Abstract
Background:
Matrix metalloproteinase (MMP) are involved in bone transformation at the extraction site postdental extraction. We examined the genetic association between single nucleotide polymorphisms of MMP-1 and continuous atrophy of edentulous mandible.
Methods:
Buccal cells from 33 edentulous patients were collected using sterile wooden spatula and were suspended in 15 ml falcon tubes containing 1.5 ml of cell lysis buffer, without proteinase K. The cells were transported to the laboratory on ice and were stored at −20°C until being processed.
Results:
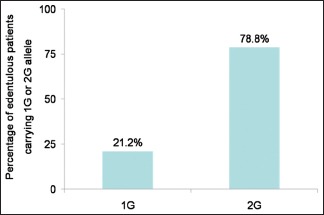
Of the samples analyzed, 26 edentulous patients (78.8%) carried 2G allele, while 7 of them (21.2%) carried 1G allele.
Conclusion:
The patients with the alveolar bone resorption exhibited more of 2G allele while only 21.2% of them showed 1G allele, associated with excessive atrophy of edentulous mandible. This study may provide genetic background to identify susceptible individuals prone to develop jawbone atrophy after dental extraction.
KEY WORDS: Matrix metalloproteinases, matrix metalloproteinase-1, polymorphism, ridge resorption, 1G/2G allele
The prevalence of edentulism increases with age and increasing life expectancies. Prosthodontic concerns are directly related to this increase, as dentists have to satisfy elderly denture wearers. Treatment in these patients is dependent upon the degree of residual ridge resorption (RRR).[1] The term residual ridge is used to describe the clinical alveolar ridge, formed as a consequence of wound healing after tooth extraction. The size of residual ridge is reduced most rapidly in the first 6 months following tooth extraction; however, bone resorption of the residual ridge continues throughout life, resulting in loss of large amount of jawbone structure. In extreme cases, pathological bone fracture may occur.[2] The etiology of RRR is presently considered to be multifactorial. Local osseous remodeling is also dependent upon adaptive immune response. Cytokines which are liberated by some lymphocytes are implicated in bone resorption.[3] In the dentulous state, most pathogenic bacteria reside in periodontal pockets and do not invade the periodontal tissue. The immune system can never efficiently eliminate the microorganism. This unique situation leads to a chronic inflammation and continuous host response, resulting in tissue destruction.[4,5]
Extracellular matrix (ECM) macromolecules are important for creating the cellular environments required during development and morphogenesis of tissues and serves as a scaffold for normal biological functions. The matrix is basically composed of fibrous proteins such as collagen and elastin, and elongated glucoproteins such as fibronectin and laminin, whose function is to provide cell-matrix adhesion.[6] Matrix Metalloproteinases (MMP's) represent the major class of enzymes responsible for ECM metabolism. MMP's are secreted by inflammatory cells in response to stimuli such as lipopolysaccharides and cytokines.[7]
Matrix metalloproteinases-1 is a major proteinase of the MMP family that specifically degrades type I collagen, which is a major component of the ECM, as well as other fibrillar collagens of types II, III, V, IX, and X at neutral pH. As these collagen types are the most abundant proteins in the body, MMP-1 is critical for remodeling of ECM.[6] MMPs have important roles in diverse physiological and pathological processes, as they regulate various cell behaviors such as angiogenesis, cell proliferation, apoptosis, alteration of cell motility, effects on the immune system and host defense, and modulation of the bioactivity of chemokines. Alteration in the activity of MMPs are related to a number of important diseases such as cartilage destruction and bone erosion in rheumatoid arthritis, osteoarthritis, implant failure, acute myocardial infarction, and in carcinogenesis.[6] MMP's are involved in degradation of ECM in intervertebral discs.[8] It has been hypothesized that single nucleotide polymorphism in genes that are involved in bone remodeling may contribute to abnormal residual ridge formation and maintenance.[1] Hence, this study was designed to find out whether polymorphism of MMP-1 can be co-related with resorption of the residual ridge in completely edentulous population.
