Abstract
Aim:
This study evaluates erosive potential of commonly used beverages, medicated syrup, and their effects on dental enamel with and without restoration in vitro.
Materials and Methods:
Test medias used in this study included carbonated beverage, noncarbonated beverage, high-energy sports drink medicated cough syrup, distilled water as the control. A total of 110 previously extracted human premolar teeth were selected for the study. Teeth were randomly divided into two groups. Test specimens were randomly distributed to five beverages groups and comprised 12 specimens per group. Surface roughness (profilometer) readings were performed at baseline and again, following immersion for 14 days (24 h/day). Microleakage was evaluated. The results obtained were analyzed for statistical significance using SPSS-PC package using the multiple factor ANOVA at a significance level of P < 0.05. Paired t-test, Friedman test ranks, and Wilcoxon signed ranks test.
Results:
For surface roughness high-energy sports drink and noncarbonated beverage showed the highly significant difference with P values of 0.000 and 0.000, respectively compared to other test media. For microleakage high-energy sports drink had significant difference in comparison to noncarbonated beverage (P = 0.002), medicated syrup (P = 0.000), and distilled water (P = 0.000).
Conclusion:
High-energy sports drink showed highest surface roughness value and microleakage score among all test media and thus greater erosive potential to enamel while medicated syrup showed least surface roughness value and microleakage among all test media.
KEY WORDS: Commonly used beverages, dental enamel, erosive potential, medicated syrup, microleakage, surface roughness
Dental erosion is defined as “an irreversible loss of dental hard tissue due to a chemical process without involvement of microorganisms.”[1] Dental erosion may be caused by either extrinsic or intrinsic factors. Extrinsic erosion is the result of exogenous acids. One of the extrinsic causes of dental erosion is excessive consumption of carbonated beverages, fruit juices, high-energy drinks, and candies. Most of the carbonated drinks, noncarbonated drinks, sports drinks, and medicated syrups have a low pH rendering them acidic in nature. Studies reporting the frequency of ingestion of soft drinks and other low pH beverages have shown increased potential in the formation of the dental erosive lesion.[2,3,4]
Soft drink consumption has increased dramatically over the past 50 years. In this era, a growing trend toward increased consumption of canned juices, sports drinks, and high-energy beverages has been seen among adolescents.[5,6] The commercial sale of soft drinks has increased by 56% over the last 10 years and now it is estimated that it will keep rising at about 2–3% a year.[7] It has been recognized as an important cause of tooth loss not only in adults but also in children. Children with chronic diseases often require long-term drug regimens. Several medicines can alter plaque composition and oral pH being potentially harmful to the teeth.
The solubility of enamel is pH dependent, and the rate at which apatite precipitates depends on certain factors like calcium binding in saliva. Since the critical pH of enamel is approximately 5.5, any solution with a lower pH may cause erosion, particularly if the attack is lengthy and intermittent over time. The total acid level (titratable acidity) of acidic drinks is considered to be more important than the pH alone as it determines the concentration of damaging hydrogen ions available to interact with the tooth surface.[8]
In the erosive lesions of the tooth, the coronal margins of restoration are usually in enamel while the cervical margins are in dentin and cementum. Erosion of enamel may also cause micro leakage between restoration and hard tissue of the tooth. Subsequent erosion poses special challenges to the dentist for their restorations.
Considering the erosive potential of various beverages and medicated syrups on dental enamel, a study was designed to evaluate the erosive potential of carbonated drink, noncarbonated drink, high-energy sports drink, medicated syrup and distilled water, and their effects on enamel surface and on the restoration of teeth.
Aims and objectives
The study was conducted in the Department of Pedodontics and Preventive Dentistry, Ahmedabad Dental College and Hospital with an aim to determine erosive potential of commonly used beverages: Carbonated beverage, noncarbonated beverage, high-energy sports drink, medicated cough syrup, and control (distilled water).
Their effect on dental enamel with following objectives;
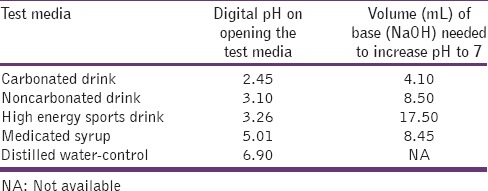
To evaluate the digital pH and titrable acidity (neutralizable acidity) of all test medias
To evaluate the effect of on enamel surface morphology using profilometer (surface roughness) analysis
To evaluate the effect of erosion on restored enamel surface, assessed by microleakage with dye penetration method.
