Abstract
Periodontal plastic surgery is the branch of periodontology that is focused mainly on the correction or elimination of mucogingival problems associated with lack of attached gingiva, a shallow vestibule and aberrant frenum. Various mucogingival surgical procedures are used to halt the progression of the gingival recession and to correct poor esthetic appearance. Free gingival autograft is one of the most common techniques used for a gingival recession in areas of inadequate attached gingiva in the mandibular anterior region. Fibrin sealants are human plasma derivatives that mimic the final stages of blood coagulation, forming a fibrin clot. Fibrin Sealants enhances the overall outcome of surgical intervention because of their hemostatic, adhesive, and healing properties. These properties of fibrin sealants may reduce operating time, prevent complications, and enhance the overall outcome of many surgical interventions. Hence, this case report aims to investigate the clinical effectiveness of free gingival graft along with the commercially available fibrin-fibronectin sealing system (Tissucol®) in the treatment of Miller's class II gingival recession.
KEY WORDS: Fibrin-fibronectin sealing system (Tissucol®/Tisseel®), free gingival graft, gingival recession
There are multiple periodontal plastic surgery approaches designed to improve the esthetics, such as the free gingival autograft,[1] the double papilla graft,[2] the laterally positioned flap,[3] the coronally positioned flap,[4] and connective tissue grafts.[5] These procedures are only the beginning of a branching out of innovations, modifications, and variations comprising at least 50 surgical solutions to the problem of gingival recessions.
Surgical techniques involving flaps from neighboring teeth with the objective of obtaining root coverage have been widely utilized.[6,7] These techniques can be used only when donor tissue is available adjacent to the gingival recession. In cases where donor tissue is not available adjacent to the gingival recession, free gingival autografts have been employed as an alternative. A longitudinal study carried out by Rateitschak et al. concluded that free gingival autografts are more effective in preventing gingival recessions by increasing the width of attached gingiva rather than in obtaining root coverage.[8] Gingival recession regions, in the absence of a mucogingival problem, in which there is an esthetic or hypersensitivity consideration, can be also managed with free gingival graft (FGG).
The desire for an agent, which will produce a biostable union between tissue planes has led to the development of tissue adhesive, fibrin sealant that is, Tissucol®.[9] Fibrin sealant is probably one of the most complex human plasma derivatives both in terms of composition and clinical applications. This product mimics the last step of the coagulation cascade through the activation of fibrinogen by thrombin, leading to the formation of a semi-rigid fibrin clot [Figure 1]. The combined effect of the substances contained in fibrin adhesive system provides prolonged stability of the coagulum with firm and persistent adhesion. The fibrin clot consolidating and adhering to the application site acts as a fluid tightness agent able to hold tissues or materials in a required configuration, while evidencing hemostatic and quicker wound healing properties.[10]
Figure 1.
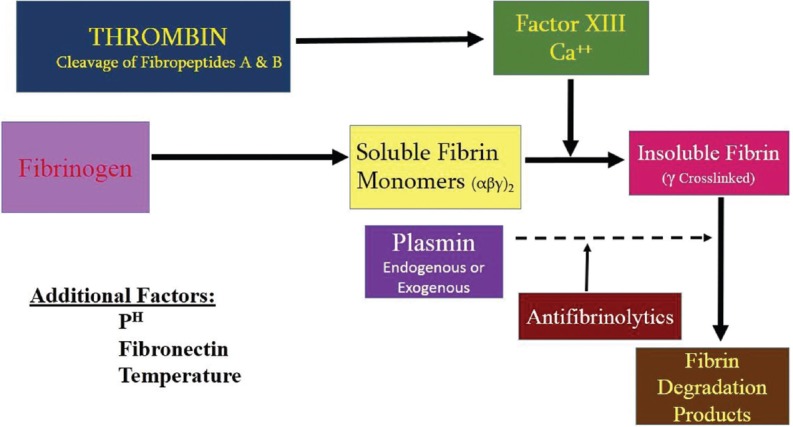
Fibrin sealant activation mechanism
This article presents a case report where the fibrin-fibronectin sealing system (FFSS) is used along with free gingival autograft in the treatment of localized gingival recession.
