Abstract
In the last few years, the interest for oral cytology as a diagnostic and prognostic methodology, for monitoring patients in oral potentially malignant disorders and oral cancer has re-emerged substantially. In 1983, buccal mucosal micronuclei assay was first proposed to evaluate genetic instability. There are biomarkers that predict if a potentially malignant disorder is likely to develop into an aggressive tumor. These genotoxic and carcinogenic chemicals have been reported to be potent clastogenic and mutagenic agents which are thought to be responsible for the induction of chromatid/chromosomal aberrations resulting in the production of micronuclei. Various studies have concluded that the gradual increase in micronucleus (MN) counts from normal oral mucosa to potentially malignant disorders to oral carcinoma suggested a link of this biomarker with neoplastic progression. MN scoring can be used as a biomarker to identify different preneoplastic conditions much earlier than the manifestations of clinical features and might specifically be exploited in the screening of high-risk population for a specific cancer. Hence, it can be used as a screening prognostic and educational tool in community centers of oral cancer.
KEY WORDS: Chromosomal aberration, Exfoliative cytology, genotoxic damage, micronucleus
Cancer is one of the major global threats to public health. Oral cancer is one of the 10 most common among all cancers and oral squamous cell carcinoma is the most common of all oral malignancies that arise directly or, is preceded by some benign lesions or conditions which is termed as potentially malignant disorders.[1]
Biological behavior of these potentially malignant disorders like leukoplakia, oral submucous fibrosis, lichen planus, etc., are unpredictable as some of them may progress to malignant transformation. The risk of malignant transformation has been reported to be between 6.6% and 36.4%.[2] It would be of practical importance to identify the risk group among them.[3] Micronuclei assay is an excellent candidate to serve as such a biomarker, which detects chromosome loss.[4]
Genomic damage is probably the most important fundamental cause of developmental and degenerative disease.[5]
It is generally accepted that oral carcinogenesis is a multi-step process of accumulated genetic damage leading to cell dysregulation with a disruption in cell signaling.[1]
Though histopathology of biopsied material is a gold standard in diagnosing cancers, biopsy being an invasive technique it loses viability, as a result of variations in blood glucose and disease itself.[4] Biopsy has limitations for some professionals and psychological implications for some patients.[3,6]
These days interest has been increasing in noninvasive diagnostic testing because of the pain associated with invasive techniques.[7] Ease of use and painless method can encourage more frequent testing.[8] In such situation, exfoliative cytology would be a relatively safe procedure, which can also be done chair-side during routine oral examination.[7] With the advancement in the field of quantitative exfoliative cytology, there is the reemergence of oral cytology as a powerful diagnostic[9] and screening tool.[9] Screening involves checking for the presence of disease in a person who is symptom-free.[10] Conventional cytopathology has improved in the early detection of changes in individual exfoliated cells.[11]
Exfoliative cytology is defined as procurement and characterization of cells from the surface of the oral mucosa.[12]
History of Micronuclei
In 1970s, the term micronucleus (MN) test was suggested for the first time by Boller and Schmidt. Heddle who showed that this assay provided a simple method to detect the the genotoxic potential of mutagens after in vivo exposure of animals using bone marrow erythrocytes.[4]
In 1976, Countryman and Heddle recommended that peripheral blood lymphocytes could be used for the micronucleus approach and they recommended using micronuclei as a biomarker in testing schemes.[13]
In 1980 exfoliated buccal mucosa cells were used to evaluate the genotoxic effects of betel nuts and quid. The MN assay in buccal cells was also used to study cancerous and precancerous lesions and to monitor the effects of a number of chemopreventive agents.[6]
Theories of Origin of Micronuclei
There are two predominant mechanisms leading to the MN in a mitotic cell:
Chromosomal breakage
Dysfunction of the mitotic apparatus.[14]
Clastogens induce chromosome breaks and yield acentric fragments. These chromosomal fragments are directly included into micronuclei. In the other mechanism, aneugenic agents prevent the formation of spindle apparatus during mitosis.[14]
