Abstract
Oral cancer is one of the most common cancers worldwide. Despite of various advancements in the treatment modalities, oral cancer mortalities are more, particularly in developing countries like India. This is mainly due to the delay in diagnosis of oral cancer. Delay in diagnosis greatly reduces prognosis of the treatment and also cause increased morbidity and mortality rates. Early diagnosis plays a key role in effective management of oral cancer. A rapid diagnostic technique can greatly aid in the early diagnosis of oral cancer. Now a day's many adjunctive oral cancer screening techniques are available for the early diagnosis of cancer. Among these, autofluorescence based diagnostic techniques are rapidly emerging as a powerful tool. These techniques are broadly discussed in this review.
KEY WORDS: Autofluorescence imaging, autofluorescence spectroscopy, autofluorescence visualization, early diagnosis, noninvasive, oral cancer, porphyrins, visually enhanced lesion scope
Oral cancer is a major health problem globally and is considered the sixth most common cancer in the humans.[1] In India, the prevalence of oral cancer and its associated mortality rates are worst among all the cancers.[2] Five years survival rate is approximately 50–55% but mostly, the survival rate is only 16% especially when diagnosed at advanced stages.[3] The diagnostic delay may be due to patient delay or professional delay. However, both can occur. Hence prevention of oral cancer and its associated morbidity and mortality relay upon the early detection.
The presence of epithelial dysplasia is generally accepted as one of the most important predictors of malignant development in premalignant lesions. If a premalignant lesion is detected and treated at their initial stage, they may not progress to cancer. Any tool that improves the early detection of lesions can effectively improvise oral cancer screening system. Many light based noninvasive diagnostic techniques has been developed that aids in the early detection of oral cancer. Among these, autofluorescence based diagnostic techniques are emerging as one of the most potential and dynamic tool in the early diagnosis of oral cancer.
Autofluorescence
When cells interact with the light of particular wavelength, they become excited and re-emit light of varying wavelength (color), which is called as autofluorescence. Autofluorescence of tissues is produced by fluorophores that naturally occurs in most of the human tissues. The naturally occurring fluorophores are collagen, tryptophan, elastin, keratin hemoglobin and NADH etc., Potentially malignant disorders and cancerous conditions cause a change in the concentration of these fluorophores.[4] They also cause alterations in the natural light scattering and absorption properties of the tissues. Changes in the spectral property of mucosa can be detected and can be used as a tool in diagnosing the potentially malignant disorders and cancerous conditions.
Autofluorescence of oral neoplastic tissue
The autofluorescence property of oral mucosa depends upon the anatomic location and the type of lesion that occurs on it. In normal mucosa, fluorescence in the ultraviolet (UV) and visible region of the spectrum is predominantly due to the collagen that is present in the connective tissue. The epithelium always shows weak autofluorescence due to the mitochondrial nicotinamide adenine dinucleotide dehydrogenase (NADH) and flavin adenine dinucleotide (FAD) present in the basal cells of the epithelium. Keratin present in the epithelium also produces fluorescence. Neoplasia causes loss of stromal collagen, which leads to loss of autofluorescence. Epithelial dysplasia increases mitochondrial fluorescence of the epithelium. Loss of both epithelial and stromal autofluorescence is observed in inflammatory lesions.[5]
The major advantage of autofluorescence technique is that they are noninvasive procedures that can minimize the need of unnecessary biopsies. They can be performed on medically compromised patients who are contraindicated for biopsies. They can be performed as in vivo chair side procedures, which can be used to define surgical margins of a lesion. They can also be used as a tool in mass screening procedures.
The autofluorescence based diagnostic techniques for oral cancer are:
Visual autofluorescence (visually enhanced lesion scope [VELscope])
Autofluorescence imaging
Autofluorescence spectroscopy.