Materials and Methods
A total of 110 previously extracted human premolar teeth were selected for the study. The selected teeth were: Free of hypocalcification, dental caries, fractures. Teeth were carefully cleaned of calculus and other debris and stored in distilled water, prior to usage in the study. Teeth were randomly divided into two groups; Group 1: 60 teeth for surface roughness test. Group 2: 50 teeth for microleakage test.
Three test beverages based upon current drinking trends and commonly prescribed medicated syrup for respiratory tract infection for children in India were selected for the study [Figures 1 and 2] Carbonated beverage (The Coca-Cola Company, India), noncarbonated beverage (Parle Agro Pvt. Ltd., India), high-energy sports drink (Rauch Fruchtsafte GmbH and Co OG, Red Bull Asia FZE, UAE), medicated cough syrup (Johnson and Johnson, India) Distilled water as the control.
Figure 1.

Measurement of surface roughness values using Profilometer (ZEISS Surfcom 5000)
Figure 2.

Microleakage score, grade 0 score
Measurement of Initial pH and titrable acidity (neutralizable acidity)
The initial pH of each test media was measured using a digital pH meter (Equip-Tronics pH Meter Model No EQ-615, India). Twenty millimeter of each freshly opened drink was placed in a glass beaker. The titrable acidity (neutralizable acidity) of each test media was measured by placing 20 ml of the product in a glass beaker. Then, 0.1 M sodium hydroxide solution was gradually pipetted into the beaker until the pH became neutral. Each test media was tested three times to give a mean measurement.
Enamel surface roughness
The average surface roughness (Ra) is the arithmetic average height of roughness component irregularities from the mean line measured within the sampling length and is expressed in microns (um). Smaller Ra values indicate smoother surfaces.
Measurement of enamel surface roughness
All measurements of enamel surface roughness (indicate enamel loss) were made using profilometer (Zeiss Surfcom 5000). Vertical resolution: 10 nm, lateral resolution: 0.125 mm, maximum scan size: 120 mm (x) × 40 mm (y) × 12 mm (z).
Sixty premolar teeth (12 teeth/media) were used for enamel surface roughness (Ra) measurement. The teeth were then embedded in acrylic resin. Half of the exposed enamel surface was painted with acrylic nail varnish while the remaining surface was left exposed to the testing media. This established a baseline evaluation prior to immersion in the beverage media. All teeth samples were checked for initial surface roughness values with Profilometer (ZEISS Surfcom 5000) before placing into test medias [Figure 3].
Figure 3.

Microleakage score, grade 1 score
The specimens were stored in glass beakers with 30ml of beverages at 37°C in an incubator for a total of 14 days. The beverages were changed daily for 14 days. The testing period or beverage immersion period adopted protocol was followed based on a study by von Fraunhofer and Rogers for dissolution of enamel in beverage solutions.[9] This protocol was based upon an average daily consumption of 25 ounces of soft drink and a residence time in the mouth of 20 s (before salivary clearance), making an annual exposure of enamel to soft drinks approximately 90,000 s (25 h) per year. The test period of 350 h used in the study is comparable to 14 years of normal beverage consumption, a reasonable time period for evaluating the erosion of enamel in adolescents and young adults. The surface roughness (Ra) of each enamel specimen was again assessed at the end of the 14-day test period.
Measurement of microleakage
Fifty extracted noncarious permanent human premolars with fully developed roots were selected. The window of 3 mm × 2 mm × 2 mm was prepared with high-speed handpiece using diamond bur with air-water spray. Then the cavities were restored with GIC (GC Fuji IX GP) according to manufacturer's instructions. All specimens were randomly divided into five groups and placed into test media for 14 days test duration along with the teeth samples for surface roughness test. The specimens were thermal stressed between 5°C and 55°C water bath for 250 cycles. A complete cycle lasted for 1-min, with 10 s transfer time.