Molecular epidemiology is an area of increasingly intense activity and interest.[4,15] Molecular biological studies have provided evidence that individual susceptibility to cancer is mediated by genetic and environmental factors. DNA damages can be assessed by chromosomal aberrations, sister chromatid exchanges, and MN. Of these, MN test is found to be the most sensitive when compared with other test as it neither requires tedious procedure like cell culture nor it requires specific DNA stains.[16]
Micronucleus is defined as a microscopically visible round to oval cytoplasmic chromatin mass next to the nucleus.[17] MN are extranuclear cytoplasmic bodies. It is situated around the main nucleus within the inner half of the cytoplasm. The diameter of MN is less than that 1/3rd of that of the main nucleus, and so it can be readily differentiated from a binucleated cell.[14]
Micronucleus scoring or assay can also be done in human erythrocytes, lymphocytes, and exfoliated epithelial cells.[14] Since 1937, micronuclei have been regarded as indicators for genotoxic exposure.[18]
Etiology of Micronuclei
In normal healthy individuals due to exposure to environmental pollutants such as drugs, chemicals, food, and free radical injuries.[14]
Occupational and environmental exposures (organic solvents, antineoplastic agents, lead containing paints solvents, and drinking water contaminated with arsenic).[4,6,13]
Ionizing radiation (for the treatment of neoplasia but it also produces genetic damage).[4,6] Lifestyle factors (smoking, alcohol consumption, diet, vitamin deficiencies.[4,6] Gupta, Mhaske et al. conducted a study on MN index: An early diagnosis of oral carcinoma. The obtained data showed that stages of oral carcinoma and erythroplakia among premalignant cases associated with chronic use of tobacco had the highest MN index. Hence, the authors concluded that this index can be used as a biomarker screening test among the risk groups particularly the tobacco users.[16]
Routine diagnostic use of X-rays. X-rays are a potent mutagenic agent causing mutations and chromosomal aberrations.[19]
Genotoxic factors
Micronuclei are induced in oral exfoliated cells by a variety of substances including genotoxic agents like, medical procedures (e.g., radiation and chemicals), carcinogenic components in tobacco, betel nut and alcohol, and genetic factors such as inherited defects in DNA metabolism and/or repair.[5]
Carcinogen released from betel nut is thought to be responsible for the promotion of reactive oxygen species from the areca nut extracts. These reactive oxygen species can in turn cause damage to the DNA.[8]
Vasudevan et al. aimed to investigate the genotoxic effect associated with occupational exposure to chromium workers using the MN test. He concluded that chronic occupational exposure could lead to increased levels of DNA damage represented by increased frequency of MN.[20]
Epithelial cells are highly proliferative.[6] Buccal cells are the first barrier for the inhalation or ingestion route and are capable of metabolizing proximate carcinogens to reactive products.[1]
More than 90% of cancers arise in epithelial tissues, and these cells can be easily collected from the mouth without causing discomfort to patients. The procedure is feasible, cheap, and accurate; final results can be obtained in less time.[18]
Therefore, it could be argued that oral epithelial cells represent a preferred target site for early genotoxic events induced by carcinogenic agents entering the body via inhalation and ingestion.[6]
Micronucleus: Formation
The basal layer of oral epithelium contains the stem cells that may express genetic damage (chromosome breakage or loss) as MN during nuclear division.[5]
Micronuclei are extra nuclear cytoplasmic bodies. They are induced in cells by numerous genotoxic agents that damage the chromosomes. The damaged chromosomes, in the form of acentric chromatics or chromosome fragments, lag behind in anaphase when centric elements move towards the spindle poles. After telophase, the undamaged chromosomes, as well as the centric fragments, give rise to regular daughter nuclei. The lagging elements are included in the daughter cells too, but a considerable proportion is transferred into one or several secondary nuclei, which are as a rule much smaller than the principal nucleus and are therefore called the micro nuclei. This takes place in the basal layer of the epithelial tissue, where cells undergo mitosis. This rapid turnover of epithelial tissue brings the cells to the surface, where they exfoliate.[21] Bigger micro nuclei result from the exclusion of whole chromosome following damage to the spindle apparatus of the cell (aneugenic effect). Whereas small micro nucleus result from structural aberrations causing chromosomal fragments (clastogenic effect).[8]
The oral epithelium maintains itself by continuous cell renewal whereby new cells produced in the basal layer by mitosis migrate to the surface replacing those that are shed.[5]
Their frequency and the number of micronuclei are known to increase with carcinogenic stimuli, long before the development of clinical symptoms.
