Abstract
Aim and Background:
A disintegrin and metalloproteinase 8 (ADAM8) is a marker belonging to the class of ADAM family of metalloproteinase which is found to be involved in inflammation and bone resorption in periodontal disease by acting as osteoclast stimulating factor. In several systemic inflammatory diseases, elevated levels of ADAM8 are detected in human serum and other body fluids. Recently, ADAM8 was even detected in gingival crevicular fluid (GCF) of patients with periodontal diseases. Hence, the aim of the study was to estimate the levels of ADAM8 in GCF of healthy and chronic periodontitis subjects.
Materials and Methods:
Periodontal examination and collection of GCF by the extracrevicular method was performed in 30 subjects selected randomly and categorized into two groups. Group I (healthy, n = 15) and Group II (chronic periodontitis, n = 15). ADAM8 levels in GCF were estimated by enzyme-linked immunosorbent assay.
Results:
ADAM8 was detected in both Group I and II. Highest mean ADAM8 concentration was obtained for Group II, whereas the lowest concentration was seen in Group I. This suggests that ADAM8 levels increase proportionally with the progression of periodontal disease. There was a significant correlation between ADAM8 levels and clinical parameters in the study group.
Conclusion:
The results of our study indicate that the ADAM8 levels in GCF are positively associated with periodontal disease, which may provide a useful tool in monitoring its progression. Nevertheless, further longitudinal studies are required with larger sample sizes in which ADAM8 levels are progressively estimated and compared to baseline values.
KEY WORDS: A disintegrin and metalloproteinase 8, gingival crevicular fluid, periodontal disease
The connective tissue breakdown in periodontitis is thought to be the result of activation of host cells by inflammatory mediators like arachidonic acid metabolites, cytokines which in turn can trigger the resorption of alveolar bone and the generation of proteases that degrade extracellular matrix, resulting in tissue destruction.[1] Metzincins are family multidomain zinc (II)-dependent endopeptidase, includes metalloproteases such as matrix metalloproteases (MMPs), a disintegrin and metalloproteases (ADAMs), the ADAMs with a thrombospondin-like motifs.[2]
A disintegrin and a metalloproteinases belong to the adamalysin family of the metzincin superfamily of Zn-dependent metalloproteinases. ADAM8 consists of a signal domain, a prodomain, a disintegrin domain, a metalloproteinase domain, a cysteine-rich domain, a transmembrane region, an epidermal growth factor-like domain and a cytoplasmic tail.[3] ADAM8 is a cell surface glycoprotein expressed primarily in immune cells such as macrophages, neutrophils, monocytes, dendritic cells, and B-cells.[4] ADAM8 is expressed in osteoclasts and has been involved in the process of osteoclast fusion and, thus plays an essential role as an osteoclast stimulating factor.[5]
As periodontal disease is an inflammatory disorder, ADAM8 is found to be elevated in the gingival crevicular fluid (GCF) of patients with periodontal diseases, including gingivitis, chronic periodontitis, and aggressive periodontitis.[6] Hence, our study was designed to estimate the levels of ADAM8 in GCF of subjects with clinically healthy periodontium and chronic periodontitis and assess the relationship of ADAM8 levels with the increase in severity of periodontal disease.
Materials and Methods
The study population consisted of 30 subjects belonging to both sexes and all the subjects selected were from the outpatient clinics of the Department of Periodontics, in JKKN Dental College. The following parameters were evaluated for the subjects: Plaque index (PI), the gingival index (GI), probing depth (PD), and clinical attachment loss (CAL). Fifteen subjects with good oral hygiene, no bleeding on probing, no visual signs of gingival inflammation, GI = 0, no CAL were included in Group I. The chronic periodontitis group (Group II) consisted of 15 subjects with PD ≥ 5 mm and CAL ≥ 2 mm. Exclusion criteria were gingivitis, aggressive forms of periodontal disease, history of periodontal treatment received in the past 6 months, medications that would affect the periodontal status, history of underlying systemic disease, anomalies of the immune system, smoking and tobacco chewing habits. Approval from the ethical committee of the institution was obtained, the nature and purpose of the study were explained to the subjects and written consent was taken. All data were recorded in a standard proforma.
