Abstract
Lamotrigine and valproic acid are well-tolerated anticonvulsants, but frequently associated with severe cutaneous reactions, such as the Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis, when used in combination. We report a case of SJS likely induced by the use of a lamotrigine and valproic acid regimen and as a dental surgeon it is important to identify such lesion and report to pharmacovigilance.
KEY WORDS: Lamotrigine, Stevens–Johnson syndrome, toxic epidermal necrolysis, valproic acid
“A new eruptive fever with stomatitis and ophthalmia” was described as a severe variant of erythema multiforme and was termed by Steven and Johnson in 1922. In 1940, it was commonly called as “Steven–Johnson's syndrome (SJS).”[1] SJS, is a serious mucocutaneous illness with systemic symptoms characterized by the presence of flat, atypical target lesions, and epidermal detachment encompassing <10% of the total body surface area with two or more mucosal sites affected usually.[2] There are various factors, which precipitate this condition, including viral infections, medications, and systemic illness.[3,4] There are more than 200 drugs that have been associated with the development of SJS. The drugs such as antibiotics, anti-epileptics, and nonsteroidal anti-inflammatory drugs are the common agents responsible for SJS.[2,3,5,6]
Lamotrigine, a novel anti-epileptic drug effective in treating partial and generalized seizures has been reported to be frequently associated with severe cutaneous reactions, such as the SJS and toxic epidermal necrolysis (TEN).[5,7] We report a case with SJS likely induced by the use of a lamotrigine and valproic acid regimen and as a dental surgeon the need for us to identify the lesion earlier and to initiate an appropriate treatment and report to pharmacovigilance.
Case Report
A 35-year-old female reported with complaints of painful ulcers in the mouth, bleeding lips, rashes throughout the body, and high fever (39°C) of 4 days duration. Her past medical history revealed that she was suffering from seizures for past 4 years and was on medication (valproate 600 mg/day) for the same. One week back, before reporting to us she was started on lamotrigine 25 mg/day along with valproate, since the seizure was not under the control only with valproate. Subsequently, she developed high grade fever with watering of eyes, followed by painful ulcers in the oral cavity, encrustations on the lips, diffuse erythematous rashes over the face, neck, trunk, arms, and legs. History revealed that no such lesions occurred earlier and that was the first time such rashes have occurred. Other personal and family history was not relevant. Her past dental and surgical histories were noncontributory. Vital signs were within the normal limits except for a temperature of 39°C.
Physical examination showed multiple round erythematous plaques on the face, neck, trunk, and extremities, some of which were tender. Some of them showed diffuse atypical target lesions such as purpuric macules and papules especially on the hands. No ophthalmic lesions were noted [Figures 1–3].
Figure 1.
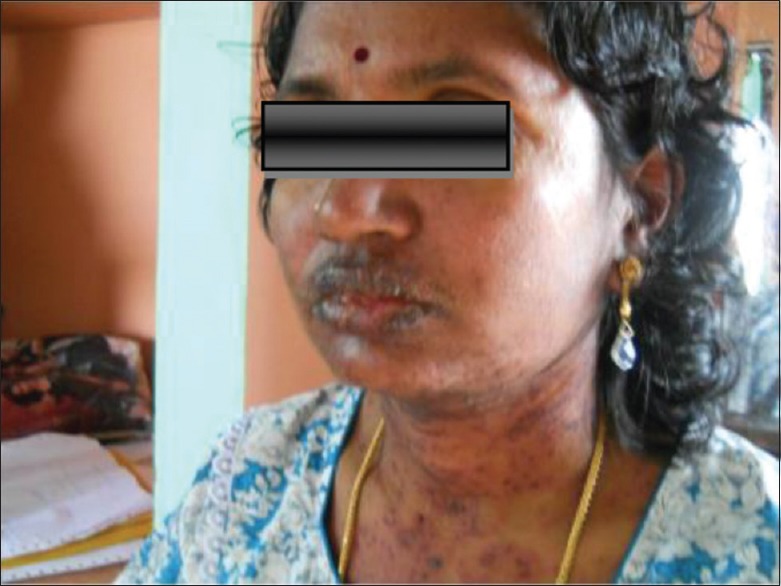
Skin lesions on the face and neck
Figure 3.
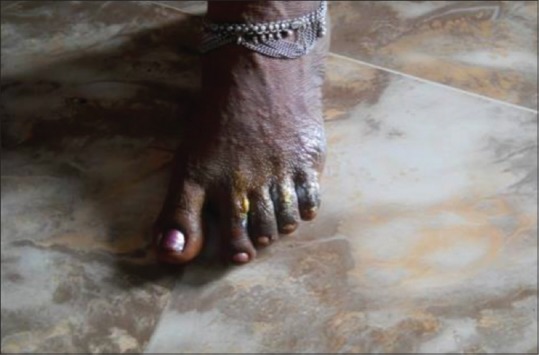
Skin lesions of foot
Figure 2.