The optimal timing between 7 and 21 days after exposure is needed because peak expression may vary depending on the effects of the particular DNA damage or chromosomal exposure on the basal cell turnover rate.[2] It seems likely that cells with more chromosomal damage, and hence more micronuclei that leads to increased frequency of MN from precancer to cancer.[22]
Various uses of micronucleus scoring [Table 1].[14]
Table 1.
Other possible applications of Micronucleus Scoring
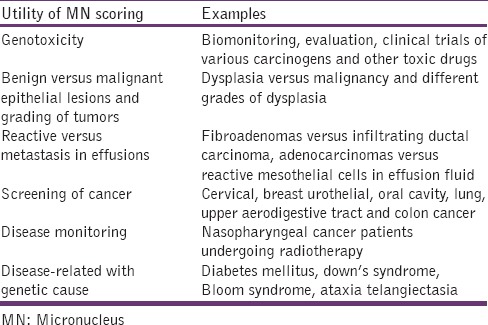
Collection of exfoliated oral mucosal cells
Exfoliated oral mucosa cells can be collected using a wooden tongue depressor, a metal spatula, toothpicks, toothbrush, and cytobrush. According to Ogden et al. the wooden spatula has been shown to be a satisfactory instrument for obtaining smears from the buccal mucosa.[23] Cytobrush sampling [Figure 1] is more frequently used which facilitates their uniform distribution onto the microscopic slide, thus probably improving sensitivity.[24]
Figure 1.
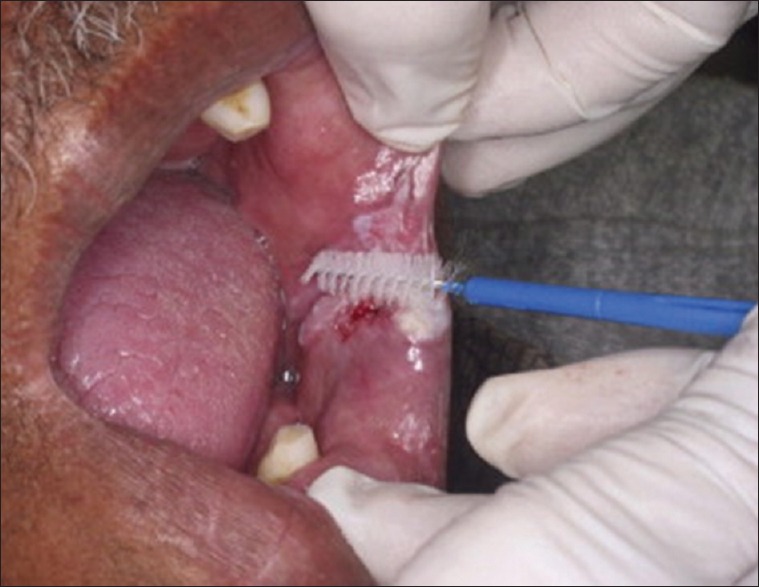
Smear obtained from potentially malignant disorder using a cytobrush
The instrument used for making smear should be easy to use in any location and provide an adequate number of epithelial cells.[25] MN frequency was higher when cells were collected by vigorous, rather than by light scraping.[4]
The smear procedure can also be done after rinsing the mouth gently with water to remove food debris. Using a slightly moistened wooden spatula [Figure 2] oral mucosal cells can be collected. The obtained cells are smeared on a sterile glass slide, fixed with a spray fixative and stained with papanicolaou stain. Smear can be observed under microscope with ×100 magnification [Figure 3].
Figure 2.