Selection of the test site, detailed case history, clinical examination, and supragingival scaling were done 1-day before the collection of GCF. On the subsequent day after drying the area with a blast of air, a supragingival plaque was removed without touching the marginal gingiva, and the GCF was collected. A standardized volume of 3 μL was collected from each test site with an extracrevicular approach (unstimulated), using volumetric capillary pipettes (Sigma-Aldrich Chemicals Company Limited, USA) that were calibrated from 1 to 10 μL. Each sample collected was allowed a maximum of 20 min. The collected GCF was transferred immediately to plastic vials and stored at −70°C until the time of assay. ADAM8 was determined using a commercially available enzyme-linked immunosorbent assay kit according to the manufacturer's instructions.
Statistical analysis
The data were analyzed statistically, to find the mean and test of significance of mean values for the various parameters between the groups. The Kruskal–Wallis one way ANOVA was used to calculate. The P < 0.05 was considered statistically significant. The Pearson correlation analysis was done to assess the relationship between the various clinical parameters and ADAM8 levels in each study group.
Results
The clinical parameters recorded were compared between Group I and II and depicted in Table 1. The ADAM8 level in the study groups is described in Table 2 and Graph 1. The ADAM8 levels in Group I ranged between 0.29 and 0.84 ng/ml and between 5.56 and 20.97 ng/ml in Group II. Comparison of the levels of ADAM8 in the two different groups showed that the mean ADAM8 level in Group II (10.35 ± 4.435) is significantly higher than the mean ADAM8 level in Group I (0.57 ± 0.179) (P < 0.05). The relationship between ADAM8 level and various clinical parameters within the study group is shown in Table 3. There was a significant relationship between ADAM8 levels and clinical parameters.
Table 1.
Comparison of clinical parameters between group I and group II
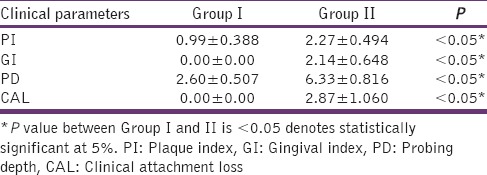
Table 2.
Comparison of ADAM8 levels between group I and II

Graph 1.
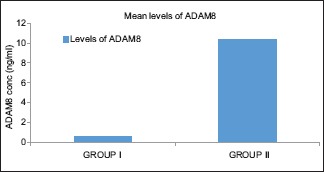
Comparison of a disintegrin and metalloproteinase 8 levels between Group I and II
Table 3.
Correlation of clinical parameters with ADAM8 levels in group II (correlation coefficient)

Discussion
Periodontal diseases are a complex group of diseases characterized by inflammation and the subsequent destruction of the tooth supporting tissue. The destruction of the periodontal tissue is caused by the bacterial infection and host immune response, either directly or indirectly by various mediators that can activate osteoclastic activity. The loss of periodontal attachment and bone destruction during periodontitis is at least partly due to MMPs. As GCF permeates through the diseased soft tissue of the periodontal pocket, contains molecules from the periodontal disease process and so it is considered the most promising source of biochemical indicators like MMPs.[7]
According to Hannas et al.,[8] MMP activity is observed in a transmembrane protein containing both ADAM domain presenting both cell adhesion and protease activity. ADAMs are glycoproteins that share homology with snake venom metalloproteinase/disintegrins and sperm surface proteins. Among other biological functions, ADAMs are involved in the release of membrane-anchored proteins, such as tumor necrosis factor-α (TNF-α), transforming growth factor-β, and L-selectin, from the plasma membrane.
A disintegrin and metalloproteinase 8 plays various role in the pathogenesis of periodontal disease. For example, the proteolytic activity is exerted by the metalloproteinase domain of ADAM8, which contributes to the destruction of the extracellular matrix.[6] The shedding of L-selectin and vascular cell adhesion molecule-1 by the metalloproteinase domain may regulate and limit the influx of some subsets of leukocytes into the periodontal tissues as determined by Gómez-Gaviro et al.[4]
Choi et al.,[5] found that the disintegrin and cysteine-rich domains of ADAM8 are involved in cell to cell fusion of osteoclast precursors to become mature multinucleated osteoclasts, may be responsible for alveolar bone resorption in periodontitis. Kataoka et al.,[9] experimented murine CD156 gene and found that ADAM8 is expressed mainly in cells of the immune system particularly monocytes and granulocytes. Furthermore, its expression has been shown to be inducible by lipopolysaccharide and γ – interferon and by TNF-α in the central nervous system.
