrous tissue. Subsequently, this condition leads the patient to progressive weakness and eventual death by respiratory failure, cardiac failure, or both.[63,64]
Current therapeutic approaches involve steroids and result in modest beneficial effects. Novel experimental approaches can be schematically grouped into three major areas.[66]
The first is gene therapy aiming at the production of new viral vectors (mainly adeno-associated and lentiviral vectors, the latter for cell-mediated gene therapy) that are designed to be less antigenic and more efficient in transducing adult muscle fibers and/or myogenic cells[66]
Novel pharmacological approaches focus on high-throughput screens for molecules that may interfere with pathogenic pathways. This aims to identify molecules that cause the skipping of termination codons or upregulate utrophin synthesis, a cognate protein that compensates for dystrophin absence when overexpressed in dystrophic mice; it also includes molecules that simply enhance muscle regeneration or delay protein degradation[67]
The final group is cell therapy, based initially on myoblast transplantation, and more recently on the transplantation of stem progenitor cells.[68]
Diabetes and Stem Cells
The complications associated with diabetes (retinopathy, nephropathy, and neuropathy) require intensive treatments like pancreas transplantation or SC therapy which could provide long-term benefits to the patients. Pancreas transplantation performed since 1978 required major surgery and its associated complications heralded the development of new technique where transplant of only pancreatic islet cells to the liver through the portal vein via transhepatic angiography and it gained more attention since late 1980s. In general, poor outcomes were obtained throughout the 1990s, but the introduction of the Edmonton protocol in 2000 provided far better results, the improvement being due to better islet preparations, transplantation of more islets and improved immunosuppression.[69]
With the advancement in SC biology, SCs obtained from human ESCs cultured in gelatine or human fibroblast served its purpose better than Edmonton protocol method. Induced pluripotent SC is a recently developed SC and ethically has fewer controversies than hESCs and acts as a better source for the production of pancreatic islets for transplants. With the acquirement of knowledge on pdx-1, a transcription factor; it has been evidenced that adult SCs and precursor cells produced pancreatic islets apart from hESCs for the treatment of diabetes.[70]
Orthopedic Applications of Stem Cells
The inherent regenerative ability of the cartilage, tendon, and ligament is comparatively less than bone which has the tremendous regenerative capacity. Hence, concentrated use of SCs is required for repair of cartilage tendon and ligaments, where injuries to these tissues result in replacement of tissues with less organized scar tissues inferior in quality to the native tissue.[71] Chondral defects penetrating the subchondral bone usually do not require extensive treatment as it is repaired by the MSCs inherently present in the bone marrow, periosteum, and synovium.[71,72,73] And in areas where the defects are superficial and do not penetrate subchondral bone, lack of MSCs and vascularity for repair demands SC therapy using cultured MSCs.[71] Injuries to the ligaments and tendons may lead to inflammation or tear to these structures where the ultimate result would be degenerative joint diseases if not treated promptly. In normal healing process, healing is accelerated with the stimulation of fibroblasts by growth factors such as platelet-derived growth factor (PDGF), TGF-β, and FGF.[74] For example, ligaments like anterior collateral ligament show less tendency for repair compared to the medial collateral ligament and requires MSC gene therapy using viral vectors to deliver the growth factors to accelerate and organize the healing process.[75,76]
Burns and Skin Ulcers
Burns and skin ulcers are major causes of morbidity and, in the case of burns, mortality, in both the developed and developing world. The traditional methods to treat burns include autologous and allogenous skin grafting. Epidermal SCs which reside in the basal layer of epidermis are responsible for the ability of the epidermis to replace itself, both in normal circumstances and in traumatic conditions (burns and skin ulceration).[14,77]
In autologous skin grafting, epidermal cells have been used to treat burns since the introduction of the split skin graft by Karl Thiersh in the late 1800s. Skin grafting to cover defects caused by burns or skin ulceration is limited by the area of skin. Full-thickness grafts (including all of the epidermis and dermis) provide good cosmetic results but require a primary closure of the donor site, limiting the area that may be grafted.[78]