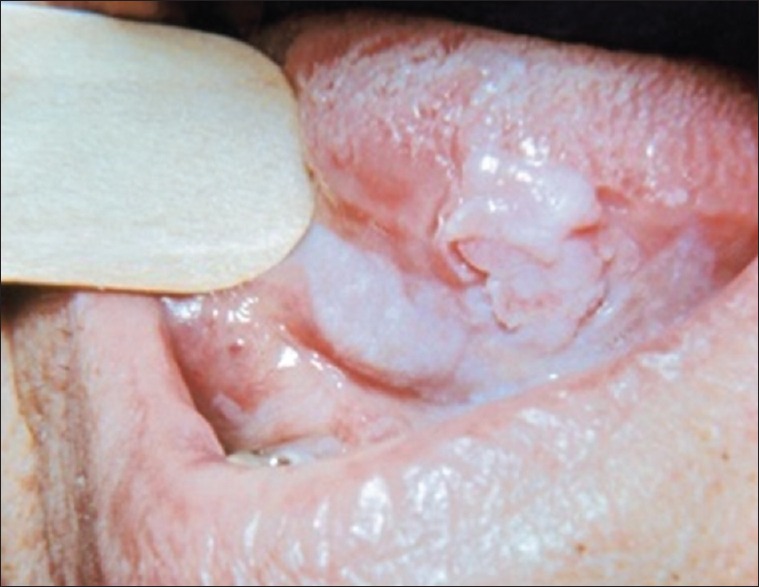
Smear obtained from the lesion using wooden spatula
Figure 3.
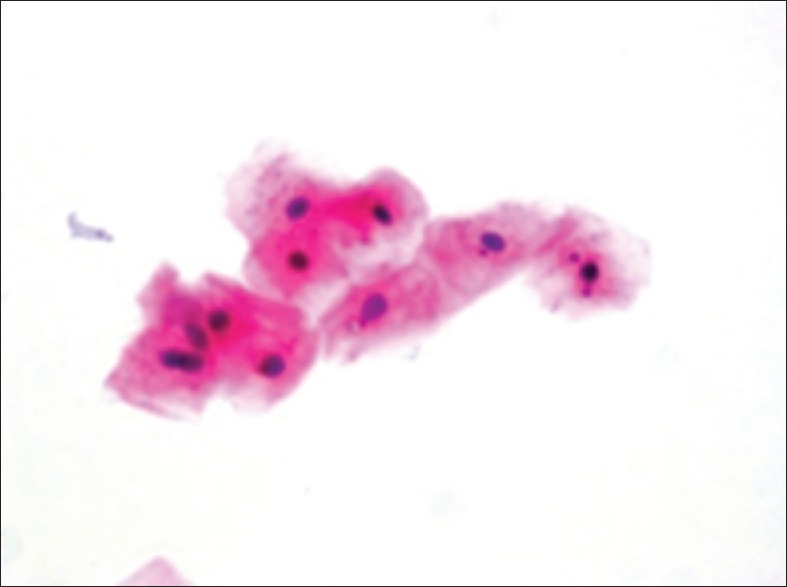
Micronuclei in exfoliated buccal epithelial cells
Dr. George N. Papanicolaou was the first to introduce Pap smear. More Details in 1928 in cervical tissues and since then this technique has helped reduce cervical cancer incidence and mortality rates by 75%. This is an easy technique and can be replicated in the oral cavity for analysis of the changes caused by smoking.[18] Palaskar and Jindal have shown that Pap is better stain for counting MN due to the fact that MN were easily seen in clear cytoplasm in regard to other stains like Giemsa stain.[24]
Many studies have shown that a variety of different stains can be used in micronuclei studies. Among the DNA-specific stains, the ones which are most widely used are Feulgen, acridine orange, 4,6-diamidino-2-phenylindole (DAPI), and propidium iodide were also used. About 30% of the studies on epithelial cells were conducted using nonspecific stains (Giemsa, May-Grunwald's Giemsa, and less frequently, Orcein).[4,6,24]
Criteria to assess micronuclei
Tolbert et al. criteria[9] parameters for identifying micronucleus are as follows:
Rounded smooth perimeter suggestive of a membrane
Less than a third the diameter of associated nucleus, but large enough to discern shape and color
Staining intensity similar to nucleus
Texture similar to nucleus
Same focal plane as nucleus
Absence of overlap with or bridge to nucleus
In general, the most commonly used is, zigzag method for screening of the slides. Cells with intact nuclei and cell boundaries should be counted.[26] Tolbert et al. recommended the scoring of at least 1000 cells in a slide so that more precise results would be obtained with increasing number of cells.[4]
Importance of micronuclei scoring in clinical pathology
Micronuclei have been used as a measurement and biomonitoring of genotoxicity of various carcinogens, heavy metal poisoning, antineoplastic drugs, pollutants, etc.