Methods
Extraction and analysis of deoxyribonucleic acid
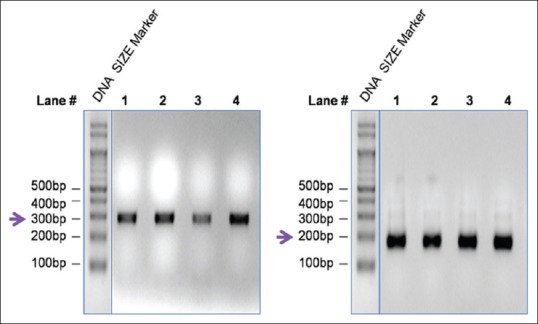
After taking up the ethical clearance from the institution and informed consent, Buccal cells collected from 33 edentulous patients with no history of systemic disease within a edentulous span more than a year within a period of 10 years, were processed to extract genomic deoxyribonucleic acid (DNA). A 10 μl aliquot of the DNA was subjected to electrophoresis in a 0.8% agarose gel to analyze the quality of the genomic DNA. A high molecular weight band without any observable smear indicates DNA of high quality [Figure 1]. The intensity of the DNA band, which reflects their concentration, varies among samples. While the DNA concentration in lane five is high, the concentration of DNA in lane 3 is less (indicated by a bold arrow). This is expected, since the amount of DNA purified, depends largely on the amount of starting tissue material.
Figure 1.
Polymerase chain reaction was performed in two stages
Analyzing the deoxyribonucleic acid concentration
The concentrations of DNA in each of the sample was measured in an ultraviolet and visible dual beam spectrophotometer, and were found to be in the range of 10–100 ng/μl. Total DNA concentrations in 50μl volume varied among samples and were in the range of 0.5–7 μg. The absorbance at 260 nm and associated DNA concentration in each of the sample is shown in Table 1.
Table 1.
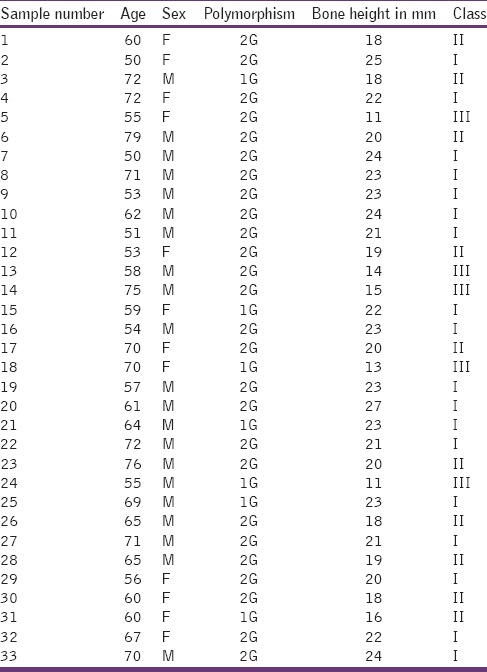
The age of the patient, sex, 1G/2G polymorphism, bone height, and classification of edentulism
Polymerase chain reaction
In order to analyze the 1G/2G polymorphism in the promoter region of MMP-1 gene, we first downloaded the promoter sequence from the public domain database (www.ncbi.nlm.nih.gov/nucleotide)[9] and designed the primers flanking the known polymorphism site at −1067 base. The minus sign indicates that the site (1067 in this study) is in the promoter region, upstream of the transcription start site of MMP-1 gene.
Detecting the 1G/2G polymorphism
The amplification products of second step of polymerase chain reaction (PCR) were digested with Alu1 restriction enzyme in a 25 μl reaction volume at 37°C for 4 h. The digested products were analyzed by running a 15 μl aliquot in a 2.5% agarose gel at 50 V for 30 min. Alu1 recognizes a tetranucleotide motif AGCT (1G) but not AGGCT (2G) pentanucleotide motif, and digests (cuts) the PCR product (DNA) after “G”. Samples carrying 1G allele being sensitive to Alu1 restriction digestion are cleaved into 169 bp and 25 bp products, which migrate faster relative to undigested (uncut) 2G allele samples of 194 bp in size. A representative of the digestion product electrophoresis is shown in Figure 1.
Of the samples analyzed, 26 of edentulous patients (78.8%) carried 2G allele, while 7 of them (21.2%) carried 1G allele [Figure 2 and Table 2]. A majority of the edentulous samples analyzed carried 2G allele.
Figure 2.