To overcome this problem, the use of split skin grafts was developed whereby epidermis and underlying dermis is shaved from the donor site to provide a graft and the donor site then re-epithelializes from the SCs present in the underlying hair follicles. The limitations of skin grafting techniques are the area that can be covered by them and the many weeks it may take to cover a large area of burn with autologous split skin. Burns of 80–90% are survivable in the short-term with resuscitation, but if the treatment is delayed because of a lack of grafts, results in a high morbidity and mortality rate.[79] To overcome these problems, Rheinwald and Green developed a technique for the serial cultivation of epidermal cells, producing a 1000-10,000 fold area of graftable epidermis than the initial biopsy. Then these epidermal sheets can then be grafted onto clean wound beds but they are sensitive to loss by bacterial infection and blistering. In full-thickness burns where the dermis has been lost, the cultured epidermal autograft may be placed directly onto muscle or fascia. These cultured epidermal autografts form a permanent covering, suggesting that the SCs initially cultured and then transplanted have been maintained as SCs and, therefore, retain their crucial role in epidermal maintenance.[80]
In allogenous skin grating technique, allografts were developed due to the lack of available donor sites for split-skin grafting in patients with massive burns as the time taken to grow cultured autologous skin from these patients is more. In order to prevent secondary sepsis and other complications associated with open burn wound, cadaveric skin is an alternative which is immediately available, plentiful, effective, and affordable. And it is always eventually rejected by the recipient as it is a true allograft. Alloderm is a processed human dermis from which the epidermal and dermal cells have been removed, leaving only the connective tissue matrix. Then alloderm can be applied to burns, and cultured autograft may be placed on top of it to prevent the early said complications. The currently available artificial dermis lacks a vascular plexus for the nourishment of the epidermis and requires host vasculogenesis into the dermis graft to supply nourishment to the grafted epidermis. Efforts have therefore been focused on encouraging the process of vasculogenesis by genetic engineering of grafts to produce growth factors and cytokines vital to this process.[81,82]
Skin ulcers
In ulcer therapy, the time constraints are not as severe and for definitive closure, split skin grafts remain the gold standard. Cultured skin has been used in the treatment of skin ulcers as a “living dressing” (particularly cultured allografts). Cultured, autologous outer root sheath cells used in the treatment of chronic decubitus ulcers have been found to produce an “edging effect” - The contraction of the chronic wounds edges in response to the graft, believed to be caused by a release of growth factors, cytokines, and hormones from the outer root sheath cells.[83] Apligraf is a cultured, bilayered living skin equivalent derived from neonatal foreskin keratinocytes, bovine type I collagen, and neonatal foreskin fibroblasts. It is indicated for the treatment of venous ulcers and neuropathic diabetic foot ulcers. Chronic wounds (e.g., those with dormant edges) re-epithelialize when exposed to living allograft material. This edge effect, like that seen with outer root sheath cells, is most probably caused by the presence of stimulatory factors. Chronic wounds heal better after repeated application of skin grafts. Hence, cultured autologous skin grafts offer better results, and suitable for larger area coverage and chronic ulcers.[84]
Tissue Engineering
Traditionally, approaches to restore tissue function have involved organ donation. However, the shortage of transplantable human tissues such as bone marrow, hearts, kidneys, livers, and pancreases required alternatives, where tissue engineering plays a vital role.[66,85] Tissue engineering-based therapies may provide a possible solution to alleviate the current shortage of organ donors. Biological and engineering principles are combined in tissue engineering techniques to produce cell-based substitutes. One of the major hurdles in engineering tissue constructs for clinical use is the limited availability of human cells as tissue source. SCs isolated from adults or developing embryos are a current source for cells for tissue engineering.[66,85]
Tissue engineering is an interdisciplinary field that applies the principles of engineering and life sciences to develop biological substitutes, usually composed of biological and synthetic components, which restore, maintain or improve tissue function. Tissue-engineered products would provide a life-long therapy which would reduce the hospitalization and healthcare costs associated with drug therapy while enhancing the patients’ quality of life. Tissue engineering uses any one of the following substitutes: (1) Isolated cells or cell substitutes as cellular replacement parts, (2) acellular materials capable of inducing tissue regeneration, (3) a combination of cells and materials (typically in the form of scaffolds).[86,87] Isolated cells have been used as a substitute for cell replacement parts for many years. In fact, the first application of SCs as a cellular replacement therapy is associated with bone marrow transplantation or blood transfusion studies, in which donor HSCs repopulated the host's blood cells.[88]