It projects as a biomarker of chromosomal damage.
This assay has been extensively used to evaluate the presence and the extent of chromosome damage in human populations exposed to genotoxic agents.[3]
The frequency of MN can help to assess the therapeutic prognosis.[9]
It represents as an “internal dosimeter” to estimate exposure to genotoxic and carcinogenic agents.[3]
Their frequency of occurrence is a measure of chromosome breakage in early cell divisions.[5]
It has been shown to have a sensitivity of 94%, specificity of 100%, and an accuracy of 95%.[4]
Importance of early detection
Early detection will help the clinician to plan the treatment thereby improving patient's survival rates. The prevention of PMD, oral cancer and its associated morbidity and mortality, hinges upon the early detection, allowing for histological evaluation and subsequent treatment depending on the stage of diagnosis[2]
Early detection of MN in a cell represents an “internal dosimeter” to estimate exposure to genotoxic and carcinogenic agents[3]
Screening of individuals who are at high risk of malignant transformation is more pivotal in preventing and reducing the costly and painful treatment later on
Micronuclei assay in exfoliated cells holds promise as, one of the biomarkers of exposure to genetic toxins and can provide as screening prognostic and educational tool in community center of PMD and cancer[9]
The MN assay in exfoliated buccal cells is potentially an excellent candidate to serve as such a biomarker as one of the early diagnostic tool in oral pre cancer and cancer.[4]
Kamboj and Mahajan conducted a study to define MN as an early diagnostic tool of leukoplakia and SCC. MN assay was performed on oral exfoliated cells of chosen subjects. They observed the frequency of mean percentage occurrence of MN cells increased significantly in comparison to controls with leukoplakia and SCC. They concluded that MN can be stated as an early indicator and an upcoming marker for diagnosing oral pre cancer and cancer.[27]
Buajeeb et al. conducted a study to determine the frequency of MN exfoliated cells in atrophic and erosive oral lichen planus (OLP). The authors revealed an increase in MN in OLP lesions. They concluded that the results of their study indicated genotoxic damage in atrophic and erosive OLP.[28]
Micronuclei assay in exfoliated cells holds promise as a one of the biomarkers of exposure to genetic toxins and provides as screening prognostic and educational tool in the community center of PMD and cancer.[9] It is noninvasive, simple, easy, clinical chairside technique, risk free that is well-accepted by the patient with no contraindications[29] and the scoring of micronuclei is rapid and does not require much expertise.[3]
Limitation of Micronuclei Scoring
Micronuclei scoring can be interfered by the bacteria that are commonly found in the mouth which can be differentiated by their characteristic shape
Small dye granules may sometime resemble MN, but usually have a slightly different refractility and color intensity
Other cellular structures such as keratohyalin granules resembling MN can lead to false positive results.[30]
Mahimkar et al. conducted a study to investigate the extent of chromosomal damage by analyzing micronucleated cell frequency in exfoliated buccal epithelial cells with oral leukoplakia. The authors concluded the chromosomal damage in target tissue was higher. MN frequency in combination with genetic polymorphism in DNA repair may serve as a better predicator of risk.[31]
Jadav et al. conducted to observe micronuclei; an essential biomarker in oral exfoliated cells for grading of oral squamous cell carcinoma. The authors concluded the MN can be candidate to serve as a biomarker for prediction of the grade of oral squamous cell carcinoma.[32]