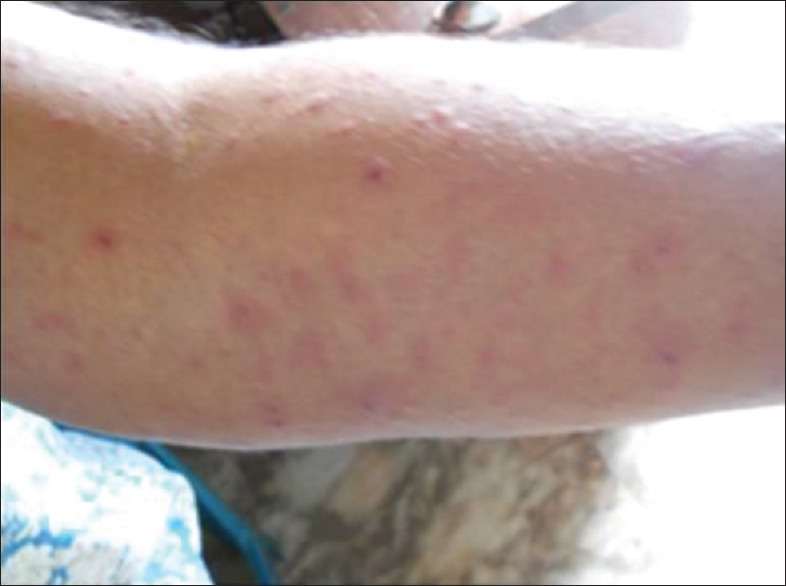
Skin lesions on hand
On extra oral examination, there was limited mouth opening and erythematous crusted areas on both the lips with a rough surface. On intraoral examination, there was generalized inflammation and ulceration, which was progressive and caused functional difficulty.
Based on the history and clinical presentation a diagnosis of SJS was made. Since the presumptive cause was lamotrigine, the drug was discontinued immediately; the patient was hospitalized and treated symptomatically with cyclosporine and methyl prednisolone. Intravenous fluids were given for rehydration. The oral lesions were treated with topical lignocaine gel and chlorhexidine mouth rinse. Her condition improved during the next 2 weeks, and she eventually recovered with continued follow-up on an outpatient basis.
Discussion
Stevens–Johnson syndrome is a rare, life-threatening, and severe blistering mucocutaneous disease, most commonly caused by drugs.[2]
Physicians, when they prescribe, must make the patients aware of the possible adverse effects. One such potential complication of drugs is SJS, a potentially life-threatening condition that manifests mainly on the skin and mucosal surfaces and also affects other vital organs.
Stevens–Johnson syndrome is a less severe variant of TEN and separates clinically from erythema multiforme (EM). EM is characterized by mucosal erosions of raised atypical target lesions usually located on the extremities and/or the face. The characteristic findings of SJS are mucosal erosions plus widespread distribution of flat atypical targets or purpuric macules. The lesions may be present on the trunk, face, and on the extremities. Compared to EM, SJS is more closely associated with medication use and mycoplasma pneumonia infection. It is potentially life-threatening with a mortality of up to 15%.[8]
The incidence of SJS is estimated to be between 1.1 and 7.7 cases per million, with 16% of cases showing a past history of short-term use of the anti-epileptic drug.[2] Lamotrigine is an analog of phenyltriazine class, a potential anti-epileptic drug, which is known to cause SJS, strengthening the probable association between lamotrigine and SJS.[5,9] The risk of skin reactions is increased when the starting dose of lamotrigine is high when the dose is increased rapidly and when used in combination with valproate.[10]
The pathogenesis of SJS is yet to be clarified. The scenario suggested by today's literature points toward drug-specific CD8+ cytotoxic T-cells utilizing perforin/granzyme B triggered apoptosis of epithelial cells. Following this, an expansion of apoptosis occurs involving the interaction of either membrane-bound or soluble Fas ligand with its receptor Fas.[3,6,11,12]
A widely accepted hypothesis regarding SJS has been that patients suffering from severe drug reactions are exposed to increased amounts of oxidative metabolites because of a lowered ability to detoxify reactive metabolites, either on a genetic basis or on a functional basis.[9,11] A genetic predisposition in different populations has been identified. Individuals with antigens human leukocyte antigen Bw44, HLA-B12, HLA-B*502, and HLADQB1*0601 appear to be more susceptible to developing SJS.[3,6,13]
Drug-induced SJS could be diagnosed according to following criteria such as mucosal erosions plus widespread distribution of atypical targets or purpuric macules and epithelial detachment involving <10% body surface area on the trunk, face, and extremities.[3] The same criteria were used in the present case for the diagnosis of SJS.
A recent study by Oflaz et al., in 2011 reported a case of lamotrigine induced SJS, especially with concomitant use of valproic acid. This holds well in our case where the patient was on lamotrigine for a week. It is known that valproic acid interacts with lamotrigine metabolism. This results in a reduced total clearance and therefore to an increased elimination half-life of lamotrigine, resulting in higher serum concentrations.[14]
Stevens–Johnson syndrome should be differentiated from other immunobullous lesions such as pemphigus vulgaris and bullous pemphigoid, acute generalized exanthematous pustulosis, linear IgA dermatosis, paraneoplastic pemphigus, disseminated fixed bullous drug eruption, and staphylococcal scalded skin syndrome.