Tissue engineering approaches that use cells and scaffolds can be categorized into two categories; open and closed systems. In open tissue engineering systems, cells are immobilized within a highly-porous, three-dimensional scaffold. Scaffold could either be comprised of synthetic or natural materials or composites of both. Ideally, this scaffold provides the cells with a suitable growth environment, optimum oxygen and nutrient transport properties, good mechanical integrity, and a suitable degradation rate. The use of scaffolds provides three-dimensional environments and brings the cells in close proximity so that it provides the cells with sufficient time to enable self-assembly and formation of various components that are associated with the tissue microenvironment. Open tissue engineering systems have been successfully used to create a number of biological substitutes, like bone, cartilage, skin and tooth, etc.[71,89,90,91,92,93,94,95,96,97,98]
The closed systems aim to overcome this difficulty by immobilizing cells within polymeric matrices that provide a barrier for the immunological components of the host. The implants can either be implanted into the patient directly or used as extra-corpical devices. Closed tissue engineering systems have been used particularly for the treatment of diabetes, liver, and Parkinson's disease.[71,89,90,91,92,93,94,95,96,97,98]
Synthetic scaffolds that support tissue growth by serving as the extracellular matrix for the cells do not represent the natural extracellular material associated with each cell type and tissue. The use of “smart” scaffolds that release particular factors and/or control the temporal expression of various molecules released from the polymer could be used like dual delivery of vascular endothelial growth factor-165 and PDGF-BB, each with distinct kinetics, could produce a mature vascular network from a single, structural polymer scaffold. Another difficulty with the current materials is their lack of control over the spatial organization within the scaffold. In order to create tissues that resemble the natural structure of biological tissues, the spatial patterning of cells must be recapitulated.[71,89,90,91,92,93,94,95,96,97,98]
Stem Cell Gene Therapy
Stem cell gene therapy uses the concept that a genetically defective SC in the body could be genetically reprogrammed to become a normal SC, where the genetically corrected cell multiplies and produces more number of genetically appropriate, normal functioning cells in the body. The currently available techniques to achieve these goals are gene addition and genomic editing. For example, the most common genetic diseases of blood, such as thalassemia and sickle cell anemia could be treated either by delivery of globin transgene to HSCs or by direct repair of a specific gene mutations in HSCs.[99,100] Likewise, a genetically modified MSCs could be used to treat the genetic diseases such as osteogenesis imperfecta, Marfan's syndrome, and muscular dystrophy.[101]
Gene addition involves the delivery of corrective DNA using viral vectors (retrovirus, lentivirus) to compensate or overrides the defective gene, either by integration into one of the existing chromosomes or by incorporation of the transgene in a synthetic microchromosome.[102,103,104] Genomic editing involves DNA repair and/or homologous recombination process to correct an existing defective gene sequence in order to restore normal DNA state by delivering small DNA fragments, modified DNA polymerases and/or hybrid DNA/RNA molecules that are homologous to the defective sequence with the expectation of the bases intended DNA alteration.[105,106]
Application of Stem Cell in Dentistry
The search for MSC like cells in specific tissues resulted in the discovery of SCs in every organ and tissue including dental SCs.[107,108] For dental tissue regeneration, several potential MSC type of dental SCs have been identified in relation to the tooth and periodontal tissues. The main source of dental SCs in orofacial region includes: (i) Dental pulp SCs (DPSCs) derived from the pulp tissue of impacted tooth, (ii) SCs from human exfoliated deciduous teeth derived from the pulp tissue of a exfoliating deciduous tooth, (iii) SCs from apical papilla isolated from the soft tissue at the apices of developing permanent teeth, (iv) periodontal ligament SCs isolated from the mixed cell population of in the periodontal ligament space, (v) dental follicle precursor cells derived from an ectomesenchymal tissue surrounding the enamel organ and the dental papilla of the developing tooth germ prior to eruption.[108,109]