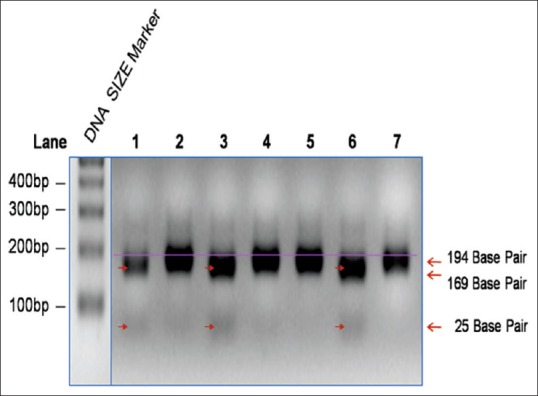
1G/2G polymorphism
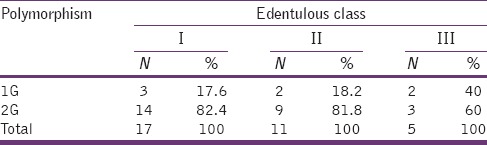
Table 2.
The number and percentage of 1G or 2G polymorphism under each edentulous classification
Determination of Bone Height
In order to analyze the possible association of bone height with the occurrence of polymorphism, the bone height was determined in each of the patient by measuring on Panoramic dental radiographs, obtained from all subjects, using which the bone height of the lowest vertical height of the edentulous mandible was measured following the protocol of the American College of Prosthodontists.[10] Depending on existing bone height, the edentulous statuses were designated as class I, II, III, and IV. Bone height measurements and their respective class designations are shown in Table 1. A majority of the patients (51.5%) belonged to class I followed by class II (33.3%) and class III (15.2%). None of them belonged to class IV in this study. The mean bone height in each of the class was found to be 23 mm for class I, 18.7 mm for class II, and 12.8 mm for class III [Bar Diagram 1].
Bar Diagram 1.
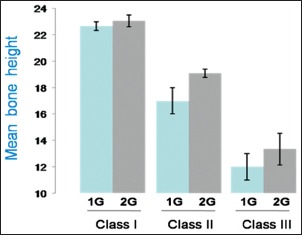
The mean bone height in comparison with 1G/2G polymorphism under each edentulous classification
Association between 1G or 2G polymorphism and bone height
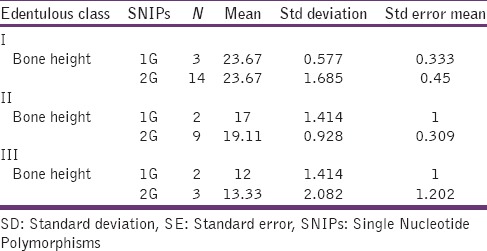
Analysis on the distribution of 1G or 2G polymorphism in each of the edentulous class is expressed in Table 3. No association between the edentulous class and 1G or 2G polymorphism was observed. When the mean value of bone height in each class was grouped with that of polymorphism, again, no significant association was found.
Table 3.
Mean, SD and SE in each polymorphism under edentulous classification
Discussion
Matrix metalloproteinases-1 is capable of degrading type I, II, and III collagens, and is one of only four MMPs able to degrade triple helical collagens. MMP-1 is a major proteinase involved in modeling and remodeling of ECM.[6] MMP-1 gene located in chromosome 11q22 has the propensity for polymorphism which has been an indicator for cancer, hip arthroplasty, implant failure, periodontitis. We attempt to first correlate between MMP-1 polymorphism and the risk of bone resorption in RRR. The result of this study showed that 78% of the experimental group showed 2G polymorphism, and 22% were of 1G polymorphism [Bar Diagram 2].
Bar Diagram 2.