A disintegrin and metalloproteinase family is involved in ectodomain shedding, by which the biologically active cytokines, growth factors, and their receptors are released from membrane-bound precursors. ADAM8 is able to cleave membrane-bound CD23, low-affinity IgE receptor as detected by Fourie et al.,[10] in transfected cells and human macrophage cell lines. According to them, ADAM8 dependent soluble CD23 release requires proteolytically active ADAM8 and a physical association of ADAM8 with the membrane-bound form of CD23. This proteolytic release of soluble CD23 by the metalloprotease activity caused up-regulation of IgE production and the induction of inflammatory cytokines. As ADAM8 is expressed in the same cell types as CD23, they finalized that ADAM8 can contribute to ectodomain shedding of CD23, and thus it is a potential target for intervention in allergy and inflammation.
Elevated levels of soluble ADAM8 are detected in serum, bronchoalveolar lavage fluid, and amniotic fluid in association with several pathologic and inflammatory-related conditions, including myocardial infarction, asthma, pneumonia, allergy, rheumatoid arthritis, and preterm delivery.[11,12,13] ADAM8 is also considered to be a novel serologic marker for lung cancer.[14]
The presence of ADAM8 and the raised ADAM8 levels in GCF of patients with periodontal diseases corresponds well with the elevated levels of ADAM17 (TNF-α-converting enzyme), another member of the adamalys in subfamily in periodontitis.[15] TNF-α, which is one of the major proinflammatory cytokines in periodontal diseases, induces the expression of ADAM8 in astrocytes and neuronal cells as determined by Schlomann et al.[16] Black et al.,[17] found that increased levels of ADAM17, which primarily cleaves membrane-anchored protein TNF-α to activate soluble TNF-α and may generate a greater than normal level of TNF-α in GCF, which may lead to an enhancement of ADAM8 production in periodontal disease.
A study by Khongkhunthian et al.,[6] concluded that there is an increase in the level of ADAM8 in periodontitis condition. The present study was undertaken to determine the potential role of ADAM8 in the subjects by quantifying its level in the GCF in periodontal health and disease. The main finding of our study was that the mean concentration of ADAM8 in GCF was found to increase progressively from health (0.57 ± 0.179 ng/ml) to periodontitis (10.35 ± 4.435 ng/ml). This was in accordance with that of a study done by Khongkhunthian et al.,[6] who reported increasing ADAM8 levels in GCF with the progression of periodontal disease.
The previous study by Khongkhunthian et al.,[6] investigated that the median ADAM8 levels were increased in accordance with severity of periodontal disease, that is, the patients with chronic and aggressive periodontitis had significantly higher median ADAM8 levels than did patients with gingivitis. This explained by the fact that ADAM8 plays a pivotal role in both inflammation and bone loss, would be expected to have higher ADAM8 levels than the sites with only gingival inflammation without periodontal tissue loss in gingivitis. It is also due to an accumulation of various types of inflammatory cells in diseased periodontal tissues because ADAM8 is mainly expressed in neutrophils,[4] B-lymphocytes, dendritic cells, and monocytes. This is the reason why we excluded gingivitis group in our present study. When the pair-wise comparison was done, a statistically significant difference was observed between healthy and chronic periodontitis group.