It has been anticipated that the SCs derived from dental tissues would be committed to dental lineage and produce only dental tissues like dentin, pulp, etc., but it has been substantiated that dental SCs have the ability to differentiate into other cell lineages. For example, DPSCs have the ability to differentiate into myogenic, adipogenic, osteogenic, chondrogenic, and neurogenic lineages. The main advantages of dental SCs over other MSCs is that ease with which teeth can be obtained and the low levels of risk and these somatic dental SCs will probably become a source of cells that will be useful not only in dentistry but also in many other regenerative therapies.[110,111,112,113]
In the therapeutic aspects, dental SCs are used in the repair of damaged dentin, pulp re-vascularization and regeneration, and for periodontal disorders.[114] In tissue engineering, whole tooth regeneration, a current dental regenerative process under progress would reduce the difficulties associated with the presently offered dental treatments such as prosthesis, implants, and tooth transplantation.[115,116,117]
Whole tooth regeneration by tissue engineering currently uses two methods; scaffold method and cell aggregates method. In scaffold method of tooth regeneration, the stem or precursor cells are arranged in proper spatial orientation using a biodegradable polymer membrane (polyglycolate/poly-L-lactate) or collagen sponge scaffolds to generate an artificial tooth germ. This tooth germ mimics tooth germ in the late stages of differentiation during normal tooth development which has proper cell polarization to achieve perfect spatial relationship in the final tooth structure (enamel, dentin, pulp, and cementum).[115,116,117] In cell aggregates method, dental epithelial tissue and mesenchymal cell pellets are dispersed in a well-controlled culture condition to create an artificial tooth germ. The tooth germ formed in this method mimics a tooth germ of the early inductive stage of tooth development where cell to cell and epithelial-mesenchymal interactions are predominant.[118,119,120,121,122,123] The hurdles associated with whole tooth regeneration with bioengineered tooth germ in the adult oral environment are fully developed neighboring tissues and lack of continuous signaling in adults in contrast to embryo where tooth germ develops along with the neighboring tissue and receives signaling molecules from there. It has been reported that a bioengineered tooth germ primordium isolated from a bioengineered tooth germ, could be transplanted into a tooth cavity after the extraction of a mandibular incisor and could develop into a tooth with typical spatial orientation of structures, such as enamel, dentin, dental pulp, root, blood vessels, periodontal ligament, and alveolar bone. These findings suggest the possibility of successful generation of whole teeth by transplantation of bioengineered tooth germs into the adult oral environment in future.[124,125]
Conclusion
Thus, we have seen the endless possibilities of what intelligent harnessing of SCs can achieve. The bioengineering technologies developed for tooth regeneration will make substantial contributions to understand the developmental process and will encourage future organ replacement by regenerative therapies in a wide variety of organs such as the liver, kidney, and heart. Further research toward the same has the potential to herald a new dawn in effective treatment of notoriously difficult diseases which could prove highly beneficial to mankind in the long run.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–63. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 2.MacCallum WG. A Text-book of Pathology. Philadelphia, PA: WB Saunders; 1924. pp. 1115–22. [Google Scholar]
- 3.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 4.Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–7. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- 5.Papaioannou VE, Rossant J. Effects of the embryonic environment on proliferation and differentiation of embryonal carcinoma cells. Cancer Surv. 1984;2:165–83. [Google Scholar]
- 6.Gunsilius E, Gastl G, Petzer AL. Hematopoietic stem cells. Biomed Pharmacother. 2001;55:186–94. doi: 10.1016/s0753-3322(01)00051-8. [DOI] [PubMed] [Google Scholar]
- 7.Arey LB. 7th ed. Philadelphia, PA: W.B. Saunders; 1974. Developmental Anatomy: A Textbook and Laboratory Manual of Embryology. [Google Scholar]
- 8.Boutwell RK. Some biological aspects of skin carcinogenisis. Prog Exp Tumor Res. 1964;4:207–50. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- 9.Wobus AM, Boheler KR. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–78. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 10.Trosko JE, Chang CC. Stem cell theory of carcinogenesis. Toxicol Lett. 1989;49:283–95. doi: 10.1016/0378-4274(89)90038-6. [DOI] [PubMed] [Google Scholar]
- 11.Human Stem Cell Research. Australian Academy of Science. 2001. [Last accessed on 2001 Apr 18]. pp. 1–35. Available from: https://www.science.org.au/sites/default/files/user-content/stemcell.pdf .