Conclusion
Detection of micronuclei and their assay is an upcoming research domain in the field of cancer prevention and therapeutics. The presence and frequency of MN represent genomic damage. These miniature nuclear offshoots, if properly identified can turn out to be important biomarkers with huge potential in screening and predicting patients with oral potentially malignant disorders and also can act as risk assessors in patient's ongoing treatment for invasive cancers. The frequency of increase in MNC's from normal mucosa to potentially malignant disorders to oral cancer can suggest a link of this biomarker with malignant neoplastic progression. Therefore, MN assay in exfoliated cells holds promise as a specific biomarker for exposure to various carcinogens, and can also be used as a screening test in oral health centers. The advantage of micronuclei assay lies in its simplicity as scoring of MN is rapid, practical, and does not require much expertise.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tanaka T, Ishigamori R. Understanding carcinogenesis for fighting oral cancer. J Oncol 2011. 2011 doi: 10.1155/2011/603740. 603740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messadi DV. Diagnostic aids for detection of oral precancerous conditions. Int J Oral Sci. 2013;5:59–65. doi: 10.1038/ijos.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jois HS, Kale AD, Kumar KP. Micronucleus as potential, biomarker of oral carcinogenesis. IJDA. 2010;2:197–202. [Google Scholar]
- 4.Kashyap B, Reddy PS. Micronuclei assay of exfoliated oral buccal cells: Means to assess the nuclear abnormalities in different diseases. J Cancer Res Ther. 2012;8:184–91. doi: 10.4103/0973-1482.98968. [DOI] [PubMed] [Google Scholar]
- 5.Dórea LT, Meireles JR, Lessa JP, Oliveira MC, de Bragança Pereira CA, Polpo de Campos A, et al. Chromosomal damage and apoptosis in exfoliated buccal cells from individuals with oral cancer. Int J Dent 2012. 2012 doi: 10.1155/2012/457054. 457054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659:93–108. doi: 10.1016/j.mrrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Athariya R, Pradeep AR. Salivary proteomic biomarkers for oral diseases. A Review of literature. AOSR. 2010;1:43–9. [Google Scholar]
- 8.Palve DH, Tupkari JV. Clinico-pathological correlations of micronuclei in oral squamous cell carcinoma by exfoliative cytology. J Oral Maxillofac Pathol. 2008;12:2–7. [Google Scholar]
- 9.Jois HS, Kale AD, Mohan Kumar KP. Micronucleus as potential biomarker of oral carcinogenesis. Indian Journal of Dental Advancements. 2010;2:1–5. [Google Scholar]
- 10.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazanowska K, Halon A, Radwan-Oczko M. The role and application of exfoliative cytology in the diagnosis of oral mucosa pathology – Contemporary knowledge with review of the literature. Adv Clin Exp Med. 2014;23:299–305. doi: 10.17219/acem/37082. [DOI] [PubMed] [Google Scholar]
- 12.Shafer's Text Book of Oral Pathology. 7th ed. Healing of oral wounds: Elseivier's Publication; 2012. pp. 591–611. [Google Scholar]
- 13.Bansal H, Sandhu VS, Bhandari R, Sharma D. Evaluation of micronuclei in tobacco users: A study in Punjabi population. Contemp Clin Dent. 2012;3:184–7. doi: 10.4103/0976-237X.96825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samantha S, Dyp P. Micro nucleus and its application. Diagn Cytopathol. 2010;40:84–90. [Google Scholar]
- 15.Chadha P, Yadav JS. Studies on the genotoxicity of gutkha. Int J Hum Genet. 2011;11:277–82. [Google Scholar]