Case Report
The present case report is about a 29-year-old man who has been referred to the Department of Periodontology, Sree Sai Dental College and Research Institute, Srikakulam (Andhra Pradesh) from the Department of Dentistry, Rajiv Gandhi Institute of Medical Sciences, Srikakulam after performing initial scaling and root planing. On examination, the patient was systemically healthy and had no history of abnormal habits. Chief complaint of the patient was sensitivity in the lower anterior teeth region. On periodontal examination and radiographic evaluation, the patient presented with a Miller's class II gingival recession in the lower central incisors region (number 31, 41) [Figure 2].
Figure 2.
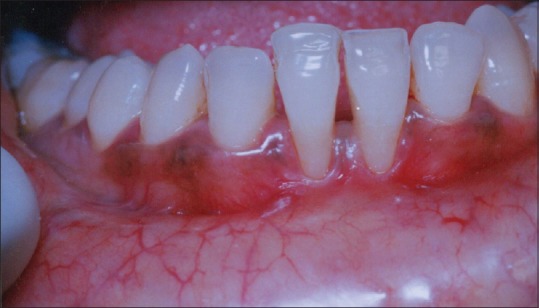
Preoperative view with Miller's class II gingival recession in 31 and 41
Initial therapy consisted of oral prophylaxis, which was repeated until the patient achieved an O’Leary et al. plaque score of 20% or below.[11] Trauma from occlusion if detected was eliminated. At 4 weeks following phase I therapy, a periodontal re-evaluation was performed to confirm the suitability of the surgical procedure. Clinical measurements were made using William's periodontal probe with graduation to a precision of 1 mm [Figure 3]. Patient was explained about the treatment design.
Figure 3.
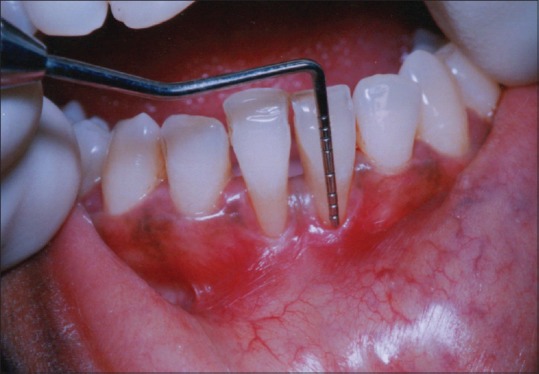
Clinical measurements with William's periodontal probe were recorded
Clinical recordings
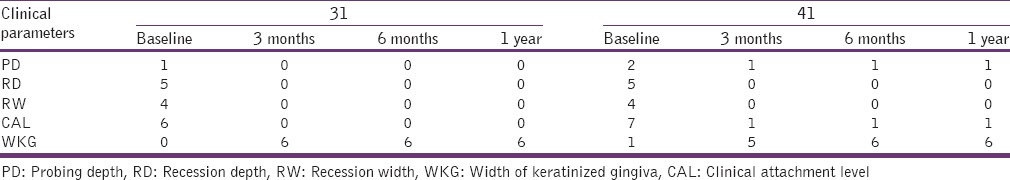
The selected site presented a healthy periodontium with the gingiva exhibiting no evidence of bleeding on probing. The following clinical parameters were taken at the mid-buccal aspect of the lower central incisors (31, 41) separately at baseline, 3 months, 6 months, and 12 months postsurgery.
Probing pocket depth (PD) was measured with a standard periodontal probe to the nearest millimeter from the gingival margin to the bottom of sulcus[12]
Clinical attachment level (CAL) was measured from the cemento-enamel junction (CEJ) to the bottom of the sulcus[13]
Recession depth (RD) measured from CEJ to the gingival margin[12]
Recession width (RW) measured across the buccal surface at the CEJ level
The width of keratinized gingiva (WKG).
