Abstract
Objectives:
To determine and compare the potential difference of nickel release from three different orthodontic brackets, in different artificial pH, in different time intervals.
Materials and Methods:
Twenty-seven samples of three different orthodontic brackets were selected and grouped as 1, 2, and 3. Each group was divided into three subgroups depending on the type of orthodontic brackets, salivary pH and the time interval. The Nickel release from each subgroup were analyzed by using inductively coupled plasma-Atomic Emission Spectrophotometer (Perkin Elmer, Optima 2100 DV, USA) model. Quantitative analysis of nickel was performed three times, and the mean value was used as result. ANOVA (F-test) was used to test the significant difference among the groups at 0.05 level of significance (P < 0.05). The descriptive method of statistics was used to calculate the mean, standard deviation, minimum and maximum. SPSS 18 software ((SPSS.Ltd, Quarry bay, Hong Kong, PASW-statistics 18) was used to analyze the study.
Result:
The analysis shows a significant difference between three groups. The study shows that the nickel releases from the recycled stainless steel brackets have the highest at all 4.2 pH except in 120 h.
Conclusion:
The study result shows that the nickel release from the recycled stainless steel brackets is highest. Metal slot ceramic bracket release significantly less nickel. So, recycled stainless steel brackets should not be used for nickel allergic patients. Metal slot ceramic brackets are advisable.
KEY WORDS: Artificial saliva, nickel, orthodontic brackets, recycled bracket
Recently there has been a major inflation in the usage of metal and nonprecious alloys in clinical dentistry. The term stainless steel is used when the chromium content is generally in the range of 12–30%. Stainless steel can be classified as ferritic, martensitic and austenitic.[1] The austenitic stainless steel contains 18% chromium and 8% nickel, it is used for making orthodontic bands wires, space maintainers, brackets,[2] etc. Nickel is added in austenitic stainless steel alloy so that the austenitic phase stabilizes; there is improvement in the anti-corrosive property of the alloy and decrease in the ductility, whereas chromium is added to facilitate the formation of an anticorrosive passive film.[3]
Nickel is a commonly used metal in orthodontic treatments because it is one of the basic elements that can easily be combined with other metals to form alloys. Different components of orthodontic appliances like arch wires (stainless steel and nickel-titanium [NiTi]) brackets, bands, face bows, stainless steel ligatures and stainless steel components of removable appliances are potential sources of nickel.[4] The 18/8 stainless steel contains 8% nickel, NiTi archwires contains 47–50% nickel, which makes it the richest source of nickel in an intraoral environment of the orthodontic patient.[5] Grimsdottir et al. observed the composition of brackets, face bows, arch wires and bands; and concluded that most components contain 8–12% nickel.[6]
The chief reason for the concern of an orthodontist about nickel is that it is released into the saliva.[1,7,8,9,10] Several in vitro studies have proved the release of nickel. The release of nickel is not necessarily proportional to the alloy's nickel content.[11] Factors such as heat treatment and soldering can accelerate nickel release. Quantity and quality of saliva,[12] salivary pH, plaque and amount of salivary proteins, physical and chemical properties of food and liquids, general and oral health conditions are certain other factors.
Nickel is a strong immunologic sensitizer and it is the most common cause of allergic contact dermatitis,[13] which is a type IV, delayed hypersensitivity immune response. Nickel allergy leads to burning sensation, rashes, swelling and painful erythematous lesions in the oral and labial mucosa. Gingivitis, gingival hyperplasia, lip desquamation, metallic taste, angular cheilitis and periodontitis may also follow the allergic reaction. Symptoms such as fever, compatible with general allergic reaction were reported at times.[14]
Bishara et al.[10] conducted a study to determine an increase in blood levels of nickel in patients under routine orthodontic therapy. Blood samples were collected at three-time intervals from 31 patients to mean age of 12 and 38 years. A baseline sample was obtained before fitting or cementation of bands. Another sample was obtained after orthodontic appliances placement for approximately 2 months and third blood sample was collected after appliance had been in place for at least 4–5 months, where stainless steel archwires were predominantly being used. The result indicated that no demonstrable increase in the blood level of nickel occurred during the 5 months course of orthodontic treatment.