The percentage of bone height in each polymorphism
Although 1% of polymorphism is previously reported, this could be associated with susceptibility to the disease. Based on these two observations, edentulous patients were examined to establish, correlate, and detect susceptibility to bone resorption in RRR.[6] Patients were categorized according to the level of bone loss and were in the age group 50–70 years. Majority of patients in the study showed 2G polymorphism, which has been associated previously with implant failure and progressive periodontal disease, both of which are associated with excessive bone resorption mechanism. 2G polymorphism is associated with bone loss in periodontitis and has altered gene resulting in increased destruction of ECM with increased mRNA in the inflamed gingival tissue.[4] While GG polymorphism of MMP-1 gene at −1607 domain led to early implant failures in patients which may be associated with osseointegration process.[10,11] Increased mRNA level in inflamed tissues as a result of MMP-1 polymorphism, leads to increase transcriptional activity and degradation of major interstitial collagenase resulting in increased bone loss.[12] The 2G polymorphism detected in the edentulous patients could be simply susceptibility to bone loss while both 1G and 2G polymorphism seem to be a common occurrence in the south Indian population. Although sample size prevents to make generalized conclusion regarding the association of bone loss and genetic mutation. Nevertheless, polymorphism is an important indicator for bone loss. 2G polymorphism could also be used as a potential marker for bone loss among the south Indian population.
Conclusion
Of the 33 samples that were analyzed for the presence of 1G or 2G, 26 carried 2G polymorphism in homozygous 2G/2G condition, which has been associated with an increase in bone resorption in chronic periodontitis. The rest of them carried 1G polymorphism in homozygous 1G/1G condition, with none of them carrying 1G/2G heterozygous polymorphism.
When the data were corroborated with the existing bone height level, there was no positive association between the occurrence of 2G/2G or 1G/1G polymorphism and bone resorption in any of the three classes of resorption. The number of 1G polymorphisms found in the study was close to one-fourth of 2Gs. In the absence of equal number of either polymorphisms or at least statistically matched number of 1G and 2G polymorphisms, it is hard to find associations between their occurrence and bone height.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nishimura I, Garrett N. Impact of Human Genome Project on treatment of frail and edentulous patients. Gerodontology. 2004;21:3–9. doi: 10.1111/j.1741-2358.2004.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Jahangiri L, Devlin H, Ting K, Nishimura I. Current perspectives in residual ridge remodeling and its clinical implications: A review. J Prosthet Dent. 1998;80:224–37. doi: 10.1016/s0022-3913(98)70116-7. [DOI] [PubMed] [Google Scholar]
- 3.Atwood DA. Essential of Complete Denture Prosthodontics. 2nd ed. INDIA: AITBS Publishers, Sheldon Winkler; 2009. The problem of reduction of residual ridges; pp. 22–39. [Google Scholar]
- 4.de Souza AP, Trevilatto PC, Scarel-Caminaga RM, Brito RB, Line SR. MMP-1 promoter polymorphism: Association with chronic periodontitis severity in a Brazilian population. J Clin Periodontol. 2003;30:154–8. doi: 10.1034/j.1600-051x.2003.300202.x. [DOI] [PubMed] [Google Scholar]
- 5.Hollá LI. Genetic variations in the matrix metalloproteinase-1 promoter and risk of susceptibility and/or severity of chronic periodontitis in the Czech population. J Clin Periodontol. 2007;33:233–42. doi: 10.1111/j.1600-051X.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 6.Arakaki PA, Marques MR, Santos MC. MMP-1 polymorphism and its relationship to pathological processes. J Biosci. 2009;34:313–20. doi: 10.1007/s12038-009-0035-1. [DOI] [PubMed] [Google Scholar]
- 7.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: Multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song YQ, Ho DW, Karppinen J, Kao PY, Fan BJ, Luk KD, et al. Association between promoter -1607 polymorphism of MMP1 and lumbar disc disease in Southern Chinese. BMC Med Genet. 2008;9:38. doi: 10.1186/1471-2350-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Internet source – National centre for biotechnology information. [Last accessed on 2011 Oct 16]. Available from: http://www.ncbi.nlm.nih.gov/nucleotide .
- 10.McGarry TJ, Nimmo A, Skiba JF, Ahlstrom RH, Smith CR, Koumjian JH. Classification system for complete edentulism. The American College of Prosthodontics. J Prosthodont. 1999;8:27–39. doi: 10.1111/j.1532-849x.1999.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 11.Santos MC, Campos MI, Souza AP, Trevilatto PC, Line SR. Analysis of MMP-1 and MMP-9 promoter polymorphisms in early osseointegrated implant failure. Int J Oral Maxillofac Implants. 2004;19:38–43. [PubMed] [Google Scholar]
- 12.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]