Surgical procedure
Step 1: Preparing the recipient site
The recipient site was prepared by horizontal papillary incisions made at right angles to the papilla at the level of CEJ. Two vertical incisions were made from the cut gingival margin to the alveolar mucosa [Figure 4]. Incisions were extended approximately twice the desired width of the keratinized tissue. A split thickness flap was separated without disturbing the periosteum at the recipient site [Figure 5]. An aluminum foil template of the recipient site was made.
Figure 4.
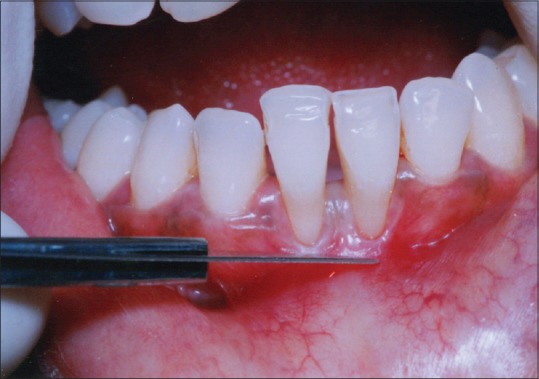
Incisions given
Figure 5.

Recipient bed prepared
Step 2: Harvesting the free gingival graft from palate
Aluminum foil template of the recipient site made was placed over the donor site, and a partial thickness FGG was harvested from the palate [Figure 6]. The graft consisted of epithelium and a thin layer of underlying connective tissue. Proper thickness between 1.0 and 1.5 mm is an important factor for survival of the graft.
Figure 6.
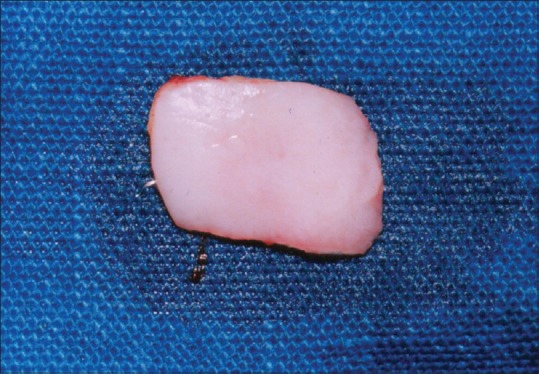
Free gingival graft harvested from the palate
Step 3: Preparation and placement of fibrin-fibronectin sealing system (Tissucol®/Tisseel) on the root surface
Each Tissucol® kit contains 5 vials with the active ingredients: It is a biological two-component sealant [Figure 7]. The two components (TISSEEL Solution and Thrombin Solution) were mixed during or immediately before application. A viscous TISSEEL-Thrombin Solution was formed that quickly sets to form a white, elastic mass (fibrin sealant), which firmly adheres to the tissue. The process simulates the key features of the physiological coagulation process and was used to achieve hemostasis, to seal or glue tissue, and to support wound healing. The solution was kept at 37°C until used.
Figure 7.
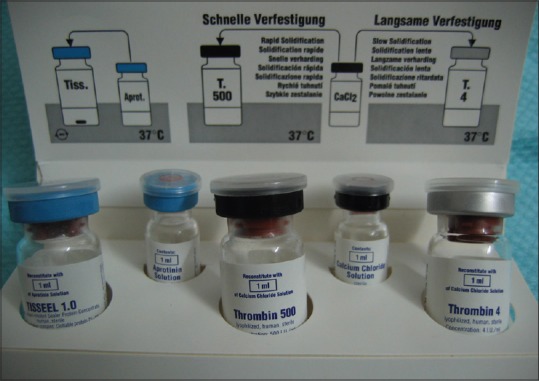
Tissucol® kit
After the required amount of FGG was harvested, a 2–3 ml film of FFSS (Tissucol®) was placed over the root surface and the surrounding bone margins and let it set in place [Figure 8].
Figure 8.