Two months after the placement of the orthodontic appliance, hiked urinary[12,15] nickel levels was reported. In a recent study by Faccioni et al.,[16] found that nickel ions can produce DNA breaks in the cell of the oral mucosa.
The aim and purpose of this study are to determine and compare the amount of nickel release from three types of orthodontic brackets in different artificial salivary pH in different time intervals.
Materials and Methods
This study was performed in the Department of Orthodontics and Dentofacial Orthopaedics, Rajah Muthiah Dental College and Hospital, Annamalai University. The artificial saliva preparation was done in association with the Department of Biochemistry and Department of Physics, Annamalai University. The chemical analysis was done in the Department of Earth Science and Marine Biology, Annamalai University using inductively coupled plasma-Atomic Emission Spectroscopy (ICP-AES) (Perkin Elmer, Optima 2100 DV, USA) [Figure 1] to determine in vitro nickel discharge from as received conventional, recycled stainless steel, and metal slot ceramic brackets (CM).
Figure 1.

Inductively coupled plasma-atomic emission spectrophotometer
Laboratory procedure
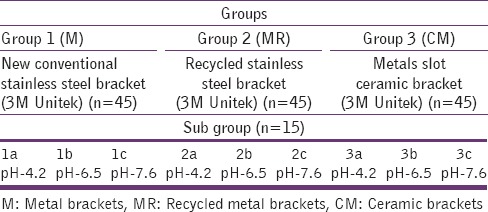
A total of 27 samples were used in this study, which are presented in Table 1. Each laboratory ware made of low-density polyethylene was cleaned by soaking in 1:1 nitric acid for at least 48 h, rinsed with Milli-Q water, then dried and stored in plastic bags.
Table 1.
Comparison of Group 1, Group 2 and Group 3 brackets under subgroups
The artificial saliva, used as the immersion electrolyte, was modified according to the method and composition described by Tanni and Zucchi[17] [Table 2]. The prepared solution was divided into three aliquots. In each the pH was measured potentiometrically using a deluxe pH meter company (model no: 1299064 394/01, Micro teknik Ambala, India) [Figure 2] and adjusted with a small amount of hydrochloric acid, and hence that the volume of the solution remained constant. The final pH values of the aliquots were 4.2 pH, 6.5 pH and 7.6 pH [Figure 3].
Table 2.
Composition of artificial saliva prepared using Tani and Zucchi method

Figure 2.

Adjusting the pH of saliva
Figure 3.

Artificial saliva with different pH
A total of 135 orthodontic brackets were divided into three groups with 45 brackets in each group; Group-1 consists of metal brackets (M) that is, new conventional Victory stainless steel brackets (slot 0.022 in MBT prescription, 3M Unitek) composition weight percentage, chromium 10–20%, nickel <4%. Group-2 consists of recycled metal brackets (MR) that is, Victory stainless steel brackets (slot 0.022” in MBT prescriptions, 3M Unitek), recycled by flaming, which is followed by ultrasonic cleaning. The gas torch flame tip was pointed at the base of the bracket for 5 s until it become red hot. It was then quenched in water at room temperature and dried in the air stream. Brackets were then ultrasonically cleaned for 10 min. Group-3 consists of CM that is, metal slot CM 0.002” MBT prescription, Clarity 3M Unitek. Composition[18] - polycrystalline with metal slot.
Each group of brackets were rinsed with a mixture of ethanol and acetone in an ultrasonic cleaning bath for 30 min, and each group of dried brackets were weighed to facilitate the metallic mass exposure to the solutions. After that, the samples (each contains 15) were immersed in cleaned, low-density polyethylene 100 ml bottles containing 10 ml of artificial saliva. The samples were gently stirred at room temperature to ensure that all the parts of the brackets were soaked thus obtaining homogeneous solutions.