Specimens were then prepared for staining by first applying two coats of nail polish to the whole surface of the teeth except the restoration and 1 mm around the restoration margins. Specimens were then placed in 1% methylene blue dye solution for 24 h to allow for dye penetration. Teeth were then sectioned using a diamond disk mounted on a straight handpiece with a continuous flow of water. The samples were sectioned buccolingually with the section dividing the restoration at its midpoint mesiodistally creating two test specimens with exposure of the tooth interface from the cavosurface margin to the pulpal wall. A total of 100 sections (20 sections in each group) were finally obtained. The specimens were observed under stereomicroscope with a magnification of × 10, depth of dye penetrations into enamel and dentin as an indication of marginal microleakage was determined by the criteria described by Khera and Chanas follows:[10]
Criteria for evaluation of dye penetration:
0°=No leakage [Figure 4].
1°=Less than and up to one-half of the depth of the cavity preparation was penetrated by the dye [Figure 1].
2°=More than one-half of the depth of the cavity preparation was penetrated by the dye but not up to the junction of the axial and occlusal or cervical wall [Figure 2].
3°=Dye penetration was up to the junction of the axial and occlusal or cervical wall but did not include the axial wall [Figure 5].
4°=Dye penetration included the axial wall [Figure 6].
Figure 4.

Microleakage score, grade 2 score
Figure 5.

Microleakage score, grade 3 score
Figure 6.

Microleakage score, grade 4 score
Dye penetration was evaluated by a single observer The results obtained were analyzed for statistical significance using SPSS-PC package SPSS- VERSION 13 software for windows (IBM, USA) using the paired t-test, one-way ANOVA, Friedman test ranks, and Wilcoxon signed ranks test.
Results
Table 1 showed that assessment of the digital pH and neutralizable acidity on opening the test media. Digital pH of all test media is as follows: Carbonated beverage > noncarbonated beverage > high-energy sports drink > medicated syrup > distilled water. Neutralizable acidity of all test media is as follows: High-energy sports drink > medicated syrup > noncarbonated beverage > carbonated beverage > distilled water.
Table 1.
Digital pH and neutralizable acidity of each test media
Surface roughness (Ra) value obtained for each test media
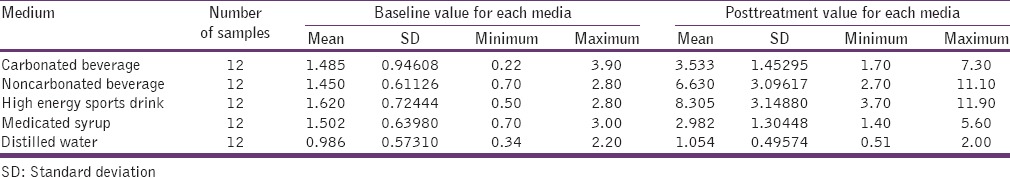
Carbonated beverage, medicated syrup showed significant difference with P values 0.001 and 0.004, respectively, while high-energy sports drink and noncarbonated beverage showed highly significant difference with P values of 0.000 and 0.000, respectively, compared to other test media [Table 2].
Table 2.
Surface roughness (Ra) value obtained in each media (μm/mm)
Intra-group comparison for surface roughness values
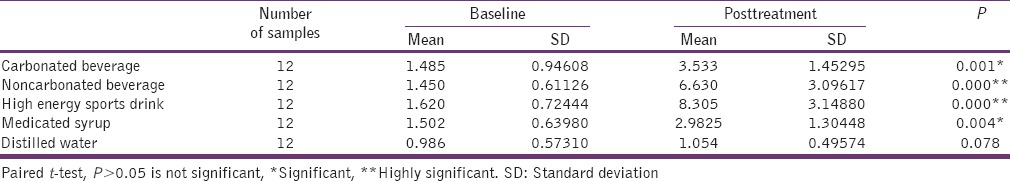
After 14 days test duration carbonated beverage, medicated syrup showed significant difference with P values 0.001 and 0.004, respectively, while high-energy sports drink and noncarbonated beverage showed highly significant difference with P values of 0.000 and 0.000, respectively, compared to other test media. No significant difference was observed in distilled water [Table 3].
Table 3.