Visual autofluorescence (Visually Enhanced Lesion Scope)
The visually enhanced lesion scope is a chair side diagnostic technique now marketed to a general dentist. It consists of a handheld device or scope which illuminated the mucosa with a fluorescent light of wavelength 400 – 460 nm and a manual unit for direct visualization.[6] Normal mucosa emits a green autofluorescence when exposed to this fluorescent light due to the presence of naturally occurring fluorophores in the mucosa. Abnormal mucosa appears dark due to the reduction or change in the quantity and quality of fluorophores in the mucosa which occurs due to abnormal or neoplastic changes of the mucosa. Many studies claim that VELscope improves the contrast between normal and lesional area, thereby easy identification of the lesion. Studies on VELscope are listed in the Table 1.
Table 1.
Studies on VELscope for oral cancer detection
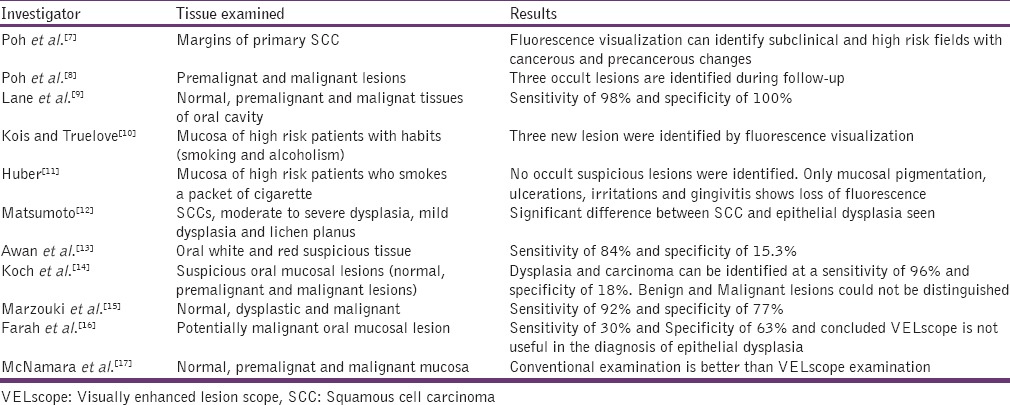
It's a rapid and easy way of detecting cancerous changes in the mucosa, which takes only few minutes, so it can be incorporated in day to day clinic practice. It's also useful in detecting tumor margin. It can be used in mass screening campaigns to facilitate easy and rapid diagnosis among a high-risk group.
Autofluorescence imaging
Autofluorescence imaging is a chairside technique that can highlight a precancerous lesion more efficiently than the normal white light examination. The advantage of autofluorescence imaging when compared to that of the visual autofluorescence VELscope is that visual autofluorescence is always subjective to the observer's skill of identifying the lesion but in cases of autofluorescence imaging digital images are captured and they can be compared with the normal algorithm chart to find out high risk or lesional areas.
In this technique, tissues are illuminated with a light source, in the near UV to the green spectral range. The images of the fluorescence produced by the tissue are recorded using a camera. These images capture the alteration in the absorption and scattering events of the tissue. The captured digital images are used to interpret the lesion. Both normal and cancerous oral mucosa appears green in color. The neoplastic tissues possess low autofluorescence intensities when compare to normal tissue due to loss of stromal collagen. Thus, the neoplastic lesions can be demarcated from the normal tissue by a darker shade of green.[18] Studies on Autofluorescence imaging are listed in the Table 2.
Table 2.