- 12.Lajtha LG. In Canadian Cancer Conference; 1967. pp. 31–9. [PubMed] [Google Scholar]
- 13.Lajtha LG. Stem cell concepts. Nouv Rev Fr Hematol. 1979;21:59–65. [PubMed] [Google Scholar]
- 14.Lajtha LG. Stem cell concepts. Differentiation. 1979;14:23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 15.Lajtha LG. Haemopoietic stem cells: Concept and definitions. Blood Cells. 1979;5:447–55. [PubMed] [Google Scholar]
- 16.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci U S A. 1997;94:5709–12. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coucouvanis E, Martin GR. Signals for death and survival: A two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–87. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 19.Scholer HR. The potential of stem cells: An inventory. In: Knoepffler N, Schipanski D, Sorgner SL, editors. Human Biotechnology as Social Challenge. Burlington (USA): Ashgate Publishing Ltd; 2007. p. 28. [Google Scholar]
- 20.Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol. 2009;114:185–99. doi: 10.1007/10_2008_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100:12–27. doi: 10.1263/jbb.100.12. [DOI] [PubMed] [Google Scholar]
- 22.Fox News; 24 August; 2006. [Last retrieved on 2010 Feb 28]. New Stem-cell Procedure Doesn't Harm Embryos, Company Claims. Available from: http://www.foxnews.com/story/2006/08/24/new-stem-cell-procedure-doesnt-harmembryos-company-claims.html . [Google Scholar]
- 23.Conner DA. Mouse Embryonic Stem (ES) Cell Culture. Current Protocols in Molecular Biology. 2001;51:1–23. doi: 10.1002/0471142727.mb2303s51. [DOI] [PubMed] [Google Scholar]
- 24.Bongso A, Lee EH, editors. Stem Cells: From Bench Top to Bedside. Haslemere (UK): World Scientific; 2005. Stem cells: Their definition, classification and sources; p. 05. [Google Scholar]
- 25.Canadian Press. Amherst Daily News; 03 January; 2009. [Last retrieved on 2010 Feb 28]. Researchers Find New Method for Turning Adult Cells into Stem Cells. Available from: http://www.cumberlandnewsnow.com/Living/2009-03-02/article-376618/Researchers-find-new-method-for-turning-adult-cellsinto-stem-cells/1 . [Google Scholar]
- 26.Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–7. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 27.Gardner RL. Stem cells: Potency, plasticity and public perception. J Anat. 2002;200:277–82. doi: 10.1046/j.1469-7580.2002.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Barrilleaux B, Phinney DG, Prockop DJ, O’Connor KC. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–19. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 30.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulati SC, Yahalom J, Portlock C. Autologous bone marrow transplantation. Curr Probl Cancer. 1991;15:1–57. [PubMed] [Google Scholar]
- 32.Kane ED. Stem-cell Therapy Shows Promise for Horse Soft-tissue Injury, Disease. DVM Newsmagazine. [Last retrieved on 2008 May 12]. Available from: http://veterinarynews.dvm360.com/stem-cell-therapy-shows-promise-horse-soft-tissue-injury-disease?rel=canonical .
- 33.Stem Cell FAQ. US Department of Health and Human Services. 2004. [Last retrieved on 2010 Mar 07]. Available from: http://www.alsindependence.com/Stem_Cell_Frequently_Asked_Questions.htm .
- 34.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs.those from other sources: Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turk J. A concise review on the classification and nomenclature of stem cells. Hematology. 2008;25:57–9. [PubMed] [Google Scholar]
- 36.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 38.Cavaleri F, Scholer H. Hand Book of Stem Cells. Ch 3. Vol. 1. USA: Elsevier academic press; 2004. Molecular facets of pluripotency; pp. 27–44. [Google Scholar]
- 39.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–90. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 40.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 41.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–60. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolch W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 43.Hibi M, Hirano T. Gab-family adapter molecules in signal transduction of cytokine and growth factor receptors, and T and B cell antigen receptors. Leuk Lymphoma. 2000;37:299–307. doi: 10.3109/10428190009089430. [DOI] [PubMed] [Google Scholar]
- 44.Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–4. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 45.Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: Rounding up the usual suspects. Cell. 2002;108:489–500. doi: 10.1016/s0092-8674(02)00644-x. [DOI] [PubMed] [Google Scholar]
- 46.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 47.Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932–41. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 49.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virchow R. Cellular Pathology as Based upon Physiological and Pathological Histology. Berlin: August Hirschwald; 1958. [DOI] [PubMed] [Google Scholar]