Analysis of samples
The chemical analysis was done in the Department of Marine Science and Marine Biology, Annamalai University. The presence of nickel in the given samples was analyzed by ICP-AES (Perkin Elmer, Optima 2100 DV, USA) [Figure 1].
Calibrated standards of nickel (10 μg/ml) were made from Merck ICP-AES, standard solution. Milli Q (blank) water was used for dilution. The samples were then introduced into the ICP-AES. Twenty percentage nitric acid was used for washing the capillary tubes after each analysis of every sample. Quantitative analysis of nickel was done three times, and the mean value was used as the result. Computer controlled data collection was used to interpret the result, the technique is based on the principal that when liquidized acid treated specimens are exposed to a temperature in the range of 5000–10,000 K, they emit light, which is recorded by multiple photodetectors.
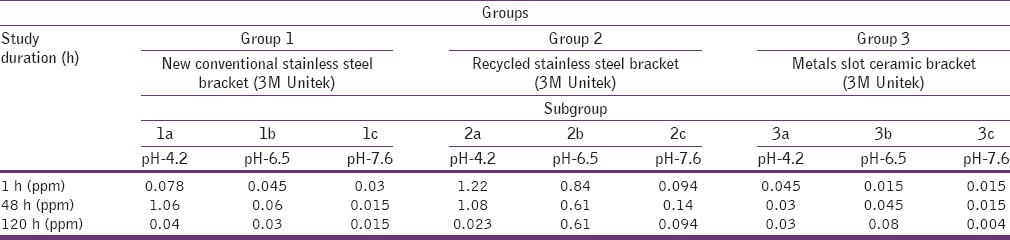
After 1-h, 48 h and 120 h, 0.1 ml of the eluent was removed and diluted and acidified at pH-2 with HNO3. Each solution was analyzed to determine the concentration of nickel release from the brackets by using the ICP-AES. The pH of the eluent[19] solutions was always re-measured after each cycle. At the end of the procedure, the brackets were rinsed with Milli-Q water, air-dried and reweighed to verify any material loss during the leaching procedure.
Statistical tools used
The following statistical tools were used to analyze the collected data. ANOVA (F-test) was used to test the significant difference among the groups. Level of significance was considered at 0.05 levels that are, P < 0.05. Furthermore, descriptive statistics was used to find mean, standard deviation, minimum, and maximum. The software used for statistical analysis was SPSS-18 software (SPSS.Ltd, Quarry bay, Hong Kong, PASW-statistics 18).
Result
All the groups were divided into three sub Groups a, b, and c [Table 1]. The materials in each subgroups, fifteen brackets were placed in separate low density polyethylene 100 ml container containing 10 ml artificial saliva at different pH levels: 4.2, 6.5 and 7.6; for the study period of 120 h.
After 1 h, 48 h and 120 h; 0.1 ml of the eluent was removed into a 10 ml low density polyethylene container and diluted and acidified at pH 2 with HNO3. Each solution was analyzed to determine the concentration of nickel released from different types of brackets by using ICP-AES (model: Perkin Elmer, Optima 2100 DV, USA). Quantitative analysis of nickel was done three times and the mean value was used as the results. Computer controlled data collection was used to interpret the results.
The amount of nickel released from each sample was measured in ppm. A total of 27 samples were used in this study, which are shown in Table 1.
In Group 1 minimum amount of nickel was observed as 0.015 ppm in sub Group-1c at 48 h and 120 h, and maximum amount of nickel was on the day of 48 h in sub Group-1a, as 1.06 ppm [Table 3]. In Group 2 minimum amount of nickel was observed to be 0.023 ppm in sub Group-2a at 120 h and the maximum amount of nickel was 1.22 ppm on the day of 1-h in sub Group-2a [Table 3]. In Group 3 minimum amount of nickel was observed at 0.004 ppm in sub Group-3c at 120 h and the maximum amount of nickel was 0.08 ppm on the day of 120 h in sub Group-3b [Table 3].