Intra-group comparison for surface roughness (Ra) value
Inter-group comparison for surface roughness values
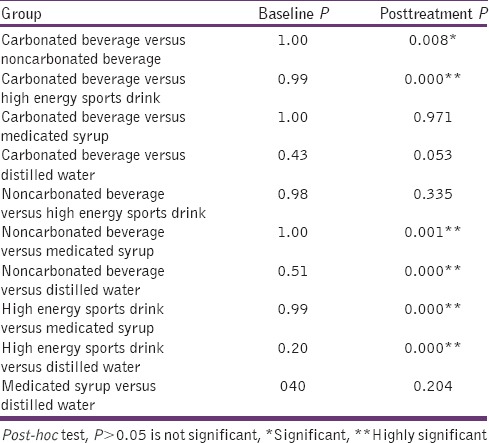
After 14 days test duration carbonated, beverage showed highly significant P values of 0.000 with high-energy sports drink followed by noncarbonated beverage (P = 0.008) and was not significant with medicated syrup and distilled water. Noncarbonated beverage showed a significant difference with a carbonated beverage, medicated syrup, and distilled water (P = 0.008, 0.001, and 0.000) [Table 4].
Table 4.
Inter-group comparison for surface roughness value
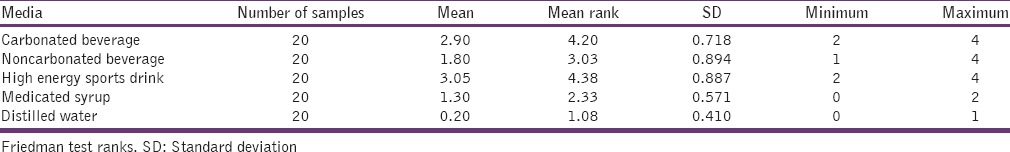
Microleakage score obtained for each test media
The highest mean score observed for high-energy sports drink 3.05 [Table 5].
Table 5.
Microleakage score obtained for each media
Inter-group comparison of microleakage score in each media
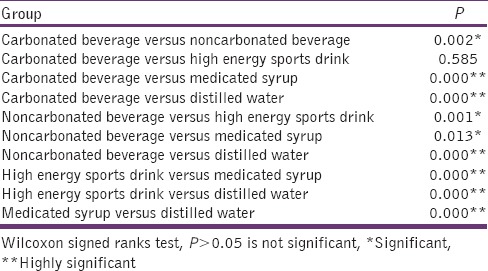
High-energy sports drink had significant difference in comparison to noncarbonated beverage (P = 0.002), medicated syrup (P = 0.000), and distilled water (P = 0.000). Noncarbonated beverage showed significant P values with high-energy sports drink (P = 0.001), medicated syrup (P = 0.013), and distilled water (P = 0.000) [Table 6].
Table 6.
Inter-group comparison of microleakage for each media
Discussion
Dental erosion is defined as an irreversible loss of dental hard tissue by a chemical process without the involvement of microorganisms and is due to either extrinsic or intrinsic sources.[1] Changing lifestyle and dietary patterns have played a major role in the increase of dental erosion in recent years. Dietary erosion may result from food or drinks containing a variety of acidic ingredients.[8,11]
Children and adolescents consume significant amounts of erosive beverages and, therefore, their risk of developing dental erosion is high. Soft drink intake in children is greater than in adults but has a huge individual variation.[12]
Carbonated and noncarbonated beverages have a low pH, are sweetened by highly refined carbohydrates (sugar and/or sugar substitute components) that are metabolized by plaque microorganisms forming organic acids, and also contain additional additives, all of which can contribute to the demineralization effect and erosive potential of dental hard tissues (enamel).[13]
In the present study, the test media selected were the commonly consumed beverages; carbonated beverage, noncarbonated beverage, high-energy sports drink, medicated syrup for respiratory tract infection for children in Indian market, and distilled water as control. As it would be unethical to create actual erosive lesions in patients, the erosive potential of these test media was evaluated in an in vitro study design.