Studies on autofluorescence imaging for oral cancer detection
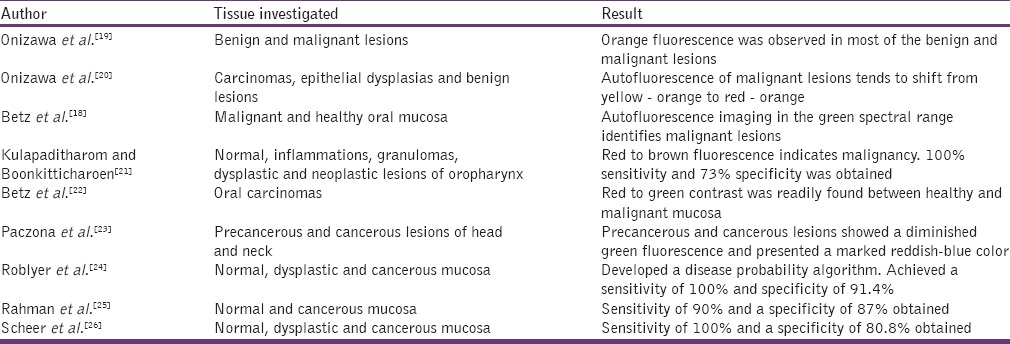
Some investigators used photosensitizers, which act as fluorescent markers and increases fluorescence of the tissue thereby favoring detection and demarcation of tumors. One of the most promising photosensitizers for an oral cancer diagnosis is 5-aminolevulinic acid. Lesions showing red fluorescence are mainly due to the porphyrins. This porphyrin fluorescence is usually seen on ulcerated and necrotic surfaces.[19] They can produce false positive results.[4]
Autofluorescence spectroscopy
Autofluorescence spectroscopy is a rapidly emerging noninvasive technique that can detect the structural and chemical alteration in oral mucosa. Potentially malignant disorders and oral cancer are always associated with structural and biochemical alterations in the mucosa. Even before the clinical evidence of these lesions, molecular level change occurs. Change in the optical property of the mucosa may give a clue about these early molecular level changes of the mucosa. Compared to autofluorescence imaging, autofluorescence spectroscopy can detect even the minor alterations of the mucosa.
This system consists of a light source usually in the near-UV to visible wavelength range that excites the tissue through a fiber. The fluorescence that is produced in the tissue is received with an analyzer probe. This probe can be disinfected with chlorhexidine gluconate and dried before use on a patient. Measurements were performed under a low ambient light level with the probe in contact with the oral mucosa. The obtained wavelengths are analyzed by a spectrograph. The recorded fluorescence spectra can be saved to a computer, which allows mathematical spectral analysis of many types.
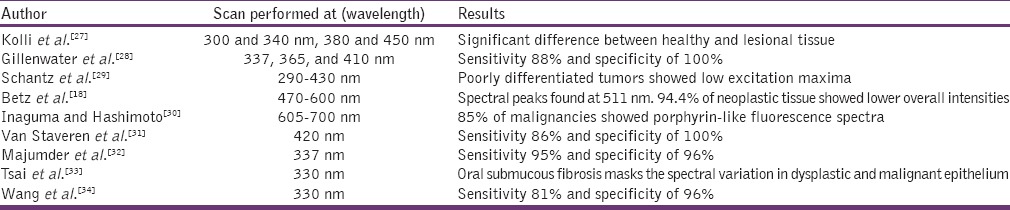
The light source used and the excitation wavelength performed in each studies vary according to each investigator. There will be a decrease in fluorescence intensity and significant difference in the fluorescence intensities between the normal and neoplastic mucosa. Studies carried out using autofluorescent spectroscope for oral cancer detection is listed in Table 3.
Table 3.