The correlation of ADAM8 concentration with the four clinical parameters of periodontal status were investigated. The ADAM8 concentration was positively correlated with PI and GI score in Group II (0.864816 and 0.857188, respectively), indicating that the ADAM8 levels are associated with the degree of periodontal tissue inflammation. This was in accordance with the study conducted by Khongkhunthian et al.[6] Moreover, the ADAM8 concentration were also positively correlated with PD and CAL in Group II (0.602501 and 0.910716, respectively), indicating that the ADAM8 levels are positively associated with the degree of periodontal tissue destruction. This was also in accordance with the study conducted by Khongkhunthian et al.[6]
The pathogenesis and progression of periodontitis are mainly caused by bacterially induced aberrant host immunity. As ADAM8 regulates the functions of neutrophils, it was concluded that it has a significant role in innate immunity.[4] Some studies have shown that cytokines produced by Th1 subsets and Th2 subsets in acquired immunity[18] can up-regulate ADAM8 expression in systemic organs.[9,16]
Since our data shows that all the GCF samples tested positive to ADAM8, it can be considered as a biomarker of periodontal disease progression. However, controlled longitudinal, with a larger population and more solid phase assays are needed to verify this possibility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Garlet GP, Martins W, Jr, Fonseca BA, Ferreira BR, Silva JS. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 2004;31:671–9. doi: 10.1111/j.1600-051X.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 2.Sekhon BS. Matrix metalloproteinases – An overview. Res Rep Biol. 2010;1:1–10. [Google Scholar]
- 3.Wolfsberg TG, Straight PD, Gerena RL, Huovila AP, Primakoff P, Myles DG, et al. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev Biol. 1995;169:378–83. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Gaviro M, Domínguez-Luis M, Canchado J, Calafat J, Janssen H, Lara-Pezzi E, et al. Expression and regulation of the metalloproteinase ADAM-8 during human neutrophil pathophysiological activation and its catalytic activity on L-selectin shedding. J Immunol. 2007;178:8053–63. doi: 10.4049/jimmunol.178.12.8053. [DOI] [PubMed] [Google Scholar]
- 5.Choi SJ, Han JH, Roodman GD. ADAM8: A novel osteoclast stimulating factor. J Bone Miner Res. 2001;16:814–22. doi: 10.1359/jbmr.2001.16.5.814. [DOI] [PubMed] [Google Scholar]
- 6.Khongkhunthian S, Techasatian P, Supanchart C, Bandhaya P, Montreekachon P, Thawanaphong S, et al. Elevated levels of a disintegrin and metalloproteinase 8 in gingival crevicular fluid of patients with periodontal diseases. J Periodontol. 2013;84:520–8. doi: 10.1902/jop.2012.120262. [DOI] [PubMed] [Google Scholar]
- 7.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 8.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka M, Yoshiyama K, Matsuura K, Hijiya N, Higuchi Y, Yamamoto S. Structure of the murine CD156 gene, characterization of its promoter, and chromosomal location. J Biol Chem. 1997;272:18209–15. doi: 10.1074/jbc.272.29.18209. [DOI] [PubMed] [Google Scholar]
- 10.Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003;278:30469–77. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- 11.Raitoharju E, Seppälä I, Levula M, Kuukasjärvi P, Laurikka J, Nikus K, et al. Common variation in the ADAM8 gene affects serum sADAM8 concentrations and the risk of myocardial infarction in two independent cohorts. Atherosclerosis. 2011;218:127–33. doi: 10.1016/j.atherosclerosis.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 12.King NE, Zimmermann N, Pope SM, Fulkerson PC, Nikolaidis NM, Mishra A, et al. Expression and regulation of a disintegrin and metalloproteinase (ADAM) 8 in experimental asthma. Am J Respir Cell Mol Biol. 2004;31:257–65. doi: 10.1165/rcmb.2004-0026OC. [DOI] [PubMed] [Google Scholar]
- 13.Malamitsi-Puchner A, Vrachnis N, Samoli E, Baka S, Iliodromiti Z, Puchner KP, et al. Possible early prediction of preterm birth by determination of novel proinflammatory factors in midtrimester amniotic fluid. Ann N Y Acad Sci. 2006;1092:440–9. doi: 10.1196/annals.1365.043. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa N, Daigo Y, Yasui W, Inai K, Nishimura H, Tsuchiya E, et al. ADAM8 as a novel serological and histochemical marker for lung cancer. Clin Cancer Res. 2004;10:8363–70. doi: 10.1158/1078-0432.CCR-04-1436. [DOI] [PubMed] [Google Scholar]
- 15.Bostanci N, Emingil G, Afacan B, Han B, Ilgenli T, Atilla G, et al. Tumor necrosis factor-alpha-converting enzyme (TACE) levels in periodontal diseases. J Dent Res. 2008;87:273–7. doi: 10.1177/154405910808700311. [DOI] [PubMed] [Google Scholar]
- 16.Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156): Implications for neuron-glia interactions during neurodegeneration. J Neurosci. 2000;20:7964–71. doi: 10.1523/JNEUROSCI.20-21-07964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 18.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: Homeostasis and autoimmunity. Periodontol. 2000;2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]