- 16.Sivashankai P, Kaur S, Reddy KS, Vivekananda S, Rao RK. Micronucleus index: An early diagnosis in oral carcinoma. J Anat Soc India. 2008;57:8–13. [Google Scholar]
- 17.Dindgire SL, Gosavi S, Kumavat RM, Ganvir S, Hazarey V. Comparitive study of exfoliated oral mucosal cells micronucleus frequency in potentially malignant and malignant lesions. Int J Oral Maxillofac Pathol. 2012;3:15–20. [Google Scholar]
- 18.Kamath VV, Anigol P, Setlur K. Micronuclei as prognostic indicators in oral cytological smears; comparison between smokers and non-smokers. Clin Cancer Investig J. 2014;3:49–54. [Google Scholar]
- 19.Cerqueira EM, Meireles JR, Lopes MA, Junqueira VC, Gomes-Filho IS, Trindade S, et al. Genotoxic effects of X-rays on keratinized mucosa cells during panoramic dental radiography. Dentomaxillofac Radiol. 2008;37:398–403. doi: 10.1259/dmfr/56848097. [DOI] [PubMed] [Google Scholar]
- 20.Vasudevan SG, Sellappa S, Prathyumnan S, Joseph S, Keyan KS. Vol. 5. Singapore: IACSIT Press; 2011. Enhanced micronuclei in exfoliative buccal cells of tannary workers exposed to chromium III in south India. International conference on Bioscience. Biochemistry and Bioinformatics IPCBEE. [Google Scholar]
- 21.Carlin V, Artioli AJ, Matsumoto MA, Filho HN, Borgo E, Oshima CT, et al. Biomonitoring of DNA damage and cytotoxicity in individuals exposed to cone beam computed tomography. Dentomaxillofac Radiol. 2010;39:295–9. doi: 10.1259/dmfr/17573156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro DA, de Oliveira G, de Castro G, Angelieri F. Cytogenetic biomonitoring in patients exposed to dental X-rays: Comparison between adults and children. Dentomaxillofac Radiol. 2008;37:404–7. doi: 10.1259/dmfr/58548698. [DOI] [PubMed] [Google Scholar]
- 23.Ogden GR, Cowpe JG, Green M. Cytobrush and wooden spatula for oral exfoliative cytology. A comparison. Acta Cytol. 1992;36:706–10. [PubMed] [Google Scholar]
- 24.Palaskar S, Jindal C. Evaluation of micronuclei using papanicolaou and May grunwald giemsa stain in individuals with different tobacco habits - A comparative study. J Clin Diagn Res. 2010;4:3607–13. [Google Scholar]
- 25.Acha A, Ruesga MT, Rodríguez MJ, Martínez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med Oral Patol Oral Cir Bucal. 2005;10:95–102. [PubMed] [Google Scholar]
- 26.Anila K, Kaveri H, Naikmasur VG. Comparitive study of oral micronucleated cell frequency in oral sub mucus fibrosis patients and healthy individuals. J Clin Exp Dent. 2011;3:201–6. [Google Scholar]
- 27.Kamboj M, Mahajan S. Micronucleus – An upcoming marker of genotoxic damage. Clin Oral Investig. 2007;11:121–6. doi: 10.1007/s00784-006-0075-y. [DOI] [PubMed] [Google Scholar]
- 28.Buajeeb W, Kraivaphan P, Amornchat C, Triratana T. Frequency of micronucleated exfoliated cells in oral lichen planus. Mutat Res. 2007;627:191–6. doi: 10.1016/j.mrgentox.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed MT, Belhus T. Cytological features of oral cytobrush smears in type II diabetes mellitus patients. J Dent Sci. 2012;1:6–12. [Google Scholar]
- 30.de Souza C, Pawar U, Chaturvedi P. Precancerous lesions of oral cavity. Int Rec Med Gen Pract Clin. 2009;1:7–14. [Google Scholar]
- 31.Mahimkar MB, Samant TA, Kannan S, Patil T. Influence of genetic polymorphism on frequency of micronucleated buccal epithelial cells in leukoplakia patients. Oral Oncol. 2010;46:761–6. doi: 10.1016/j.oraloncology.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Jadhav K, Gupta N, Ahmed MB. Micronuclei: An essential biomarker in oral exfoliated cells for grading of oral squamous cell carcinoma. J Cytol. 2011;28:7–12. doi: 10.4103/0970-9371.76941. [DOI] [PMC free article] [PubMed] [Google Scholar]