Studies carried out using autofluorescent spectroscope for oral cancer detection
Conclusion
Early detection of oral premalignant and malignant lesions is the most effective step of improving the survival rate of oral cancer patients. The main purpose of visual autofluorescence, fluorescence imaging and autofluorescence spectroscopy is to highlight oral lesions and to assist the physicians to locate better the surgical margins. These techniques cannot act as substitute for conventional oral biopsy. A biopsy is the gold standard technique for the diagnosis of oral cancer. Autofluorescence techniques can only act as an adjuvant and cannot be used as a confirmatory test in the diagnosis of oral cancer. Still advancements and standardization protocol is required in this field. As the technology and techniques evolve, these modalities may progressively reduce the need for conventional biopsy techniques, can define the surgical margins, and may emerge as a powerful chair side diagnostic tool for oral cancer.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: An overview of the literature. J Oral Pathol Med. 2008;37:1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumari S, Sathiyajeeva J, Kumar CS, Sunil PM, Thayumanavan B. Molecular predictors in the early diagnosis of oral cancer. J Clin Diagn Res. 2013;7:942–4. doi: 10.7860/JCDR/2013/5058.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–31. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Shin D, Vigneswaran N, Gillenwater A, Richards-Kortum R. Advances in fluorescence imaging techniques to detect oral cancer and its precursors. Future Oncol. 2010;6:1143–54. doi: 10.2217/fon.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balevi B. Evidence-based decision making: Should the general dentist adopt the use of the VELscope for routine screening for oral cancer? J Can Dent Assoc. 2007;73:603–6. [PubMed] [Google Scholar]
- 7.Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12:6716–22. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 8.Poh CF, Ng SP, Williams PM, Zhang L, Laronde DM, Lane P, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29:71–6. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 9.Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11:024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 10.Kois JC, Truelove E. Detecting oral cancer: A new technique and case reports. Dent Today. 2006;25:94–96. 7. [PubMed] [Google Scholar]
- 11.Huber MA. Assessment of the VELscope as an adjunctive examination tool. Tex Dent J. 2009;126:528–35. [PubMed] [Google Scholar]
- 12.Matsumoto K. Detection of potentially malignant and malignant lesions of oral cavity using autofluorescence visualization device. Kokubyo Gakkai Zasshi. 2011;78:73–80. [PubMed] [Google Scholar]
- 13.Awan KH, Morgan PR, Warnakulasuriya S. Evaluation of an autofluorescence based imaging system (VELscope™) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 2011;47:274–7. doi: 10.1016/j.oraloncology.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Koch FP, Kaemmerer PW, Biesterfeld S, Kunkel M, Wagner W. Effectiveness of autofluorescence to identify suspicious oral lesions – A prospective, blinded clinical trial. Clin Oral Investig. 2011;15:975–82. doi: 10.1007/s00784-010-0455-1. [DOI] [PubMed] [Google Scholar]
- 15.Marzouki HZ, Tuong Vi Vu T, Ywakim R, Chauvin P, Hanley J, Kost KM. Use of fluorescent light in detecting malignant and premalignant lesions in the oral cavity: A prospective, single-blind study. J Otolaryngol Head Neck Surg. 2012;41:164–8. [PubMed] [Google Scholar]
- 16.Farah CS, McIntosh L, Georgiou A, McCullough MJ. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck. 2012;34:856–62. doi: 10.1002/hed.21834. [DOI] [PubMed] [Google Scholar]
- 17.McNamara KK, Martin BD, Evans EW, Kalmar JR. The role of direct visual fluorescent examination (VELscope) in routine screening for potentially malignant oral mucosal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:636–43. doi: 10.1016/j.oooo.2012.07.484. [DOI] [PubMed] [Google Scholar]