Table 3.
Comparison of Group 1, Group 2 and Group 3 brackets under subgroups at different time interval
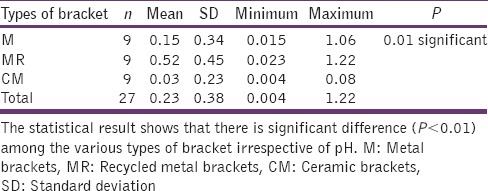
The statistical analysis states that there is a significant difference (P < 0.01) among the various types of bracket irrespective of pH [Table 4], which is represented in [Graph 1]. In 4.2 pH, among the different types of brackets there is a statistical significant difference (P < 0.05) with MR higher mean score, followed by M and CM [Table 5], [Graph 2]. In 6.5pH there is a statistical significant difference (P < 0.001) among the various types of brackets with higher value to MR [Table 6], [Graph 3]. In 7.6 pH there is a statistical significant (P < 0.001) difference among various types of brackets with higher value to MR [Table 7], [Graph 4]. In 1-h there is a statistical significant difference (P < 0.05) among various types of brackets with higher value to MR [Table 8], [Graph 5]. In 48 h, there is a significant difference (P < 0.03) among various types of brackets with higher value to MR [Table 9], [Graph 6]. In 120 h there is a significant difference (P < 0.03) among various types of brackets with higher value to MR [Table 10], [Graph 7].
Table 4.
Mean, SD, minimum, maximum and P value for comparison of different types of brackets
Graph 1.
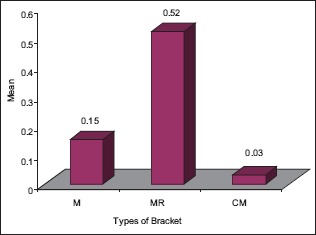
The significant difference among the various types of bracket irrespective of pH
Table 5.
4.2 pH and different types of brackets
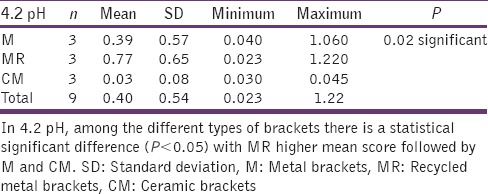
Graph 2.
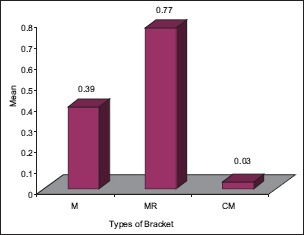
The significant difference in 4.2 pH, among the different types of brackets with higher MR mean score followed by M and CM
Table 6.
6.5 pH and different types of brackets
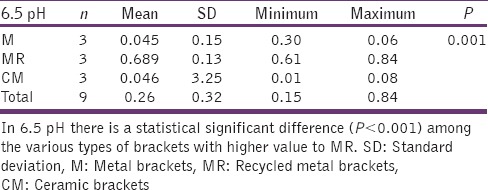
Graph 3.
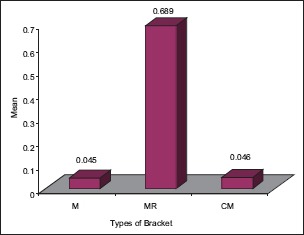
The significant difference in 6.5 pH among various types of brackets with higher MR
Table 7.
7.6 pH and different types of brackets
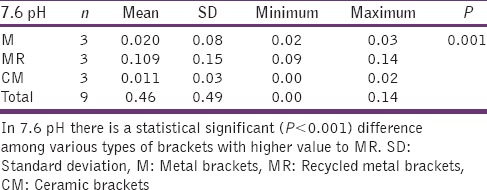
Graph 4.
The significant difference in 7.6 pH among the various types of brackets with higher MR
Table 8.