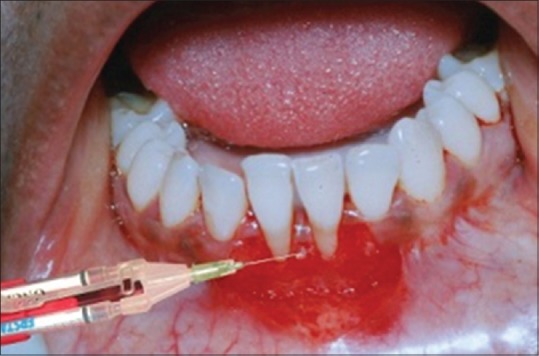
A film of Tissucol® placed over the root surface
Step 4: Placement of free gingival graft on the recipient bed coated with Tissucol®
The harvested FGG from the palate was then placed at the recipient site and sutured at the lateral borders and to the underlying periosteum [Figure 9]. The graft was firmly held in place using digital pressure for 5 min and Coe-Pak was placed at the donor site and over the recipient site. Periodontal dressing was applied over the surgical area for 10 days.
Figure 9.

Free gingival graft sutured
Step 5: Postoperative care
The patient was advised not to brush the treated site for 4 weeks. 0.12% chlorhexidine mouth rinse was prescribed twice a day for 4 weeks. Antibiotics and analgesics were administered as needed. Sutures were removed 10 days after surgery. Patient was examined weekly for the 1st month and then once a month for the next 3 months. Patient was recalled at 3 months intervals for oral hygiene instructions and supragingival scaling until the end of the study period. The case was followed up for a period of 1 year [Figures 10 and 11].
Figure 10.

Two weeks postoperative
Figure 11.
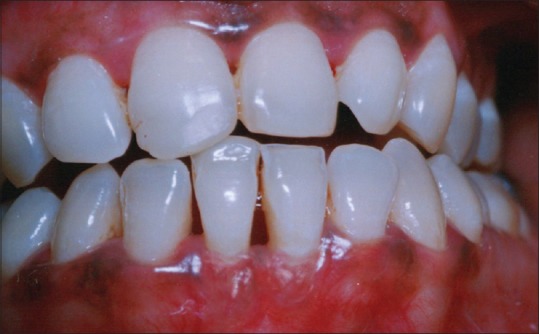
One year postoperative
Results
There was a significant reduction in RD, RW, and probing PD. A significant gain of 6 mm WKG and a 6 mm decrease in the CAL at the end of 1 year compared to baseline values in relation to lower central incisors (31 and 41) was observed. The results of all the clinical parameters obtained were summarized in Table 1.
Table 1.
Clinical parameters
Discussion
Successful coverage of exposed roots for esthetic and functional reasons has been the objective of various mucogingival procedures. This generates a need for the clinician to develop newer techniques to fulfill these requirements without compromising esthetics and comfort. There are currently different techniques for root coverage, but it is often difficult to predict the success rate of root coverage procedures since it depends on multiple factors, including the location and classification of the recession and the type of surgical technique used.[14]
Previous studies have reported a coverage of 40–70% using FGG in class I and II recessions.[15] Although Miller[16] proposed that FGG is a predictable method of root coverage, the apparent disadvantage of the poor color match and donor site morbidity render it unsuitable for use as a root coverage procedure. However, even with the emergence of subepithelial connective tissue graft and allogenous grafts like Alloderm, FGG continues to be the most anticipated method to increase the apico-coronal dimension of the keratinized mucosa.[17]
In this case report, a FGG was used for successful root coverage along with FFSS. The use of this technique is justified because it provides highly predictable results when root coverage of large gingival recessions is desired. Miller[18] and Holbrook and Ochsenbein have demonstrated the successful utilization of free gingival autografts in covering large gingival recessions.[19] FGG is the ideal technique, which can be used in the lower anterior teeth region where there is a gingival recession with an inadequate amount of keratinized gingiva.