- 51.Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: Evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. 1999;96:7029–34. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci. 2001;21:8108–18. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–70. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 54.Gunzalez R, Teng YD, Park KI, Lee JP, Ourednik J, Ourednik V, Imitola J, et al. Ch 52. Vol. 1. USA: Elsevier academic press; 2004. Neural stem cells: Therapeutic application in neurodegenerative diseases. Essentials of Stem Cell Biology; pp. 383–94. [Google Scholar]
- 55.Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–50. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 56.Hassink RJ, Dowell JD, Brutel de la Rivière A, Doevendans PA, Field LJ. Stem cell therapy for ischemic heart disease. Trends Mol Med. 2003;9:436–41. doi: 10.1016/j.molmed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Rubart M, Wang E, Dunn KW, Field LJ. Two-photon molecular excitation imaging of Ca2+ transients in Langendorff-perfused mouse hearts. Am J Physiol Cell Physiol. 2003;284:C1654–68. doi: 10.1152/ajpcell.00469.2002. [DOI] [PubMed] [Google Scholar]
- 58.Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92:1217–24. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- 59.Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation. 1991;48:173–82. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 60.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–53. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 61.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–86. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zweigerdt R, Burg M, Willbold E, Abts H, Ruediger M. Generation of confluent cardiomyocyte monolayers derived from embryonic stem cells in suspension: A cell source for new therapies and screening strategies. Cytotherapy. 2003;5:399–413. doi: 10.1080/14653240310003062. [DOI] [PubMed] [Google Scholar]
- 63.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 64.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 65.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niklason LE, Langer R. Prospects for organ and tissue replacement. JAMA. 2001;285:573–6. doi: 10.1001/jama.285.5.573. [DOI] [PubMed] [Google Scholar]
- 67.Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: Challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–6. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 70.Weir GC, Haggensen A, Bonnerweir S. Handbook of Stem Cells. Vol. 1. Academic Press; 2004. Insulin producing cells derived from embryonic stem cells. A potential treatment for diabetes; pp. 723–9. [Google Scholar]
- 71.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 72.Nakahara H, Bruder SP, Goldberg VM, Caplan AI. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop Relat Res. 1990;259:223–32. [PubMed] [Google Scholar]
- 73.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 74.Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999;367:S312–23. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 75.Schultz GS, White M, Mitchell R, Brown G, Lynch J, Twardzik DR, et al. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987;235:350–2. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- 76.Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–6. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- 77.Pellegrini G, Bondanza S, Guerra L, De Luca M. Cultivation of human keratinocyte stem cells: Current and future clinical applications. Med Biol Eng Comput. 1998;36:778–90. doi: 10.1007/BF02518885. [DOI] [PubMed] [Google Scholar]
- 78.Límová M, Grekin RC. Synthetic membranes and cultured keratinocyte grafts. J Am Acad Dermatol. 1990;23:713–9. doi: 10.1016/0190-9622(90)70279-q. [DOI] [PubMed] [Google Scholar]
- 79.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–45. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 80.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–43. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 81.Pellegrini G, Ranno R, Stracuzzi G, Bondanza S, Guerra L, Zambruno G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–79. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 82.Stern R, McPherson M, Longaker MT. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J Burn Care Rehabil. 1990;11:7–13. doi: 10.1097/00004630-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Jeschke MG, Richter W, Ruf SG. Cultured autologous outer root sheath cells: A new therapeutic alternative for chronic decubitus ulcers. Plast Reconstr Surg. 2001;107:1803–6. doi: 10.1097/00006534-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 84.Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf, a human skin equivalent. Cutis. 1998;62:1–8. [PubMed] [Google Scholar]
- 85.Platt JL. New directions for organ transplantation. Nature. 1998;392:11–7. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- 86.Nerem RM. Cellular engineering. Ann Biomed Eng. 1991;19:529–45. doi: 10.1007/BF02367396. [DOI] [PubMed] [Google Scholar]
- 87.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 88.Till JE, McCulloch EA. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980;605:431–59. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- 89.Levenberg S, Khademhosseini A, Langer R. Embryonic stem cells in tissue engineering. Handbook of Stem Cells. 2004;1:737–46. [Google Scholar]