Two ways to quantify the acid content of a foodstuff or beverage include pH and total or titratable acidity. The pH or actual acidity is the negative logarithm of the hydrogen ion concentration (actual hydrogen ion concentration) and is measured on a scale of 0 to 14 with a reading below 7 indicating an acid content or environment. Neutralizable acidity is the total number of acid molecules, both protonated and nonprotonated and determines the actual hydrogen ion availability for interaction with the tooth surface. Beverages with lower pH values have greater erosive effect; However the neutralizable acidity level may be a more realistic and accurate method for measuring the potential acidity in a given beverage.[8,14]
The present study showed that high-energy sports drink needed the most base (17.50 ml) to neutralize it thereby having greater erosive potential than the carbonated and the other noncarbonated beverage. This was quite similar to the findings of other studies by Jensdottir et al., 2006; Bamaise et al., 2007; Touyz 1994.[15,16,17] The most common type of acid used in sports drinks is citric acid, which has greater erosive potential.[18] Actually citric acids and/or citrates are added as buffering and flavoring agents, but they can concurrently bind to calcium and phosphorus thereby promoting increased titratable acidity levels.
The medicated syrup contains diphenhydramine hydrochloride, ammonium chloride, sodium citrate, menthol, and ethanol. As mentioned by Maganur that a larger quantity of citric acid is added in the form of sodium citrate to mask the unpleasant taste of medication, which adversely affects medicine.[19] Furthermore, diphenhydramine hydrochloride and ethanol may contribute for its increased neutralizable acidity. Similarly, different concentration of acid may be the reason for different neutralizable acidity in a high-energy sports drink and medicated syrup.
Quantitative (profilometer) analysis was performed verifying the erosive potential of all test medias. Arithmetic average roughness has been conventionally evaluated on enamel as an indicator of acid erosion using the contact stylus surface profilometry and the most frequently reported surface roughness parameter in the dental literature.[20,21] Ra value in present study significant differences was exhibited between different beverages.
At baseline, the mean surface roughness values ranged between 0.986 and 1.620 with no significant inter-group difference was present. In case, of high-energy sports drink study shows significant posttreatment Ra value (8.305) as compared to baseline Ra values (1.620). It contains sodium citrate (sodium salt of citric acid), which is added as buffering and flavoring agents; these additions can concurrently bind to calcium, promoting increased erosion potential.[22] Study by Tahmassebi et al., Edwards et al., Duggal and Curzon have reported that beverages containing citric acid have shown an increased potential for the dissolution of hydroxyapatite due to the formation of calcium citrate by the chelating (calcium binding) action of citric acid that withdraws Ca ions from the beverage, resulting in an increased dissolution tendency.[7,14,23] Its posttreatment Ra values are significant higher compared to other test media.
In case, of noncarbonated beverages, there was significant posttreatment Ra value (6.630) as compared to baseline (1.450) after the test period of 14 days. These findings could be due to the presence of mango pulp, sugar, citric acid, ascorbic acid, salts, anti-oxidants which are responsible for the erosive potential of noncarbonated beverage.
The present study shows a significant increase in Ra value (3.533) as compared to baseline (1.485) after the test period of 14 days in case of carbonated beverages. The carbonated beverage despite having the lowest pH on opening was easy to neutralize than the high-energy sports drink, noncarbonated beverage, and medicated syrup. The carbonated beverage contains inorganic acids such as phosphoric acid to stimulate taste and counteract the sweetness. Edward et al. reported that even when the carbon dioxide has been blown off, and the drinks have become “flat” still the pH remains low.[14] This indicates that soft drinks have inherent acidity due to other acids that are added to stimulate taste and counter sweetness. Carbonated beverages contain carbonic acid formed by carbon dioxide in solution that is, carbonated. Carbonic acid is present in the carbonated beverage; it acts as a mild acid and does not readily bind with cations and forms chelate. As mentioned earlier, beverages containing citric acid have shown an increased potential for the dissolution of hydroxyapatite due to the formation of calcium citrate and the chelating (calcium binding) action of citric acid.
The present study shows a significant increase in Ra value (2.9825) as compared to baseline (1.485) after the test period of 14 days in case of medicated syrup. A long term use of sugar containing drugs has been considered a cause of dental caries in children.[24] As the sugar content in liquid medication tend to dissolve enamel quickly when they remain for a longer time. This contributes toward the increase in surface roughness (Ra) value. For medicated syrup, this could be related to chelation. Chelation is independent of pH of the medium so that removal of such metallic ions as calcium from even a biological calcium phosphorous system may occur at a neutral or even alkaline pH.[25] Besides, the acid components found in such medications, other factors such as high frequency of ingestion (two of more times a day), bedtime consumption, high viscosity, presence of ingredients such as hypromellose, microcrystalline cellulose, polyethylene glycol, stearic acid, and titanium dioxide may contribute to the increase in the Ra values.