Fibrin sealant was first used to fix periodontal flaps and grafts in 1982.[20] Later, the FFSS was used with guided tissue regeneration in the treatment of gingival recession.[9] In a split-mouth clinical study, no statistically significant clinical differences were seen in terms of RD between defects treated with fibrin sealant plus a coronally positioned flap and those treated with a coronally positioned flap alone.[21] However, recently in 2012, it was used along with bioresorbable type I collagen membrane to treat gingival recession and found that the results obtained from collagen membrane with Tisseel® group were slightly better, although statistically not significant compared to collagen membrane alone.[22]
The promising results obtained with the application of FFSS on FGG can be explained by the fact that the fibrin contains factors such as thrombin, fibrin, fibronectin, and platelet-derived growth factor, which have biologic activities on cell proliferation and differentiation during wound healing. These factors potentially enhance the wound healing and regeneration of new tissues. Fibrin sealant simulates key features of the physiological blood clotting mechanism. Factor XIII, activated by thrombin, catalyses the formation of cross-links within the fibrin clot and a stable clot is formed at the site of application.[23]
The observation, in this case, was that the application of a tissue adhesive overcame mechanical and anatomical limitations of graft adaptation and stabilization. As reported by Caton et al. the collagen fibrils exposed during tetracycline HCL conditioning may effectively bind the FFSS and this in turn, secures the gingival flap to the root.[24] It has been previously observed by Ripamonti et al. that the amount of flap contraction during wound healing was minimized by the adjunctive application of a fibrin-fibronectin adhesive system to demineralized root surfaces in experimentally induced osseous defects in nonhuman primates.[25]
The results of this case report have shown a significant decrease in RD, RW, probing depth at baseline to 1 year, and there was a significant gain in the WKG and CAL at the end of 1-year. However, still longitudinal studies need to be done to evaluate the clinical efficiency of FFSS with FGG in the treatment of gingival recession.
Conclusion
The results of this case report indicate that FFSS (Tissucol®) provides an additional armamentarium in periodontal therapy and it can be beneficially used as an adjunct in various periodontal surgical procedures for gaining new attachment and for accelerated postoperative healing.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sullivan HC, Atkins JH. Free autogenous gingival grafts 3. Utilization of grafts in the treatment of gingival recession. Periodontics. 1968;6:152–60. [PubMed] [Google Scholar]
- 2.Cohen DW, Ross SE. The double papillae repositioned flap in periodontal therapy. J Periodontol. 1968;39:65–70. doi: 10.1902/jop.1968.39.2.65. [DOI] [PubMed] [Google Scholar]
- 3.Grupe HE, Warren RF. Repair of gingival defects by a sliding flap operation. J Periodontol. 1956;27:290–5. [Google Scholar]
- 4.Bernimoulin JP, Lüscher B, Mühlemann HR. Coronally repositioned periodontal flap. Clinical evaluation after one year. J Clin Periodontol. 1975;2:1–13. doi: 10.1111/j.1600-051x.1975.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 5.Edel A. Clinical evaluation of free connective tissue grafts used to increase the width of keratinised gingiva. J Clin Periodontol. 1974;1:185–96. doi: 10.1111/j.1600-051x.1974.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 6.Cole R, Nilvéus R, Ainamo J, Bogle G, Crigger M, Egelberg J. Pilot clinical studies on the effect of topical citric acid application on healing after replaced periodontal flap surgery. J Periodontal Res. 1981;16:117–22. doi: 10.1111/j.1600-0765.1981.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 7.Common J, McFall WT., Jr The effects of citric acid on attachment of laterally positioned flaps. J Periodontol. 