- 90.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 91.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 92.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 93.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 94.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–33. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 95.Dorfman J, Duong M, Zibaitis A, Pelletier MP, Shum-Tim D, Li C, et al. Myocardial tissue engineering with autologous myoblast implantation. J Thorac Cardiovasc Surg. 1998;116:744–51. doi: 10.1016/S0022-5223(98)00451-6. [DOI] [PubMed] [Google Scholar]
- 96.Storch A, Schwarz J. Neural stem cells and Parkinson's disease. J Neurol. 2002;249(Suppl 3):30–2. doi: 10.1007/s00415-002-1306-z. [DOI] [PubMed] [Google Scholar]
- 97.Fricker J. Human neural stem cells on trial for Parkinson's disease. Mol Med Today. 1999;5:144. doi: 10.1016/s1357-4310(99)01454-9. [DOI] [PubMed] [Google Scholar]
- 98.Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355:S247–56. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 99.McInerney JM, Nemeth MJ, Lowrey CH. Erythropoiesis: Review Article: Slow and Steady Wins The Race? Progress in the Development of Vectors for Gene Therapy of beta-Thalassemia and Sickle Cell Disease. Hematology. 2000;4:437–55. [PubMed] [Google Scholar]
- 100.McCune SL, Reilly MP, Chomo MJ, Asakura T, Townes TM. Recombinant human hemoglobins designed for gene therapy of sickle cell disease. Proc Natl Acad Sci U S A. 1994;91:9852–6. doi: 10.1073/pnas.91.21.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Damme A, Vanden Driessche T, Collen D, Chuah MK. Bone marrow stromal cells as targets for gene therapy. Curr Gene Ther. 2002;2:195–209. doi: 10.2174/1566523024605645. [DOI] [PubMed] [Google Scholar]
- 102.Bradfute SB, Goodell MA. Adenoviral transduction of mouse hematopoietic stem cells. Mol Ther. 2003;7:334–40. doi: 10.1016/s1525-0016(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 103.May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–6. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 104.Olivares EC, Hollis RP, Chalberg TW, Meuse L, Kay MA, Calos MP. Site-specific genomic integration produces therapeutic Factor IX levels in mice. Nat Biotechnol. 2002;20:1124–8. doi: 10.1038/nbt753. [DOI] [PubMed] [Google Scholar]
- 105.Goncz KK, Gruenert DC. Site-directed alteration of genomic DNA by small-fragment homologous replacement. Methods Mol Biol. 2000;133:85–99. doi: 10.1385/1-59259-215-5:85. [DOI] [PubMed] [Google Scholar]
- 106.Davis BR, Nicol L. Handbook of Stem Cells. Ch 72. Vol. 1. USA: Elsevier academic press; 2004. Stem cell gene therapy; pp. 793–804. [Google Scholar]
- 107.Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–54. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 108.Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, et al. Pluripotency regulators in human mesenchymal stem cells: Expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915–23. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- 109.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 110.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33:703–8. doi: 10.1016/j.joen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–23. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 113.Koyama N, Okubo Y, Nakao K, Bessho K. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501–6. doi: 10.1016/j.joms.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 114.Jamal M, Chogle S, Goodis H, Karam SM. Dental stem cells and their potential role in regenerative medicine. J Med Sci. 2011;4:53–61. [Google Scholar]
- 115.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–8. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 116.Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27:3238–48. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 117.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28:680–9. doi: 10.1016/j.biomaterials.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 119.Layer PG, Robitzki A, Rothermel A, Willbold E. Of layers and spheres: The reaggregate approach in tissue engineering. Trends Neurosci. 2002;25:131–4. doi: 10.1016/s0166-2236(00)02036-1. [DOI] [PubMed] [Google Scholar]
- 120.Yamamoto H, Kim EJ, Cho SW, Jung HS. Analysis of tooth formation by reaggregated dental mesenchyme from mouse embryo. J Electron Microsc (Tokyo) 2003;52:559–66. doi: 10.1093/jmicro/52.6.559. [DOI] [PubMed] [Google Scholar]
- 121.Hu B, Nadiri A, Bopp-Kuchler S, Perrin-Schmitt F, Wang S, Lesot H. Dental epithelial histo-morphogenesis in the mouse: Positional information versus cell history. Arch Oral Biol. 2005;50:131–6. doi: 10.1016/j.archoralbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069–75. doi: 10.1089/ten.2006.12.2069. [DOI] [PubMed] [Google Scholar]
- 123.Song Y, Zhang Z, Yu X, Yan M, Zhang X, Gu S, et al. Application of lentivirus-mediated RNAi in studying gene function in mammalian tooth development. Dev Dyn. 2006;235:1334–44. doi: 10.1002/dvdy.20706. [DOI] [PubMed] [Google Scholar]
- 124.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–30. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 125.Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–22. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]