The erosive effect of these beverages causes loss of minerals from teeth surface and restoration.[26] Long term use of these beverages cause adverse effect leading to microleakage in between tooth and restoration, which ultimately lead to restoration failure or secondary caries. When the microleakage scores were evaluated, the results obtained for microleakage are as follows: High-energy sports drink 3.05 >carbonated beverage had 2.90, >noncarbonated beverage it was 1.80, >medicated syrup 1.30, >for distilled water the score was 0.20.
The samples placed in high-energy sports drinks showed significantly higher microleakage scores compared to the noncarbonated beverage, medicated syrup, and distilled water. This could be due to the higher degree of erosion between restoration and tooth surface. This correlates with the highest surface roughness values obtained in this group. Carbonated beverages had shown significant higher microleakage score compared to noncarbonated beverages, medicated syrup, and distilled water. This finding was similar to study by Maganur et al.[19] Surface roughness value of noncarbonated beverage is higher compared to carbonated beverages. The main reason was higher surface roughness that is, the higher erosive potential for the enamel of noncarbonated beverages, but microleakage is lower than a carbonated beverage. This shows a lesser erosive effect of noncarbonated beverage on tooth and restoration interface. Among four test group least the microleakage score was found in medicated syrup, mainly because of significantly lower surface roughness values as compared to other test groups.
Conclusion
From the present study, the following conclusion can be drawn:
Digital pH of all test media is as follows: Carbonated beverage > noncarbonated beverage > high-energy sports drink > medicated syrup > distilled water. Neutralizable acidity of all test media is as follows: High-energy sports drink > medicated syrup > noncarbonated beverage > carbonated beverage > distilled water
Carbonated beverage, noncarbonated beverage, High energy sports drink, Medicated syrup all showed significant surface roughness changes to enamel; distilled water did not show significant surface changes
High energy sports drink showed highest surface roughness value and microleakage score among all test media and thus greater erosive potential to enamel while medicated syrup showed least surface roughness value and microleakage among all test media.
However, it is inappropriate to extrapolate the findings of our study to the conditions existing in vivo in humans. In the oral cavity, any drink or foodstuff will be instantaneously mixed with saliva with a subsequent rise in its pH. After consuming a low pH drink, the pH on the tongue stays low only for a short duration. A possible limitation of this study would include a small sample size. The group size was determined to be small enough to facilitate the logistics of the experimental design, yet large enough to demonstrate significant differences statistically. This study attempted to identify which drinks were the most aggressive toward enamel using quantitative analysis and explore and/or confirm the possible reasons for the erosive effect of these beverages.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by the MSME Tool Room, Indo German Tool Room, Ahmedabad and by Chemistry Department, School of Sciences, Gujarat University, Ahmedabad.