1 h and different types of brackets
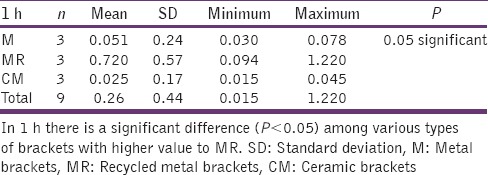
Graph 5.
The significant difference among the various types of brackets with higher MR value after ‘1’hour time
Table 9.
48 h and different types of brackets
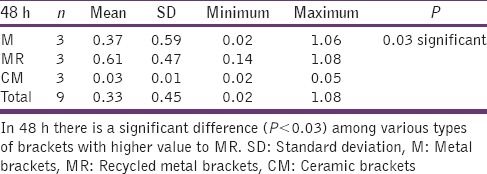
Graph 6.
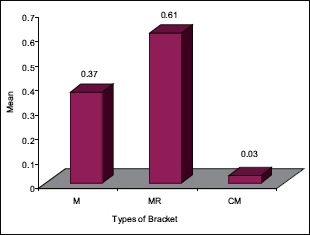
The significant difference among the various types of brackets with higher MR value after 48 hours
Table 10.
120 h and different types of brackets
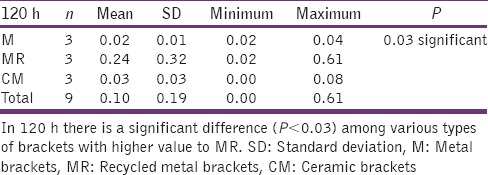
Graph 7.
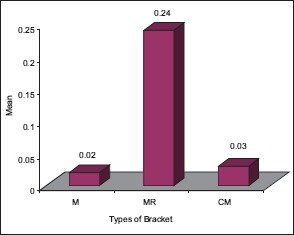
The significant difference among the various types of brackets with higher MR value after 120 hours
Overall in the present study the total discharge of nickel was more in Group 2 [Table 3].
Discussion
The present study was conducted to evaluate the in vitro nickel release from different types of orthodontic brackets by immersion of the samples in artificial saliva at various pH (4.2, 6.5, and 7.6) over an extended time interval of 1-h, 48 h and 120 h. 6.5 and 7.6 are the range of natural salivary pH; whereas 4.2 pH was used to investigate a stimulated condition that occurs only when people consumes acidic foods or drinks (e.g.,: Lime juice, cola drinks).
When compared to the previous studies done by Maria et al.[19] this investigation also shows greatest amount of nickel release in recycled stainless steel brackets. In our study we have found significantly less amount of nickel release from metal slot CM.
Ionic leaching can be accelerated by heat treatment during the reconditioning process.[9,20] It has been demonstrated that the release of metals from stainless steel appliances increases after heating from 400°C to 800°C. This is a reason why recycled brackets release a higher amount of ions than new conventional stainless steel brackets.
In the previous studies, Maria et al. used Alphident recycling process,[19] which involves washing the brackets in a nonacid solution, followed by drying and heating to 350°C for 24 h. The brackets were then washed twice in a nonacid solution, dried, electro polished for 20 s and sterilized at 250°C.
In the current study, the recycling process used is simple and effective for reconditioning stainless steel orthodontic brackets. Orthodontists still flame the bracket with micro torch, followed by ultrasonic cleaning. Here the tip of the flame will be pointed at the bracket base for 5 s until the base becomes red hot, then quenched in water at room temperature and dried in the air stream. Thus treated brackets were then ultrasonically cleaned for a time period of 600 s.[21]
In this study, the comparison was done for all kinds of brackets; the highest nickel was released in the experiment at 4.2 pH from recycled stainless steel brackets and the least nickel was in 6.5 pH and 7.6 pH from metal slot CM. These results agree with those put forward by Huang et al.[22] According to them the acidic conditions provide a reducing environment in which the stainless steel oxide film required for corrosion resistance is less stable.