Treatment of SJS is primarily supportive and symptomatic which includes strict isolation, increasing caloric intake, preventing superinfection and sepsis, and correcting electrolyte disturbance.[6,11] Even though the role of systemic corticosteroids is still controversial, they have been the mainstay therapy for SJS in most cases, as in our case. However, some complications are inevitable. Complications include skin lesions secondary to the infection, organ dysfunction, mucocutaneous fibrosis, and ocular and esophageal strictures.[6,8] In our case, no such complications have been reported in a 2 years follow-up period.
Pharmacovigilance is the pharmacological science relating to the detection, assessment, understanding and prevention of adverse effects, and the duty of this sector is to perform clinical trials to check the action of drugs. Reporting of adverse reactions must be done frequently and quickly.[15]
Cases should be notified to regulatory agencies. Patient and their first-degree relatives should be alerted to their elevated risk of reaction to the same drug. Genotyping should be undertaken as a screening measure for the particular population to know for which drug they are more prone for. There was concern over the lack of documentation. The system of communication between the hospital laboratory and the general practitioner caused concern.
The incidence of reports about drug-induced SJS and TEN made through the central reporting system (the database of the Indian Pharmacovigilance Centre) is less which may be due to negligence in documentation and publishing.
Conclusion
Stevens–Johnson syndrome a potentially life-threatening condition with an elevated morbidity and mortality requires immediate intervention and adequate management. In addition, we would like to alert that the use of lamotrigine should be supervised.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Freeberg IM, Eisen AZ, Wolff K, Austin KF, Goldsmith LA, Katz SI, editors . Fitzpatrick's Dermatology in General Medicine. 6th ed. McGraw-Hill (Asia), Jurong, Singapore: McGraw Hill; 2003. pp. 543–57. [Google Scholar]
- 2.Jao T, Tsai TH, Jeng JS. Aggrenox (Asasantin retard)-induced Stevens–Johnson syndrome. Br J Clin Pharmacol. 2009;67:264–5. doi: 10.1111/j.1365-2125.2008.03340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deore SS, Dandekar RC, Mahajan AM, Shiledar VV. Drug induced – Stevens–Johnson syndrome: A case report. Int J Sci Stud. 2014;2:84–7. [Google Scholar]
- 4.Qureshi D, Dave L, Dixit R. Stevens Johnson syndrome due to nevirapine. Indian J Allergy Asthma Immunol. 2014;28:49–51. [Google Scholar]
- 5.Lehloenya R. Management of Stevens–Johnson syndrome and toxic epidermal necrolysis. Curr Allergy Clin Immunol. 2007;20:124–8. [Google Scholar]
- 6.Mockenhaupt M. The current understanding of Stevens–Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7:803–13. doi: 10.1586/eci.11.66. [DOI] [PubMed] [Google Scholar]
- 7.Tseng HW, Chang CH. Toxic epidermal necrolysis due to lamotrigine monotherapy for bipolar disorder. TZU Chi Med J. 2009;21:165–8. [Google Scholar]
- 8.Oflaz S, Kalkan HS, Gokce E, Karsidag C, Gurel MS. Stevens–Johnson syndrome – toxic epidermal necrolysis induced by A combination of lamotrigine and valproic acid: A case report. Bull Clin Psychopharmacol. 2011;21:150–3. [Google Scholar]
- 9.Gaeta F, Alonzi C, Valluzzi RL, Viola M, Elia M, Romano A. Hypersensitivity to lamotrigine and nonaromatic anticonvulsant drugs: A review. Curr Pharm Des. 2008;14:2874–82. doi: 10.2174/138161208786369713. [DOI] [PubMed] [Google Scholar]
- 10.Sogut A, Yilmaz A, Kilinc M, Gsogut A, Demiralay E, Uzar H. Suspected lamotrigine-induced toxic epidermal necrolysis. Acta Neurol Belg. 2003;103:95–8. [PubMed] [Google Scholar]
- 11.Ghaffarpour M, Hejazie SS, Harirchian MH, Pourmahmoodian H. Phenytion, carbamazepine, sodium valproate and lamotrigine induced cutaneous reactions. Acta Med Iran. 2005;43:37–42. [Google Scholar]
- 12.Khalili B, Bahna SL. Pathogenesis and recent therapeutic trends in Stevens–Johnson syndrome and toxic epidermal necrolysis. Ann Allergy Asthma Immunol. 2006;97:272–80. doi: 10.1016/S1081-1206(10)60789-2. [DOI] [PubMed] [Google Scholar]
- 13.French LE. Toxic epidermal necrolysis and Stevens–Johnson syndrome: Our current understanding. Allergol Int. 2006;55:9–16. doi: 10.2332/allergolint.55.9. [DOI] [PubMed] [Google Scholar]
- 14.Harr T, French LE. Toxic epidermal necrolysis and Stevens–Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Härmark L, van Grootheest AC. Pharmacovigilance: Methods, recent developments and future perspectives. Eur J Clin Pharmacol. 2008;64:743–52. doi: 10.1007/s00228-008-0475-9. [DOI] [PubMed] [Google Scholar]