References
- 1.Imfeld T. Dental erosion. Definition, classification and links. Eur J Oral Sci. 1996;104:151–5. doi: 10.1111/j.1600-0722.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 2.Millward A, Shaw L, Smith AJ, Rippin JW, Harrington E. The distribution and severity of tooth wear and the relationship between erosion and dietary constituents in a group of children. Int J Paediatr Dent. 1994;4:151–7. doi: 10.1111/j.1365-263x.1994.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett DW, Coward PY. Comparison of the erosive potential of gastric juice and a carbonated drink in vitro. J Oral Rehabil. 2001;28:1045–7. doi: 10.1046/j.1365-2842.2001.00780.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Dlaigan YH, Shaw L, Smith A. Dental erosion in a group of British 14-year-old school children. Part II: Influence of dietary intake. Br Dent J. 2001;190:258–61. doi: 10.1038/sj.bdj.4800943. [DOI] [PubMed] [Google Scholar]
- 5.Shenkin JD, Heller KE, Warren JJ, Marshall TA. Soft drink consumption and caries risk in children and adolescents. Gen Dent. 2003;51:30–6. [PubMed] [Google Scholar]
- 6.Coombes JS. Sports drinks and dental erosion. Am J Dent. 2005;18:101–4. [PubMed] [Google Scholar]
- 7.Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Softdrinks and dental health: A review of the literature. J Dent. 2005;36:1–10. doi: 10.1016/j.jdent.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Zero DT. Etiology of dental erosion – Extrinsic factors. Eur J Oral Sci. 1996;104:162–77. doi: 10.1111/j.1600-0722.1996.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 9.von Fraunhofer JA, Rogers MM. Dissolution of dental enamel in soft drinks. Gen Dent. 2004;52:308–12. [PubMed] [Google Scholar]
- 10.Welsh EL, Hembree JH., Jr Microleakage at the gingival wall with four Class V anterior restorative materials. J Prosthet Dent. 1985;54:370–2. doi: 10.1016/0022-3913(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 11.Scheutzel P. Etiology of dental erosion – Intrinsic factors. Eur J Oral Sci. 1996;104:178–90. doi: 10.1111/j.1600-0722.1996.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 12.Linnett V, Seow WK. Dental erosion in children: A literature review. Pediatr Dent. 2001;23:37–43. [PubMed] [Google Scholar]
- 13.Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Soft drinks and dental health: A review of the current literature. J Dent. 2006;34:2–11. doi: 10.1016/j.jdent.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Edwards M, Creanor SL, Foye RH, Gilmour WH. Buffering capacities of soft drinks: The potential influence on dental erosion. J Oral Rehabil. 1999;26:923–7. doi: 10.1046/j.1365-2842.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006;85:226–30. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 16.Bamaise CT, Ogunbodede EO, Olusile AO, Esan TA. Erosive potential of soft drinks in Nigeria. World J Med Sci. 2007;2:116–9. [Google Scholar]
- 17.Touyz LZ. The acidity (pH) and buffering capacity of Canadian fruit juice and dental implications. 1994;60:454–8. [PubMed] [Google Scholar]
- 18.Meurman JH, Härkönen M, Näveri H, Koskinen J, Torkko H, Rytömaa I, et al. Experimental sports drinks with minimal dental erosion effect. Scand J Dent Res. 1990;98:120–8. doi: 10.1111/j.1600-0722.1990.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 19.Maganur PC, Prabhakar AR, Sugandhan S, Srinivas N. Evaluation of microleakage of RMGIC and Flowable composite immersed in soft drink and fresh fruit juice: An in vitro study. Int J Paediatr Dent. 2010;3:153–61. doi: 10.5005/jp-journals-10005-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grenby TH. Dental properties of antiseptic throat lozenges formulated with sugars or Lycasin. J Clin Pharm Ther. 1995;20:235–41. doi: 10.1111/j.1365-2710.1995.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 21.Turssi CP, Messias DC, de Menezes M, Hara AT, Serra MC. Role of dentifrices on abrasion of enamel exposed to an acidic drink. Am J Dent. 2005;18:251–5. [PubMed] [Google Scholar]
- 22.Kitchens M, Owens BM. Effect of carbonated beverages, coffee, sports and high energy drinks, and bottled water on the in vitro erosion characteristics of dental enamel. J Clin Pediatr Dent. 2007;31:153–9. doi: 10.17796/jcpd.31.3.1157l653t8206100. [DOI] [PubMed] [Google Scholar]
- 23.Duggal MS, Curzon ME. An evaluation of the cariogenic potential of baby and infant fruit drinks. Br Dent J. 1989;166:327–329. doi: 10.1038/sj.bdj.4806828. 30. [DOI] [PubMed] [Google Scholar]
- 24.Mackie IC, Bentley E. Sugar-containing or sugar-free paediatric medicines: Does it really matter? Dent Update. 1994;21:192–4. [PubMed] [Google Scholar]
- 25.Ireland AJ, McGuinness N, Sherriff M. An investigation into the ability of soft drinks to adhere to enamel. Caries Res. 1995;29:470–6. doi: 10.1159/000262117. [DOI] [PubMed] [Google Scholar]
- 26.Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. Effect of acidic agents on surface roughness of dental ceramics. Dent Res J (Isfahan) 2011;8:6–15. [PMC free article] [PubMed] [Google Scholar]