Our results showed that the amount of nickel released in all test solutions were below the critical value for causing nickel allergy (600–2500 μg)[19,23] and below the daily intake (300–600 μg). Our results have the same opinion with the previous study (comparison of nickel release from new conventional recycled stainless steel and nickel free brackets) of nickel release.
In this study, the artificial saliva developed by Matos de Souza and Macedo de Menezes.[9], and Tanni and Zucchi[17] was used. This solution was adjusted with 0.1 g/L of urea and 0.1 g/L of alpha-amylase to make it more similar to the human saliva. Another important factor is the flow rate of saliva in relation to metal corrosion.[24] In most of the previous studies by Hwang et al., saliva was used in a static condition, but more metal release could be observed in real life because of the composition and fluidity of the saliva in the mouth and also because the oxide layers are removed by tooth brushing.[2,24]
The samples collected were subjected to nickel analysis through mass spectroscopy, ICP-AES, atomic absorption spectroscopy,[1,6,7,8] etc. Most of the previous clinical studies as well as in vitro studies[12,25] employed atomic absorption spectrophotometer for nickel analysis. However, Park and Shearer[2], Eliades et al.,[26] have employed AES for a similar clinical study. While comparing with atomic absorption, ICP-AES has an advantage of providing information about a wide spectrum of elements in the same sample.[11]
In our study, after immersion of the samples they were gently stirred on a shaking plate at room temperature so that all the parts of the brackets were soaked, and homogeneous solutions were obtained. It should be analyzed that this movement could have accelerated the degradation and increase the metal release.
In this study, the nickel release from the recycled stainless steel brackets has the highest at all 4.2 pH except in 120 h. Thus, the recycled stainless steel brackets should be avoided for patients with allergies. Metal slot ceramic bracket's nickel release is significantly low, so it could be advised. However, it is still matters of controversy and argument whether hypersensitivity occurs from dental nickel-based alloys and whether patients with hypersensitivity show forbearance, reoccurrence or cytotoxic reactions during orthodontic treatment. The point of debate is that to develop an allergic reaction in the oral mucosa, the level of an antigen must be 5–12 times greater than that needed for a skin allergy.[19]
Conclusion
The nickel release in the present study during the time period and groups was statistically significant, and the total release of nickel was more in recycled-stainless steel brackets. It can be said that though all the new advances promise a superior performance than the ones before. It needs clinical studies with large number of patients with long intervals, which should be conducted in the biological intraoral environment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Agaoglu G, Arun T, Izgi B, Yarat A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001;71:375–9. doi: 10.1043/0003-3219(2001)071<0375:NACLIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Park HY, Shearer TR. In vitro release of nickel and chromium from simulated orthodontic appliances. Am J Orthod Dentofacial Orthop. 1983;84:156–9. doi: 10.1016/0002-9416(83)90180-x. [DOI] [PubMed] [Google Scholar]
- 3.Philips, Ralph W. Skinner's Science of dental materials. 7th ed. Philadelphia, London and Toronto: W. B. Saunders Co; 1973. Dental Materials; pp. 637–53. [Google Scholar]
- 4.Mikulewicz M, Chojnacka K, Wozniak B, Downarowicz P. Release of metal ions from orthodontic appliances: An in vitro study. Biol Trace Elem Res. 2012;146:272–80. doi: 10.1007/s12011-011-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Grimsdottir MR, Gjerdet NR, Hensten-Pettersen A. Composition and in vitro corrosion of orthodontic appliances. Am J Orthod Dentofacial Orthop. 1992;101:525–32. doi: 10.1016/0889-5406(92)70127-V. [DOI] [PubMed] [Google Scholar]