- 18.Betz CS, Mehlmann M, Rick K, Stepp H, Grevers G, Baumgartner R, et al. Autofluorescence imaging and spectroscopy of normal and malignant mucosa in patients with head and neck cancer. Lasers Surg Med. 1999;25:323–34. doi: 10.1002/(sici)1096-9101(1999)25:4<323::aid-lsm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Onizawa K, Saginoya H, Furuya Y, Yoshida H. Fluorescence photography as a diagnostic method for oral cancer. Cancer Lett. 1996;108:61–6. doi: 10.1016/s0304-3835(96)04388-1. [DOI] [PubMed] [Google Scholar]
- 20.Onizawa K, Saginoya H, Furuya Y, Yoshida H, Fukuda H. Usefulness of fluorescence photography for diagnosis of oral cancer. Int J Oral Maxillofac Surg. 1999;28:206–10. [PubMed] [Google Scholar]
- 21.Kulapaditharom B, Boonkitticharoen V. Performance characteristics of fluorescence endoscope in detection of head and neck cancers. Ann Otol Rhinol Laryngol. 2001;110:45–52. doi: 10.1177/000348940111000109. [DOI] [PubMed] [Google Scholar]
- 22.Betz CS, Stepp H, Janda P, Arbogast S, Grevers G, Baumgartner R, et al. A comparative study of normal inspection, autofluorescence and 5-ALA-induced PPIX fluorescence for oral cancer diagnosis. Int J Cancer. 2002;97:245–52. doi: 10.1002/ijc.1596. [DOI] [PubMed] [Google Scholar]
- 23.Paczona R, Temam S, Janot F, Marandas P, Luboinski B. Autofluorescence videoendoscopy for photodiagnosis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2003;260:544–8. doi: 10.1007/s00405-003-0635-6. [DOI] [PubMed] [Google Scholar]
- 24.Roblyer D, Kurachi C, Stepanek V, Williams MD, El-Naggar AK, Lee JJ, et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev Res (Phila) 2009;2:423–31. doi: 10.1158/1940-6207.CAPR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman MS, Ingole N, Roblyer D, Stepanek V, Richards-Kortum R, Gillenwater A, et al. Evaluation of a low-cost, portable imaging system for early detection of oral cancer. Head Neck Oncol. 2010;2:10. doi: 10.1186/1758-3284-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheer M, Neugebauer J, Derman A, Fuss J, Drebber U, Zoeller JE. Autofluorescence imaging of potentially malignant mucosa lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:568–77. doi: 10.1016/j.tripleo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Kolli VR, Shaha AR, Savage HE, Sacks PG, Casale MA, Schantz SP. Native cellular fluorescence can identify changes in epithelial thickness in-vivo in the upper aerodigestive tract. Am J Surg. 1995;170:495–8. doi: 10.1016/s0002-9610(99)80338-9. [DOI] [PubMed] [Google Scholar]
- 28.Gillenwater A, Jacob R, Ganeshappa R, Kemp B, El-Naggar AK, Palmer JL, et al. Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Arch Otolaryngol Head Neck Surg. 1998;124:1251–8. doi: 10.1001/archotol.124.11.1251. [DOI] [PubMed] [Google Scholar]
- 29.Schantz SP, Kolli V, Savage HE, Yu G, Shah JP, Harris DE, et al. In vivo native cellular fluorescence and histological characteristics of head and neck cancer. Clin Cancer Res. 1998;4:1177–82. [PubMed] [Google Scholar]
- 30.Inaguma M, Hashimoto K. Porphyrin-like fluorescence in oral cancer: In vivo fluorescence spectral characterization of lesions by use of A near-ultraviolet excited autofluorescence diagnosis system and separation of fluorescent extracts by capillary electrophoresis. Cancer. 1999;86:2201–11. doi: 10.1002/(sici)1097-0142(19991201)86:11<2201::aid-cncr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.van Staveren HJ, van Veen RL, Speelman OC, Witjes MJ, Star WM, Roodenburg JL. Classification of clinical autofluorescence spectra of oral leukoplakia using an artificial neural network: A pilot study. Oral Oncol. 2000;36:286–93. doi: 10.1016/s1368-8375(00)00004-x. [DOI] [PubMed] [Google Scholar]
- 32.Majumder SK, Ghosh N, Kataria S, Gupta PK. Nonlinear pattern recognition for laser-induced fluorescence diagnosis of cancer. Lasers Surg Med. 2003;33:48–56. doi: 10.1002/lsm.10191. [DOI] [PubMed] [Google Scholar]
- 33.Tsai T, Chen HM, Wang CY, Tsai JC, Chen CT, Chiang CP. In vivo autofluorescence spectroscopy of oral premalignant and malignant lesions: Distortion of fluorescence intensity by submucous fibrosis. Lasers Surg Med. 2003;33:40–7. doi: 10.1002/lsm.10180. [DOI] [PubMed] [Google Scholar]
- 34.Wang CY, Tsai T, Chen HM, Chen CT, Chiang CP. PLS-ANN based classification model for oral submucous fibrosis and oral carcinogenesis. Lasers Surg Med. 2003;32:318–26. doi: 10.1002/lsm.10153. [DOI] [PubMed] [Google Scholar]