1983;54:9–18. doi: 10.1902/jop.1983.54.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Rateitschak KH, Egli U, Fringeli G. Recession: A 4-year longitudinal study after free gingival grafts. J Clin Periodontol. 1979;6:158–64. doi: 10.1111/j.1600-051x.1979.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 9.Trombelli L, Schincaglia GP, Zangari F, Griselli A, Scabbia A, Calura G. Effects of tetracycline HCl conditioning and fibrin-fibronectin system application in the treatment of buccal gingival recession with guided tissue regeneration. J Periodontol. 1995;66:313–20. doi: 10.1902/jop.1995.66.5.313. [DOI] [PubMed] [Google Scholar]
- 10.Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–43. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Lynch SE. Methods for evaluation of regenerative procedures. J Periodontol. 1992;63:1085–92. doi: 10.1902/jop.1992.63.12s.1085. [DOI] [PubMed] [Google Scholar]
- 13.Clark DC, Chin Quee T, Bergeron MJ, Chan EC, Lautar-Lemay C, de Gruchy K. Reliability of attachment level measurements using the cementoenamel junction and a plastic stent. J Periodontol. 1987;58:115–8. doi: 10.1902/jop.1987.58.2.115. [DOI] [PubMed] [Google Scholar]
- 14.Zucchelli G, Testori T, De Sanctis M. Clinical and anatomical factors limiting treatment outcomes of gingival recession: A new method to predetermine the line of root coverage. J Periodontol. 2006;77:714–21. doi: 10.1902/jop.2006.050038. [DOI] [PubMed] [Google Scholar]
- 15.Borghetti A, Gardella JP. Thick gingival autograft for the coverage of gingival recession: A clinical evaluation. Int J Periodontics Restorative Dent. 1990;10:216–29. [PubMed] [Google Scholar]
- 16.Miller PD., Jr Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in areas of deep-wide recession. Int J Periodontics Restorative Dent. 1985;5:14–37. [PubMed] [Google Scholar]
- 17.Harris RJ. Gingival augmentation with an acellular dermal matrix: Human histologic evaluation of a case - Placement of the graft on periosteum. Int J Periodontics Restorative Dent. 2004;24:378–85. [PubMed] [Google Scholar]
- 18.Miller PD., Jr Root coverage using a free soft tissue autograft following citric acid application. Part 1: Technique. Int J Periodontics Restorative Dent. 1982;2:65–70. [PubMed] [Google Scholar]
- 19.Holbrook T, Ochsenbein C. Complete coverage of the denuded root surface with a one-stage gingival graft. Int J Periodontics Restorative Dent. 1983;3:8–27. [PubMed] [Google Scholar]
- 20.Bartolucci EG, Prato GP. Preliminary observations on the use of a biologic sealing system (Tissucol) in periodontal surgery. J Periodontol. 1982;53:731–5. doi: 10.1902/jop.1982.53.12.731. [DOI] [PubMed] [Google Scholar]
- 21.Trombelli L, Scabbia A, Wikesjö UM, Calura G. Fibrin glue application in conjunction with tetracycline root conditioning and coronally positioned flap procedure in the treatment of human gingival recession defects. J Clin Periodontol. 1996;23:861–7. doi: 10.1111/j.1600-051x.1996.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 22.Sunkavalli A, Murthy KR. A comparative evaluation of bioresorbable type I collagen membrane with and without fibrin fibronectin sealing system in the treatment of gingival recession: A clinical study. J Dr NTR Univ Health Sci. 2012;1:239–44. [Google Scholar]
- 23.Martinowitz U, Saltz R. Fibrin sealant. Curr Opin Hematol. 1996;3:395–402. doi: 10.1097/00062752-199603050-00011. [DOI] [PubMed] [Google Scholar]
- 24.Caton JG, Polson AM, Prato GP, Bartolucci EG, Clauser C. Healing after application of tissue-adhesive material to denuded and citric acid-treated root surfaces. J Periodontol. 1986;57:385–90. doi: 10.1902/jop.1986.57.6.385. [DOI] [PubMed] [Google Scholar]
- 25.Ripamonti U, Petit JC, Lemmer J, Austin JC. Regeneration of the connective tissue attachment on surgically exposed roots using a fibrin-fibronectin adhesive system. An experimental on the baboon (Papio ursinus) J Periodontal Res. 1987;22:320–6. doi: 10.1111/j.1600-0765.1987.tb01592.x. [DOI] [PubMed] [Google Scholar]