- 7.Kerosuo H, Odont DR, Moe G, Henbten-Pettersen A. Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 1997;111:595–8. doi: 10.1016/s0889-5406(97)70310-x. [DOI] [PubMed] [Google Scholar]
- 8.Kocadereli L, Ataç PA, Kale PS, Ozer D. Salivary nickel and chromium in patients with fixed orthodontic appliances. Angle Orthod. 2000;70:431–4. doi: 10.1043/0003-3219(2000)070<0431:SNACIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Matos de Souza R, Macedo de Menezes L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008;78:345–50. doi: 10.2319/111806-466.1. [DOI] [PubMed] [Google Scholar]
- 10.Bishara SE, Barrett RD, Selim MI. Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am J Orthod Dentofacial Orthop. 1993;103:115–9. doi: 10.1016/S0889-5406(05)81760-3. [DOI] [PubMed] [Google Scholar]
- 11.Eliades T, Trapalis C, Eliades G, Katsavrias E. Salivary metal levels of orthodontic patients: A novel methodological and analytical approach. Eur J Orthod. 2003;25:103–6. doi: 10.1093/ejo/25.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Kerosuo H, Odont DR, Moe G, Kleven E. In vitro release of nickel and chromium from different types of simulated orthodontic appliances. Angle Orthod. 1995;2:111–6. doi: 10.1043/0003-3219(1995)065<0111:IVRONA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Rahilly G, Price N. Nickel allergy and orthodontics. J Orthod. 2003;30:171–4. doi: 10.1093/ortho/30.2.171. [DOI] [PubMed] [Google Scholar]
- 14.Senkutvan RS, Jacob S, Charles A, Vadgaonkar V, Jatol-Tekade S, Gangurde P. Evaluation of nickel ion release from various orthodontic arch wires: An in vitro study. J Int Soc Prev Community Dent. 2014;4:12–6. doi: 10.4103/2231-0762.130921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menezes LM, Quintão CA, Bolognese AM. Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofacial Orthop. 2007;131:635–8. doi: 10.1016/j.ajodo.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003;124:687–93. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Tanni G, Zucchi F. Electrochemical measurement of the resistance to corrosion of some commonly used metals for dental prosthesis. Minerva Stomatol. 1967;16:710–3. [PubMed] [Google Scholar]
- 18.Kusy RP, Whitley JQ. Frictional resistances of metal-lined ceramic brackets versus conventional stainless steel brackets and development of 3-D friction maps. Angle Orthod. 2001;71:364–74. doi: 10.1043/0003-3219(2001)071<0364:FROMLC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Sfondrini MF, Cacciafesta V, Maffia E, Scribante A, Alberti G, Biesuz R, et al. Nickel release from new conventional stainless steel, recycled, and nickel-free orthodontic brackets: An in vitro study. Am J Orthod Dentofacial Orthop. 2010;137:809–15. doi: 10.1016/j.ajodo.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Gjerdet NR, Herø H. Metal release from heat-treated orthodontic archwires. Acta Odontol Scand. 1987;45:409–14. doi: 10.3109/00016358709096365. [DOI] [PubMed] [Google Scholar]
- 21.Chetan GB, Reddy M. Comparative evaluation of four office reconditioning methods for orthodontic stainless steel brackets on shear bond strength, an in vitro study. Ann Essence Dent. 2011;3:1–8. [Google Scholar]
- 22.Huang HH, Chiu YH, Lee TH, Wu SC, Yang HW, Su KH, et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials. 2003;24:3585–92. doi: 10.1016/s0142-9612(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaaber K, Veien NK, Tjell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol. 1978;98:197–201. doi: 10.1111/j.1365-2133.1978.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 24.Hwang CJ, Shin JS, Cha JY. Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2001;120:383–91. doi: 10.1067/mod.2001.117911. [DOI] [PubMed] [Google Scholar]
- 25.Huang TH, Yen CC, Kao CT. Comparison of ion release from new and recycled orthodontic brackets. Am J Orthod Dentofacial Orthop. 2001;120:68–75. doi: 10.1067/mod.2001.113794. [DOI] [PubMed] [Google Scholar]
- 26.Christos TE, George, Elias K. Salivary metal levels of orthodontic patients: A novel methodological and analytical approach. Eur J Orthod. 2003;25:103–6. doi: 10.1093/ejo/25.1.103. [DOI] [PubMed] [Google Scholar]


